Abstract
Tumor metastasis is the most common cause of death in cancer patients. Here, we show that two, fully synthetic migrastatin analogues, core macroketone and core macrolactam, are potent inhibitors of metastasis in a murine breast tumor model. Administration of these readily accessible compounds nearly completely inhibits lung metastasis of highly metastatic mammary carcinoma cells. Treatment of tumor cells with core macroketone and core macrolactam blocks Rac activation, lamellipodia formation, and cell migration, suggesting that these chemical compounds interfere with the invasion step of the metastatic process. These compounds also inhibit the migration of human metastatic breast cancer cells, prostate cancer cells, and colon cancer cells but not normal mammary-gland epithelial cells, fibroblasts, and leukocytes. These data demonstrate that the macroketone and macrolactam core structures are specific small-molecule inhibitors of tumor metastasis. These compounds or their analogues could potentially be used in cancer-therapy strategies.
Keywords: cancer therapy, cell migration, chemical biology
Metastasis is the multistep process wherein a primary tumor spreads from its initial site to secondary tissues/organs (1, 2). This metastatic process is selective for cells that succeed in cell migration/invasion, embolization, survival in the circulation, arrest in a distant capillary bed, and extravasation into and multiplication within the organ parenchyma. Failure at any of these steps could block the entire metastatic process. Because tumor spreading is responsible for the majority of deaths of cancer patients, the development of therapeutic agents that inhibit tumor metastasis is very desirable. Such agents could be effective in restraining new tumor formation when earlier therapy or surgery has failed or in increasing the successful containment of solid tumors in combination therapy with other agents.
Migrastatin is a macrolide natural product first isolated from a cultured broth of Streptomyces, and its structure features a 14-membered macrolactone ring (Fig. 1A) (3, 4). At high μM concentrations, the natural product inhibits the migration of several types of tumor cells in vitro but has no effect on the biosyntheses of DNA, RNA, and protein in these cells (5). Recently, we reported that some synthetic migrastatin analogues are much more potent (by 3 orders of magnitude) than the migrastatin-parent natural product in inhibiting tumor cell migration in vitro (6, 7). Here, we have tested two synthetic migrastatin analogues, a core macroketone and a core macrolactam, on inhibiting breast tumor metastasis in a mouse model. We now report that these two readily synthesized compounds are potent inhibitors of breast tumor metastasis, reducing 91–99% of tumor spreading to the lung. Furthermore, we have shown that the cellular basis for this effect is interference with the formation of lamellipodia, which, in turn, inhibits migration of tumor cells.
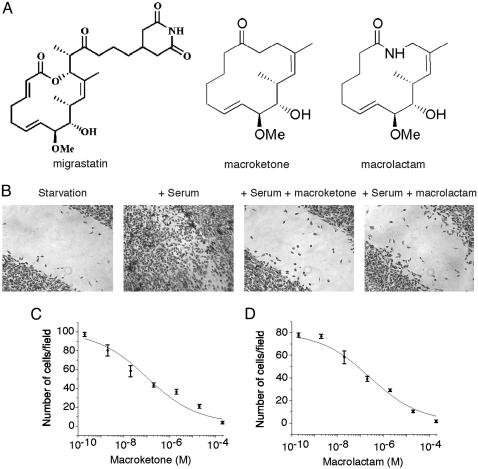
Fig. 1.
Inhibition of mouse breast tumor 4T1 cell migration by core macroketone and core macrolactam. (A) Chemical structures of migrastatin, core macroketone, and core macrolactam. (B) Wound-healing assay showed that core macroketone (2 μM) and macrolactam (2 μM) inhibited the migration of mouse breast tumor 4T1 cells induced by serum. (C) Chamber assay of the effect of various concentrations of core macroketone on serum-induced 4T1 cell migration. (D) Chamber assay of the effect of core macrolactam on the serum-induced migration of 4T1 cells. Data represent mean ± SD of three experiments.
