Abstract
To determine whether culturing peripheral blood mononuclear cells at atmospheric oxygen levels skews responses in comparison with culturing lymphocytes at physiologic oxygen levels, we cultured peripheral blood mononuclear cells at 5%, 10%, and atmospheric (20%) gas-phase oxygen for 5 days. We found that incubator oxygen levels influenced lymphocyte proliferation stimulated by two commonly used stimuli: Con A and antibodies that crosslink surface CD3 and CD28 to mimic antigen presentation. In both cases, proliferation increased as gas-phase oxygen levels increased. In contrast, oxygen levels did not influence proliferation stimulated by phytohemagglutinin, another commonly used mitogen. Similarly, oxygen levels did not impact cell viability in unstimulated cultures. Thus, we conclude that the influence of oxygen levels on proliferation depends on the stimulus, and, most importantly from the standpoint of immune responses, culturing cells at atmospheric rather than physiologic oxygen levels results in significantly increased proliferation responses to the CD3/CD28 crosslinking, a proliferation stimulus commonly used to mimic T cell antigen receptor signaling.
Keywords: CD3/CD28, incubator, T cell, tissue culture
Proliferation of lymphocytes in response to antigen or mitogen stimuli is a common measure of ex vivo lymphocyte function. Virtually all of our ideas about the autoimmune and protective mechanisms in which human lymphocytes participate are derived from studies based on this methodology. However, the CO2 incubators in which nearly all of these studies have been conducted largely contain air to which a small percentage of CO2 has been added. Thus, it is perhaps surprising that nearly all of what has been concluded to date about human lymphocyte function is based on evidence from cell-culture studies conducted at atmospheric oxygen levels (20% oxygen) that are well above the levels of oxygen to which cells are exposed in the human body.
Determining the oxygen levels available to cells in the body is a difficult task. Blood has been shown to have a partial pressure of oxygen (pO2) of 80–100 mmHg (1 mmHg = 133 Pa) (1), which is equal to 10–12.5% O2. pO2 levels in healthy tissue are considered to be in the range of 30–50 mmHg (2), which is equal to 3–6% O2. Thus, we estimate that standard culture conditions (20% oxygen) expose cells to ≈2- to 5-fold higher concentrations of oxygen than they would likely encounter in vivo.
The disparity between the in vivo pO2 and the pO2 in incubators maintained at atmospheric oxygen levels clearly raises the question of whether current culture conditions are appropriate for the study of lymphocyte and other cell functions. In fact, several studies have already examined the effect of incubator oxygen levels on lymphocyte functions such as proliferation (3), cytolytic activity (4), cytokine production (4, 5), and antibody secretion (6). However, because the methods used and the findings obtained in these studies vary considerably, no clear picture has yet emerged as to whether the behavior of cells cultured at atmospheric oxygen reflects the in vivo behavior of the cells.
To address these issues, we compared human peripheral blood mononuclear cell (PBMC) proliferation in response to standard stimuli delivered to cells cultured in incubators at which the gas phase is set at atmospheric oxygen levels (20% oxygen) or two lower oxygen levels (5% and 10%) that bracket typical oxygen levels in vivo. Briefly, we find that proliferative responses to the mitogen Con A are significantly higher at 20% than at 5% and 10% oxygen, whereas responses to the mitogen phytohemagglutinin (PHA) are affected only minimally.
We show here that PBMC proliferation in response to CD3/CD28 crosslinking, which is commonly used to simulate T cell activation by antigen-presenting cells, is significantly higher at 20% oxygen than at 5% and 10% oxygen. This finding suggests that the well studied response to CD3/CD28 stimulation in cells cultured at atmospheric oxygen levels may be considerably greater than the responses obtainable in vivo at comparable levels of stimulation.