Materials and Methods
Tumor and Primary Cells. Mouse breast tumor 4T1 cells, human breast-tumor MDA-MB-231 cells, and human mammary-gland epithelial MCF-10A cells were obtained from American Type Culture Collection. Human colon tumor Lovo-229 cells and human prostate tumor PC-3 cells were provided by L. Ma and P. P. Pandolfi (Sloan–Kettering Institute for Cancer Research). Primary mouse leukocytes (neutrophils) were provided by W. Muller (Weill Medical College of Cornell University).
Animals. Studies using mice were performed in compliance with the Institutional Animal Care and Use Committee of the Weill Medical College.
In Vitro Wound-Healing Assay. Tumor or primary cells in medium containing 10% FBS were seeded into wells of 24-multiwell plates (Becton Dickinson). After the cells grew to confluence, wounds were made by sterile pipette tips. Cells were washed with PBS and refreshed with medium with or without 10% FBS. After overnight incubation at 37°C, the cells were fixed and photographed (6).
Chamber Cell-Migration Assay. Cell migration was assayed in Boyden chambers [8.0-μm-pore-size polyethylene terephthalate membrane with Falcon cell-culture insert (Becton Dickinson)]. Cells were trypsinized and counted. A total of 5 × 104 to 10 × 104 cells in serum-free medium (300 μl) were added to the upper chamber, and 500 μl of appropriate medium with 10% FBS were added to the lower chamber. Transwells were incubated for 4–6 h at 37°C. Cells on the inside of the transwell inserts were removed with a cotton swab, and cells on the underside of the insert were fixed and stained. Photographs of three random fields were taken, and the cells were counted to calculate the average number of cells that had transmigrated (6).
Rac-Activation Assay. 4T1 cells were serum-starved overnight. After incubation with 10% FBS for 4 h, the cells were washed with PBS and lysed with lysis buffer (20 mM Tris·HCl, pH 8.0/150 mM NaCl/1 mM EDTA/1 mM EGTA/1% Triton X-100/1 μg/ml Leupeptin/1 mM PMSF). Thirty micrograms of GST-PBD (Pak Rac/Cdc42-binding domain) attached to beads were added to the cell lysates. After incubation at 4°C for 60 min, the beads were washed three times with lysis buffer. SDS sample buffer was added to the beads, and the samples were boiled at 90°C for 10 min and run on 12% SDS/PAGE gels. Western blotting of Rac was done with anti-Rac antibody (clone 23A8, Upstate Biotechnology, Lake Placid, NY).
Fluorescence Microscopy. Staining and observation of F-actin polymers were performed as described in ref. 8. Cells were plated onto coverslips coated with gelatin. The cells were then fixed with 3.7% formaldehyde, and the fixed cells were permeabilized in 0.1% Triton X-100 for 5 min. After washing in PBS, phalloidin conjugated to rhodamine (Molecular Probes) in a solution containing PBS and 1% BSA was added to stain actin. After incubation for 30 min at room temperature, the cells were washed extensively to reduce nonspecific interactions. The coverslips were then fixed onto slides and imaged by using a Zeiss fluorescence microscope. For cortactin staining, an anti-cortactin antibody (Upstate Cell Signaling Solutions, Charlottesville, VA) was used. Immunostaining was done as described in ref. 8.
Breast Tumor Metastasis in Mice. Female BALB/c mice (6–8 weeks old) were purchased from The Jackson Laboratory. 4T1 tumor cells (1 × 105) were injected s.c. into the abdominal mammary-gland area of mice by using 0.1 ml of a single-cell suspension in PBS on day 0 (9). The dosage of tumor implantation was empirically determined to give rise to tumors of ≈10 mm in diameter in untreated wild-type mice within 21–23 days. Starting on day 7, when the tumors averaged ≈4–5 mm in diameter, test compounds or control PBS saline were given every day by i.p. injection at 10 mg/kg or 20 mg/kg per mouse until day 25. On day 28, the mice were killed. This dosage regimen was well tolerated with no signs of overt toxicity. Each group included five mice. On the day the mice were killed, primary tumors were measured by using electronic calipers. The numbers of metastatic 4T1 cells in lungs were determined by clonogenic assay (10). In brief, on day 28, the lungs were removed from each mouse, finely minced, digested in 5 ml of enzyme mixture containing 1× PBS and 1 mg/ml collagenase type IV, and incubated for 2 h at 37°C on a platform rocker. After incubation, samples were filtered through 70-μm nylon cell-strainers and washed twice with PBS. The resulting cells were suspended, plated with a series of dilutions in 10-cm tissue-culture dishes in RPMI medium 1640 containing 60 μM thioguanine for clonogenic growth. Because 4T1 tumor cells are resistant to 6-thioguanine, metastasized tumor cells formed foci after 14 days, at which time they were fixed with methanol and stained with 0.03% methylene blue for counting.
Statistical Analysis. Data are expressed as mean ± SE from five experiments and analyzed by one-way ANOVA followed by Dunnett's multiple comparison test with significance defined as P < 0.05.
Results and Discussion
Inhibition of Migration of Metastatic Tumor Cells by Migrastatin Analogues. Before studying the effects of the core macroketone and the core macrolactam analogues in animals, we first investigated the effects of these chemical compounds on the migration of tumor cells in vitro. As shown in Fig. 1, whereas serum induced the migration of metastatic mouse breast tumor 4T1 cells, the addition of the macroketone or macrolactam congeners inhibited serum-induced 4T1 cell migration as measured by both the wound-healing assay and the Boyden chamber assay (Fig. 1 B–D). The macroketone and macrolactam core structures were quite effective with IC50 values of 100 and 255 nM, respectively (Fig. 1 C and D). The parent-compound migrastatin effect was with an IC50 of 29 μM (6, 7). These compounds had little effect on the proliferation of 4T1 cells in culture (6, 7).
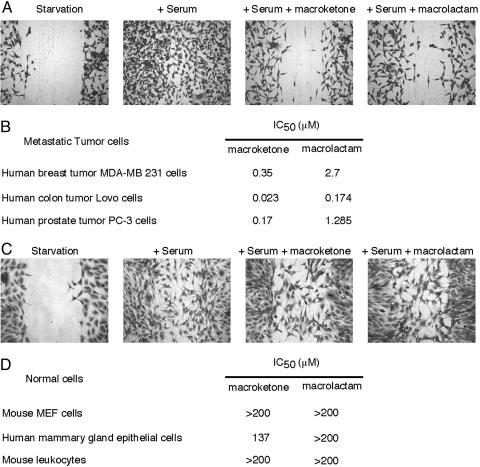
The macroketone and macrolactam congeners also inhibited the migration of several invasive and metastatic human tumor cell lines, such as human breast tumor MDA-MB-231 cells, human colon tumor Lovo cells, and human prostate tumor PC-3 cells (Fig. 2 A and B). In contrast, migration of normal human mammary-gland epithelia MCF-10A cells, mouse embryonic fibroblast cells, or primary mouse leukocytes was rather insensitive to these compounds (Fig. 2 C and D). These cellular studies demonstrated that the macroketone and macrolactam core structures are highly selective for mouse and human metastatic tumor cells vs. normal cells. These results also suggest that the activity level of the biochemical target of these compounds might be high in metastatic tumor cells, thus sensitizing these tumor cells to the compounds.
Fig. 2.
Inhibition of human tumor cell migration by core macroketone and core macrolactam. (A) Wound-healing assay showed that core macroketone (20 μM) and macrolactam (20 μM) inhibited the migration of human breast tumor MDA-MB-231 cells induced by serum. (B) IC50 of core macroketone and core macrolactam on serum-induced migrations of human breast, colon, and prostate tumor cells. (C) Wound-healing assay showed that core macroketone (200 μM) and macrolactam (200 μM) had no effect on the migration of mouse embryonic fibroblast (MEF) cells induced by serum. (D) IC50 of core macroketone and core macrolactam on serum (10% FBS)-induced migration of MEF and human mammary-gland epithelial MCF-10A cells and on N-formyl-peptide-induced (100 nM) migration of primary mouse leukocytes (neutrophils). Data are representative of three experiments.