Materials and Methods
Materials. All monoclonal antibodies (either purified or preconjugated to fluorochromes), BD Trucount tubes, and BD CompBeads were procured from Becton Dickinson Biosciences (San Jose, CA). Phycoerythrin and allophycocyanin were obtained from Prozyme (San Leandro, CA). Alexa dye 594, 5- (and 6-) carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE), and monochlorobimane were obtained from Molecular Probes. Con A, PHA, probenecid, and other fine chemicals were obtained from Sigma–Aldrich. RPMI medium 1640 was procured from GIBCO/BRL (Invitrogen). FCS was obtained from Gemini Bio-Products (Calabasas, CA).
Human Subjects. After informed consent, 10–20 ml of blood was drawn from healthy volunteers in evacuated tubes with heparin (Vacutainer, Becton Dickinson). All blood draws were performed between 9 and 11 a.m. to minimize the effects of circadian variation on the endpoints assayed.
Tri-Gas Incubators. Cells were incubated at three levels of incubator oxygen. Five percent and 10% incubator oxygen tensions were generated in Sanyo MCO-175M O2/CO2 incubators (Sanyo Scientific, Bensenville, IL). Gas-phase oxygen tensions were controlled by continuous injection of appropriate amount of medical-grade N2 to reach the target oxygen level. Cells cultured at atmospheric oxygen levels (20% oxygen) were incubated in a standard incubator without additional supply of nitrogen. CO2 levels were maintained at 7% in all cases.
Media. Cells were cultured in RPMI medium 1640 supplemented with 10% FCS (heat-inactivated)/100 units/ml penicillin/100 μg/ml streptomycin/nonessential amino acids. All media were equilibrated to the target oxygen levels at least 12 h before use. All media used for the isolation and preparations of PBMCs were equilibrated to 10% oxygen (oxygen levels found in the blood).
PBMC Isolation. PBMCs were isolated by Ficoll/Hypaque gradient separation.
CFDA-SE Staining. Human PBMCs were stained with CFDA-SE according to Mannering et al. (7) with some modifications. Briefly, Ficoll gradient-separated PBMCs were suspended at 106 cells per ml in serum-free RPMI medium 1640 and stained with 0.5 μM CFDA-SE for 10 min at 37°C. The reaction was terminated by addition of a 3-fold excess volume of RPMI medium 1640 with 10% FCS. After two washes, the cells were incubated for 15 min in RPMI medium 1640 with 10% FCS, centrifuged, and resuspended at 106 cells per ml in RPMI medium 1640 with 10% FCS.
Cell Culture and in Vitro Stimulation. CFDA-SE-labeled cells were suspended at 106 cells per ml and cultured in 24-well plates for 5 days. PBMCs were stimulated with 2.5 μg/ml PHA, 20 μg/ml Con A, or plates coated with anti-CD3 (10 μg/ml; UCTH1 clone, Becton Dickinson Biosciences) and anti-CD28 (5 μg/ml; CD28.2 clone; Becton Dickinson Biosciences). PHA and Con A were used at concentrations that were determined to be optimal for proliferation for cells at 20% oxygen.
Cell Counts and Identification of Viable Cells. Cell number at the beginning and end of the 5-day culture was determined by FACS by using BD Trucount beads according to the instructions provided by the manufacturer. Cell viability was determined by propidium iodide exclusion method.
High-Dimensional FACS Analysis. Fresh or cultured PBMCs from healthy volunteers were stained with CFDA-SE as described above. Subsequently, the cells were stained with cocktails of f luorochrome-conjugated antibodies (CD3, CD4, CD8, CD45RA, and CD11a) prepared in our laboratory or obtained from BD Pharmingen. Surface staining was performed as described (8, 9). “Fluorescence-minus-one” controls (10) were included to determine the level of nonspecific staining and autofluorescence associated with subsets of cells in each fluorescence channel. BD CompBeads (anti-mouse Ig, κ beads) were used for single-stain controls for fluorescence compensation. Propidium iodide was added to all samples before data collection to identify dead cells. High-dimensional FACS data were collected on a modified triple-laser FACS instrument. flowjo software (Treestar, San Carlos, CA) was used for fluorescence compensation and analysis.
Statistical Analysis. Analyses of FACS data, including calculation of cell division and proliferation indices, were performed by using flowjo software. Statistical analyses were performed with the jmp statistical software package (SAS Institute, Cary, NC). Sample (subject) and gas-phase oxygen level were entered as model effects in all analyses by using the jmp least-square-fit model platform. Visual representations of these analyses are shown in figures generated with the “matched columns” utility of the jmp “fit Y by X” platform, which connects the data points shown for each subject in the graph. The sample (subject) had a significant effect on the analysis (P < 0.001). For all least-square-fit models, P < 0.01.
Results
T Cell Proliferative Responses to Con A and CD3/CD28 Crosslinking Are Higher in Cells Cultured at 20% Oxygen. We evaluated the influence of physiological and atmospheric gas-phase oxygen levels on T lymphocyte proliferation in cultures of human PBMCs stimulated with CD3/CD28, Con A, or PHA. We evaluated cell proliferation by initially staining with CFDA-SE, a FACS-detectable dye that covalently binds to cellular proteins, and then measuring the amount of carboxyfluorescein fluorescence per cell at the end of a 5-day culture period. We computed division and proliferation indices with software that defines the division index as the average number of divisions that a cell has undergone and the proliferation index as the average number of division that those cells that divided underwent. We also counted the CD4 and CD8 T cells in each culture at day 1 and at the end of the 5-day culture period and calculated the change in the number of T cells in each subset as the ratio of these cell counts (fold change).
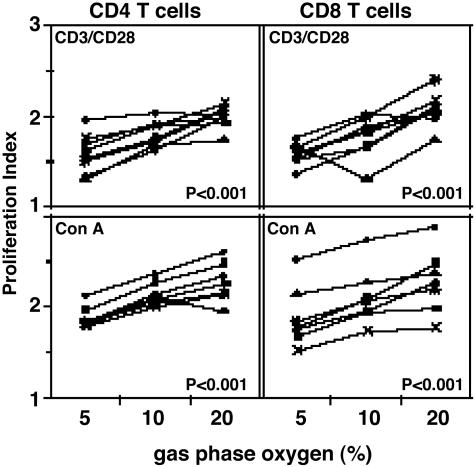
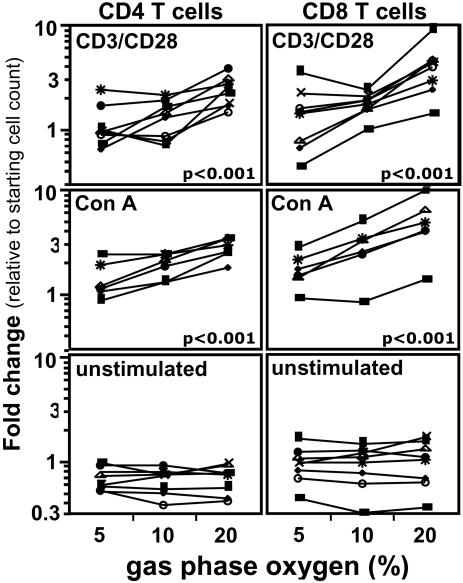
Results of these analyses demonstrate that proliferation indices (Fig. 1) and increases in CD4 and CD8 T cell numbers (Fig. 2) in response to both CD3/CD28 and Con A stimulation are significantly higher (P < 0.001) for cells cultured at atmospheric oxygen (20% O2) than for cells cultured at physiologic oxygen levels (5% and 10% O2). At 20% oxygen, total T lymphocytes increase ≈3-fold (2.8- to 3.4-fold) in relation to the number of T lymphocytes in the starting culture; at 5% oxygen, total T cells increased ≈1.4-fold (1.2- to 1.6-fold).
Fig. 1.