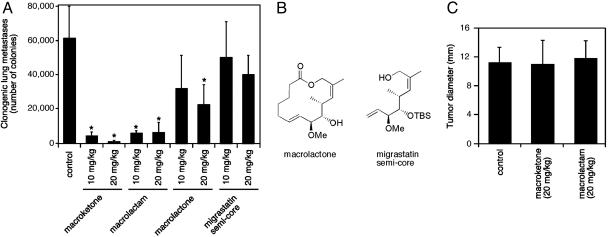
Near-Complete Inhibition of Lung Metastasis of Highly Metastatic Mammary Carcinoma Cells by Migrastatin Analogues in Mice. To assess the potential of these migrastatin analogues as therapeutic agents, we next investigated whether these analogues could affect tumor metastasis in the 4T1 mouse mammary tumor model. We examined the lung metastasis of 4T1 tumor cells in mice with or without treatment with these chemical compounds. The mouse 4T1 tumor closely mimics human breast cancer in its anatomical site, immunogenicity, growth characteristics, and metastatic properties (10). From the mammary gland, the 4T1 tumor spontaneously metastasizes to a variety of target organs including the lung, bone, brain, and liver, primarily through a hematogenous route (11). Ten days after implantation of 4T1 cells in the mammary glands of BALB/c mice, we injected the mice with the macroketone and the macrolactam core structures or control saline PBS. The dosages of the macroketone or macrolactam core structures were 10 mg/kg or 20 mg/kg. The compounds were injected daily. After 20 days, the mice were killed and examined for metastasis to the lung. Whereas mice injected with the control saline (vehicle alone) showed large numbers of metastasized 4T1 cells in the lung, the number of metastasized 4T1 cells in the lungs of mice treated with either macroketone or macrolactam was reduced by 91–99% (Fig. 3A). Mice treated with macroketone or macrolactam formed primary mammary tumors similar in size to those of mice treated with saline (Fig. 3C), implying that these chemical compounds did not interfere with primary-tumor formation by 4T1 cells. These compounds did not cause obvious side effects because the mice appeared normal with no evidence of weight loss, lethargy, or ruffled fur. Further studies are needed to establish the dose–response relationship in mice. Nevertheless, our results demonstrate that macroketone and macrolactam are potent inhibitors of 4T1 tumor cell metastasis from the mammary gland to the lung.
Fig. 3.
Inhibition of breast tumor metastasis by core macroketone and core macrolactam in a mouse model. (A) Lung metastasis was measured by the 6-thioguanine clonogenic assay. (B) Chemical structures of macrolactone and migrastatin semicore. (C) On the day the mice were killed, tumor diameter (in mm) was measured with an electronic caliper. Results are mean ± SD (n = 5). *, P < 0.01.
As further controls for the specific effects of these core structures, we examined two other compounds: migrastatin semicore and macrolactone (Fig. 3B). Upon testing with 4T1 cells for its ability to inhibit cell migration in vitro, migrastatin semicore showed a significantly lower activity than macroketone and macrolactam with an IC50 of 40 μM (6, 7). Although the macrolactone was very effective at inhibiting 4T1 cell migration (IC50 of 24 nM), our previous metabolic-stability studies showed that macrolactone was very unstable in mouse plasma with a half-life of <5 min (7). As shown in Fig. 3A, treatment of mice with migrastatin semicore (10 and 20 mg/kg) did not significantly reduce the 4T1 tumor metastasis in these mice. Although the effect of 20 mg/kg macrolactone on 4T1 tumor metastasis was statistically significant, the effect was much less than those of macroketone and macrolactam (Fig. 3A). The reduced effectiveness of macrolactone was likely due to its instability in mice.