Proliferation indices of human lymphocytes stimulated with CD3/CD28 crosslinking and Con A are higher at 20% oxygen. Human PBMC stained with CFDA-SE were cultured at 5%, 10%, and 20% oxygen for 5 days (see Materials and Methods). Shown are lymphocytes stimulated by CD3/CD28 crosslinking (Upper) and with Con A (20 μg/ml, Lower). Statistics were calculated by using jmp software by least-square-fit model with sample and oxygen as independent variables (see Materials and Methods). Each set of connected points represents one subject (n = 8).
Fig. 2.
Increase in total number of CD4 and CD8 T cells stimulated with CD3/CD28 crosslinking and Con A is higher at 20% oxygen. Human PBMC stained with CFDA-SE were stimulated at 5%, 10%, and 20% oxygen for 5 days (see Materials and Methods). Shown are cells stimulated with CD3/CD28 crosslinking (Top), cells stimulated with Con A (20 μg/ml, Middle), and unstimulated cells (Bottom). Cell counts were performed by using BD Trucount beads (see Materials and Methods). Fold change is calculated for each subject for CD4 and CD8 T cell subsets as the ratio of the number of live cells in the subset at day 5 to the number of live cells in the subset at the beginning of the culture. Statistics were calculated using jmp software by least-square-fit model with sample and oxygen as independent variables (see Materials and Methods). Each set of connected points represents one subject (n = 6). The numbers of live CD4 and CD8 T cells present in unstimulated cultures decreased 0.3-fold on average by the end of the culture period (e.g., CD4 T cells for a typical subject decreased from 3.6 × 105 cells at the start to 2.6 × 105 cells at the end of the culture period). This decrease was independent of the incubator oxygen tension (P > 0.05).
Proliferation of lymphocytes stimulated by PHA, however, shows no significant differences at the three oxygen levels (under the cell-culture conditions in this study), although cell numbers in the PHA-stimulated cultures increased 2- to 4-fold. Cell numbers in unstimulated cultures decreased ≈0.3-fold (0.26- to 0.37-fold) relative to the cell number at the beginning of the culture independent of the oxygen level at which the cells were cultured. Thus, CD3/CD28 and Con A stimulations under our culture conditions are selectively sensitive to the incubator oxygen levels.
It is interesting to note that under all stimulation conditions, comparison of the increase in CD4 T cell numbers with the increase in CD8 T cell number indicates that CD8 T cells either proliferate more or survive better than CD4 T cells at all oxygen levels (see Fig. 2). Because the division and proliferation indices at 20% oxygen are roughly the same for the two types of T cells, this finding suggests that the CD8 T cells tend to survive better at the higher oxygen level.
Other T Cell Properties. The physical properties (forward and side scatter) and cell surface marker expression (CD3, CD4, and CD8) of the T cells in all cultures were comparable and were not influenced by culture at different oxygen levels. As expected, the frequency of naive T cells decreased in stimulated cultures; however, these measurements were made only for several donors and did not allow for comparison of the decrease as a function of oxygen level.
Discussion
We have shown here that T cell proliferation in response to a stimulus (CD3/CD28 crosslinking) that mimics signaling initiated by antigen-presenting cells is significantly higher at atmospheric gas-phase oxygen levels (20% oxygen) than at physiological oxygen levels (5% or 10% oxygen). In addition, we have shown that response to stimulation with Con A, a widely used T cell mitogen, is also higher at 20% oxygen than at 5% or 10% oxygen, whereas the response to a second well known mitogen, PHA, is comparable at all oxygen levels under the experimental conditions in this study. Cell survival in the absence of stimulation is also similar at all oxygen levels. Thus, we conclude that although responses to some stimuli are independent of culture oxygen level, responses to key stimuli are strongly influenced by the amount of oxygen available to the cells.
These findings have important consequences for interpretation of data from ex vivo studies of lymphocytes. Although most functional studies with lymphocytes are performed in cell cultures at 20% gas-phase oxygen, culturing cells at 5–10% oxygen more closely approximates the in vivo conditions. Thus, our findings demonstrate that responses to stimulation are likely to be overestimated at 20% oxygen, at least when Con A and CD3/CD28 crosslinking are used as stimulating agents.