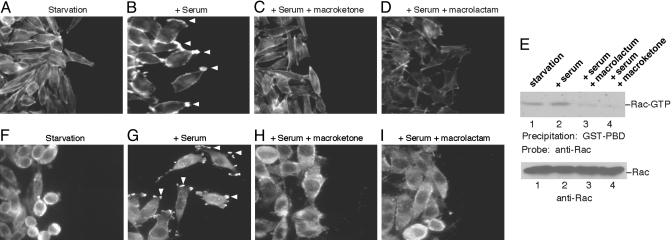
Inhibition of Rac Activation and Lamellipodia Formation by Migrastatin Analogues. To understand the cellular basis for the inhibition of macroketone and macrolactam in tumor metastasis and cell migration, we investigated the effects of these substances on the actin cytoskeleton and microtubules in 4T1 cells. Cell migration is a sequential, interrelated multistep process (12). It involves the formation of lamellipodia at the front edge, cycles of adhesion and detachment, cell body contraction, and tail retraction (12). We found that the core macroketone and the core macrolactam inhibited the formation of lamellipodia at the leading edge. Fig. 4A shows cortical staining of actin polymers in serum-starved 4T1 cells. Whereas the addition of serum induced the formation of lamellipodia (Fig. 4B), addition of either the macroketone or macrolactam cores disrupted the formation of lamellipodia (Fig. 4 C and D). Because the small GTPase Rac controls lamellipodia formation (13), we further studied the mechanism of these migrastatin analogues by examining their effects on the activation of Rac. As shown in Fig. 4E, the basal activity of Rac was high in the metastatic 4T1 cells; addition of serum slightly increased the Rac activity. Remarkably, pretreatment of 4T1 cells with core macroketone or core macrolactam blocked serum-induced Rac activation and significantly reduced the Rac activity below the basal level (Fig. 4E). These data suggest that the core macroketone and macrolactam inhibition is at, or upstream of, Rac activation. To confirm this finding, we further studied the subcellular localization of cortactin. Cortactin is a cytosolic protein and is translocated by activated Rac to the lamellipodia, where cortactin stimulates Arp2/3-mediated actin polymerization (14, 15). As shown in Fig. 4 F–I, similar to F-actin polymer staining, serum treatment induced the translocation of cortactin to the lamellipodia (Fig. 4G). Notably, pretreatment of 4T1 cells with core macroketone or core macrolactam blocked cortactin translocation (Fig. 4 H and I). These findings of altered cortactin subcellular localization are consistent with the notion that the migrastatin analogues inhibit Rac activation. Moreover, neither compound had any effect on microtubule organization (data not shown). These data demonstrate that the cellular basis of the action of these migrastatin analogues on tumor metastasis involves the blocking of Rac activation and, thus, the disruption of lamellipodia formation.
Fig. 4.
Inhibition of lamellipodia formation by core macroketone and core macrolactam. A wound was made in plates with mouse breast tumor 4T1 cells, and actin polymers were stained (A–D). (A) 4T1 cells in starvation medium. (B) 4T1 cells in the presence of 10% FBS. Arrowheads indicate lamellipodia. (C) 4T1 cells in the presence of 10% FBS and 2 μM core macroketone. (D) 4T1 cells in the presence of 10% FBS and 2 μM core macrolactam. (E) Rac activation assay. (F–I) Subcellular localization of cortactin. (F) Cortactin immunostaining in serum-starved 4T1 cells. (G) Serum-induced cortactin translocation to lamellipodia (arrowheads). (H) 4T1 cells in the presence of 10% FBS and 2 μM core macroketone. (I) 4T1 cells in the presence of 10% FBS and 2 μM core macrolactam. Data are representative of three experiments.
Conclusion
Our data have shown that macroketone and macrolactam are potent, minimally toxic inhibitors of tumor metastasis. The cellular basis for this effect rests on the inhibition of Rac activation and lamellipodia formation, which are required for cell migration as initial steps in the multistep process of tumor metastasis. The biochemical basis of the inhibition needs further investigation, and the identification of the biological target(s) of these small molecules might provide new target domains/sites for other chemical compounds. Given the fact that these agents also inhibit human tumor cell migration, one can foresee the use of such compounds in various cancer therapies in combination with tumor-growth inhibitors such as the well explored antiangiogenesis agents.
Acknowledgments
We thank L. Ma, P. P. Pandolfi, and W. Muller for tumor or primary cells; and J. Bromberg, T. Maack, D. McGarrigle, L. Palmer, and X. Zhou for comments on the manuscript. This work was supported by grants from the National Institutes of Health (to X.-Y.H. and S.J.D.).
References
- 1.Weiss, L. (2000) Cancer Metastasis Rev. 19, 193-383. [PubMed] [Google Scholar]
- 2.Fidler, I. J. (2003) Nat. Rev. Cancer 3, 453-458. [DOI] [PubMed] [Google Scholar]
- 3.Nakae, K., Yoshimoto, Y., Ueda, M., Sawa, T., Takahashi, Y., Naganawa, H., Takeuchi, T. & Imoto, M. (2000) J. Antibiot. (Tokyo) 53, 1228-1230. [DOI] [PubMed] [Google Scholar]
- 4.Woo, E. J., Starks, C. M., Carney, J. R., Arslanian, R., Cadapan, L., Zavala, S. & Licari, P. (2002) J. Antibiot. (Tokyo) 55, 141-146. [DOI] [PubMed] [Google Scholar]
- 5.Nakae, K., Yoshimoto, Y., Sawa, T., Homma, Y., Hamada, M., Takeuchi, T. & Imoto, M. (2000) J. Antibiot. (Tokyo) 53, 1130-1136. [DOI] [PubMed] [Google Scholar]
- 6.Njardarson, J. T., Gaul, C., Shan, D., Huang, X. Y. & Danishefsky, S. J. (2004) J. Am. Chem. Soc. 126, 1038-1040. [DOI] [PubMed] [Google Scholar]
- 7.Gaul, C., Njardarson, J. T., Shan, D., Dorn, D. C., Wu, K. D., Tong, W. P., Huang, X. Y., Moore, M. A. & Danishefsky, S. J. (2004) J. Am. Chem. Soc. 126, 11326-11337. [DOI] [PubMed] [Google Scholar]
- 8.Lowry, W. E., Huang, J., Ma, Y. C., Ali, S., Wang, D., Williams, D. M., Okada, M., Cole, P. A. & Huang, X. Y. (2002) Dev. Cell 2, 733-744. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuhashi, M., Liu, J., Cao, S., Shi, X. & Ma, X. (2004) J. Leukocyte Biol. 76, 322-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulaski, B. A. & Ostrand-Rosenberg, S. (1998) Cancer Res. 58, 1486-1493. [PubMed] [Google Scholar]
- 11.Aslakson, C. J. & Miller, F. R. (1992) Cancer Res. 52, 1399-1405. [PubMed] [Google Scholar]
- 12.Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. & Horwitz, A. R. (2003) Science 302, 1704-1709. [DOI] [PubMed] [Google Scholar]
- 13.Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. (1992) Cell 70, 401-410. [DOI] [PubMed] [Google Scholar]
- 14.Weed, S. A., Du, Y. & Parsons, J. T. (1998) J. Cell Sci. 111, 2433-2443. [DOI] [PubMed] [Google Scholar]
- 15.Uruno, T., Liu, J., Zhang, P., Fan, Y., Egile, C., Li, R., Mueller, S. C. & Zhan, X. (2001) Nat. Cell Biol. 3, 259-266. [DOI] [PubMed] [Google Scholar]