Previous studies with PBMCs from healthy human donors have also reported increased mitogen responsiveness by lymphocytes cultured at atmospheric oxygen levels (20% oxygen). Andersen et al. (11) showed that maximal thymidine incorporation occurs more in PHA-stimulated human PBMCs at 20% gas-phase oxygen than at 1% oxygen and that viability depends less on oxygen level than replication. Loeffler et al. (12) showed that IL-2-induced human PBMC proliferation was higher at 20% oxygen than in hypoxic or anoxic conditions. A similar study using mouse splenocytes showed that although cytotoxic T lymphocytes develop in higher numbers at 20% oxygen than at 2.5% oxygen, development of cytotoxic T lymphocytes at 2.5% gas-phase oxygen is more sustained, and the cytotoxic T lymphocytes are more lytic than at 20% oxygen (4).
In contrast, Krieger et al. (3) found that Con A and pokeweed mitogen induced higher proliferation at 5% oxygen than at 20% oxygen. Similarly, Carswell et al. (13) and Haddad and Papoutsakis (14) found that PHA and CD3/CD28 crosslinking in the presence of IL-2 (in the culture medium) induce higher proliferation at 5% than at 20% oxygen. The reasons underlying the conflict between our findings and the findings reported in these studies are unclear. They could reflect differences in culture conditions and/or the PBMC source, because donors used in the latter two studies were potentially redox-compromised.
Findings from the Con A studies agree with the findings reported by Anderson et al. (11) and by Loeffler et al. (12) and thus, we believe, tip the balance in favor of responsiveness to certain mitogens being increased at 20% oxygen. However, because PHA stimulation is not affected by oxygen levels, it will be necessary to examine various mitogens on a case-by-case basis to determine whether stimulating cells cultured at 20% oxygen results in higher responsiveness than stimulating cells at oxygen levels that more closely approximate those encountered in a healthy physiological environment. As a practical matter, standardly culturing cells at 5–10% oxygen to avoid the effects of higher oxygen levels may be the best course.
Consistent with this argument, a recent report by Haddad et al. (15) showed that the expression of genes important in lymphocyte function (e.g., granzyme A) is increased in cells cultured at 5% oxygen, whereas the expression of genes involved in stress response, cell death, and cellular repair was elevated at 20% oxygen. Although these studies were conducted with PBMCs from hemachromatosis subjects, the findings point to differences in gene expression that are likely to occur in cultures of cells from healthy donors and are consistent with the differential responsiveness of lymphocytes that we observed at different oxygen levels. If so, then the additional responsiveness we see in CD3/CD28- and Con A-stimulated cells cultured in 20% oxygen may reflect the ability of these stimulations to capitalize on the expression of stress-response genes.
Overall, the differences we observed in lymphocytes stimulated at different oxygen level with agents commonly used to stimulate T cells pose a key challenge to investigators using these types of assays to study lymphocyte function. By and large, we have assumed that results obtained when cells are cultured at atmospheric oxygen levels reflect the behavior of lymphocytes in vivo. This assumption seems reasonably safe, because much of the data obtained with culture systems is compatible with findings from in vivo studies. However, as we now push further into explorations of signaling pathways and other molecular mechanisms underlying lymphocyte development and function, it may well be wise to move to culture systems that more closely approximate the internal milieu in which lymphocytes function.
In beginning this task, we decreased the gas-phase oxygen in our culture incubators to levels that would provide cells with oxygen at pO2 values in the range of those that we expect cells to encounter in vivo. The pO2 levels that are actually encountered by different types of lymphocytes in different lymphoid locations, however, have yet to be defined. It is clear that decreasing the incubator oxygen to levels below atmospheric oxygen is a move in the right direction, because tissue oxygenation must be below the oxygenation achieved in cultures exposed to atmospheric oxygen.
The work now will be to develop a more precise view of tissue-oxygenation levels and to adapt lymphocyte-culture conditions to simulate these levels and provide a nutrient mix that comes close to the in vivo environment. The findings we have presented indicate that criteria used to establish these culture conditions most likely should not include optimal lymphocyte proliferation in response to stimuli because, as we have shown, this proliferation is favored at atmospheric oxygen.
Acknowledgments
We thank the following members of the Herzenberg laboratory: Bahram Aram for excellent and devoted technical support; Dr. Rabindra Tirouvanziam and Dr. David Parks for critical and informative discussions; and John J. Mantovani for administrative help, including the preparation of the manuscript. We also owe a debt to Dr. Jonathan S. Stamler, whose work influenced the conception of this project (16). These studies were supported by National Institutes of Health Grant AI 566223.
Author contributions: K.R.A. and Leonore A. Herzenberg designed research; K.R.A. planned the experiments and performed research; K.R.A., Leonore A. Herzenberg, and Leonard A. Herzenberg analyzed data; and K.R.A., Leonard A. Herzenberg, and Leonore A. Herzenberg wrote the paper.
Abbreviations: PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin; CFDA-SE, 5- (and 6-) carboxyfluorescein diacetate, succinimidyl ester.
References
- 1.Steurer, J., Hoffmann, U., Dur, P., Russi, E. & Vetter, W. (1997) Respiration 64, 200-205. [DOI] [PubMed] [Google Scholar]
- 2.Chow, D. C., Wenning, L. A., Miller, W. M. & Papoutsakis, E. T. (2001) Biophys. J. 81, 675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger, J. A., Landsiedel, J. C. & Lawrence, D. A. (1996) Int. J. Immunopharmacol. 18, 545-552. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell, C. C., Kojima, H., Lukashev, D., Armstrong, J., Farber, M., Apasov, S. G. & Sitkovsky, M. V. (2001) J. Immunol. 167, 6140-6149. [DOI] [PubMed] [Google Scholar]
- 5.Derevianko, A., D'Amico, R. & Simms, H. (1996) Clin. Exp. Immunol. 106, 560-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishell, R. I. & Dutton, R. W. (1967) J. Exp. Med. 126, 423-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannering, S. I., Morris, J. S., Jensen, K. P., Purcell, A. W., Honeyman, M. C., van Endert, P. M. & Harrison, L. C. (2003) J. Immunol. Methods 283, 173-183. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa, S. C. & Roederer, M. (2001) Clin. Lab. Med. 21, 697-712, vii. [PubMed] [Google Scholar]
- 9.Sahaf, B., Heydari, K. & Herzenberg, L. A. (2003) Proc. Natl. Acad. Sci. USA 100, 4001-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roederer, M. (2001) Cytometry 45, 194-205. [DOI] [PubMed] [Google Scholar]
- 11.Andersen, V., Hellung-Larsen, P. & Sorensen, S. F. (1968) J. Cell. Physiol. 72, 149-152. [DOI] [PubMed] [Google Scholar]
- 12.Loeffler, D. A., Juneau, P. L. & Masserant, S. (1992) Br. J. Cancer 66, 619-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carswell, K. S., Weiss, J. W. & Papoutsakis, E. T. (2000) Cytotherapy 2, 25-37. [DOI] [PubMed] [Google Scholar]
- 14.Haddad, H. & Papoutsakis, E. T. (2001) Cytotherapy 3, 435-447. [DOI] [PubMed] [Google Scholar]
- 15.Haddad, H., Windgassen, D., Ramsborg, C. G., Paredes, C. J. & Papoutsakis, E. T. (2004) Biotechnol. Bioeng. 87, 437-450. [DOI] [PubMed] [Google Scholar]
- 16.Eu, J. P., Hare, J. M., Hess, D. T., Skaf, M., Sun, J., Cardenas-Navina, I., Sun, Q. A., Dewhirst, M., Meissner, G. & Stamler, J. S. (2003) Proc. Natl. Acad. Sci. USA 100, 15229-15234. [DOI] [PMC free article] [PubMed] [Google Scholar]