Abstract
Human brain evolution involved both neurological reorganization and an increase in overall brain volume relative to body mass. It is generally difficult to draw functional inferences about the timing and nature of brain reorganization, given that superficial brain morphology recorded on fossil endocasts is functionally ambiguous. However, the cerebellum, housed in the clearly delineated posterior cranial fossa, is functionally and ontologically discrete. The cerebellum is reciprocally connected to each of 14 neocortical regions important to human cognitive evolution. Cerebellar volume varies significantly relative to overall brain volume among mammalian orders, as well as within the primate order. There is also significant diachronic variation among fossil human taxa. In the australopithecines and early members of the genus Homo, the cerebral hemispheres were large in proportion to the cerebellum, compared with other hominoids. This trend continued in Middle and Late Pleistocene humans, including Neandertals and Cro-Magnon 1, who have the largest cerebral hemispheres relative to cerebellum volume of any primates, including earlier and Holocene humans. In recent humans, however, the pattern is reversed; the cerebellum is larger with respect to the rest of the brain (and, conversely, the cerebral hemispheres are smaller with respect to the cerebellum) than in Late Pleistocene humans. The cerebellum and cerebral hemispheres appear to have evolved reciprocally. Cerebellar development in Holocene humans may have provided greater computational efficiency for coping with an increasingly complex cultural and conceptual environment.
Keywords: human evolution, Plio-Pleistocene hominins, Upper Paleolithic transition
Paleoneurologists agree that increased encephalization is an important dynamic of human brain evolution. However, brain evolution involved more than brain expansion. It also involved reorganization to support specific, uniquely human cognitive tasks, including those involved in linguistic processing and a highly developed facility for manufacturing and manipulating tools. Nevertheless, despite more than a century of effort, there is little consensus about how and when such reorganization occurred.
One approach to exploring functional reorganization of the brain in humans has been the analysis of impressions of the brain's surface convolutions (sulci and gyri) on the inner table of the endocranium. However, endocranial markings are notoriously difficult to identify reliably (1, 2). Even where the impressions are fairly clear, taphonomic processes may distort the evidence. In addition, the functional correlates of the brain's surface convolutions, especially in fossils, are literally superficial. Cognitive functions occur through internal connections among brain regions, as well as through the distribution of neuroreceptors that cannot be detected by examining the surface of the brain, let alone from endocranial markings. Therefore, functional inferences based on sulcal patterns are problematic.
Another approach to endocranial analysis has focused on changes in endocranial volume relative to body size. Unfortunately, even when endocranial volume can be unambiguously determined, body mass estimates for fossil humans are often based on incomplete specimens, extrapolated from body sizes of living taxa (which may not be strictly analogous), and/or undermined by the problem of determining actual brain size from endocranial measurements.
However, one anatomically, ontologically, and functionally discrete brain region can be analyzed in a way that sidesteps many of the problems posed by regional brain analyses and volumetric studies. The cerebellum occupies the posterior cranial fossa (PCF), which is well delineated by the petrous crests, sella turcica, and lateral sulci. If the volume of the posterior cranial fossa can be determined relative to overall endocranial volume, and if there are significant differences in cerebellar proportions relative to the rest of the brain that correlate with morphological and behavioral aspects of the paleontological record, then certain inferences may be drawn about the relative contribution of the cerebellum and the rest of the brain (primarily the cerebral hemispheres) to overall cognitive function.
Recent neuroanatomical studies and radiographic observations have demonstrated that the cerebellum plays a role in many cognitive functions. Moreover, the cerebellum has reciprocal connections, through the thalamus, with each of the major neocortical regions listed by Holloway (3) as having changed in the course of human cognitive evolution (Table 1).
Table 1. Neocortical regions with reciprocal cerebellar connections.
| Region and Brodman's areas | Selected functions |
|---|---|
| Parietal cortex: 5, 7, 39, 40 | Visually guided hand movements; motor planning; verbal processing and storage; spatial navigation |
| Temporal cortex: 22, 37 | Cognitive and articulatory aspects of language processing |
| Frontal and prefrontal cortex: 8, 9, 10, 44, 45 | Language functions; working memory; directed attention; motor and cognitive planning |
| Occipital cortex: 17, 18, 19 | Visual processing |
Although developmental constraints appear to impose limits on the independent evolution of distinct brain regions (4–6), numerous researchers have shown significant, systematic variation in cerebellar proportions among and within mammalian orders, including within the primate order (7–15).
Methods
The present study is an analysis of changes in relative cerebellar volume in humans compared with other mammals, other primates, and each other. Measurements of total brain volume, cerebellar volume, and body mass for 14 orders of extant mammals were assembled from the literature, including Monotremata (16), Marsupalia (16, 17), Insectivora, Macroscelidae and Scandentia (18), Chiroptera (19), Rodentia (17), Edentata (20), Lagomorpha (21), Cetacea (22, 23), Proboscidea (24), Sirenia (25), Rodentia (17), Artiodactyla (26), Carnivora (17, 21), and Primates (12–14, 18, 21, 27).
The primate data were augmented with data for humans taken from the literature, including 51 data points representing 1,416 recent humans (13, 14, 21, 27, 28), as well as original measurements from 18 fossil humans.
For specimens where cerebellar volume was available but total brain volume and/or body mass for the same specimens was not available, the mean value for the species was used. The present analysis conforms to the majority of studies in which cerebellar volume, rather than mass, is used as a variable. Where masses were given instead of volumes, a conversion factor based on the specific gravity of brain tissue was used: 1.04 brain (or cerebellum) volume = brain (or cerebellum) mass × 0.96. Brain volume for the fossil endocasts was calculated as 0.88 × endocranial volume, after Pickering (29).
Because the cerebellum constitutes a significant portion of overall brain volume, cerebellum volume (CBLM) was subtracted from overall brain volume to obtain a net brain value (NetBrain). Reduced major axis linear regression of CBLM on NetBrain produced predicted values and residuals for the sample and formed the basis for calculation of a cerebellar quotient (CQ) = actual/predicted value for each specimen.
Data from the fossil humans were derived from digital scans of latex, resin, and plaster endocasts of 18 specimens, including 1 chimpanzee, 2 australopithecines (AL 23000 and STS 19), 3 early members of the genus Homo (KNM-ER 1813, KNM-ER 1805, and KNM-ER 1470), 7 Homo erectus (Zhoukoudian E3 and Zhoukoudian L3, KNM-WT 15000, Sangiran 17, and KNM-ER 3733 and KNM-ER 3883), 2 early archaic Homo sapiens (Kabwe/Broken Hill, Swanscombe), and 3 late archaic H. sapiens, all Neandertals (La Chapelle-aux-Saints 1, La Ferrassie 1, and Forbes' Quarry 1). The endocasts were digitized with a noncontact laser digitizer (Vivid 700, Minolta, Ramsey, NJ) and measured with innovmetric software by PolyWorks (Sainte-Foy, QC, Canada). The PCF was digitally dissected from the scanned model by using the plane defined by the transverse sinuses, the petrous crests, and the dorsum sellae.
In addition, computed tomography (CT) scans of three Late Pleistocene specimens (La Chapelle-aux-Saints 1, La Ferrassie 1, and Cro-Magnon 1) were measured by using nih image software, version 1.62 (30). Equivalence of the CT data and the endocast data was confirmed when PCF volumes obtained from the CT scans and the digital endocasts for La Ferrassie 1 and La Chapelle-aux-Saints 1 were found to be identical.
Cerebellar volumes for the endocasts were estimated from PCF volume as follows: PCF volume and cerebellum volume were measured from MRI scans of 34 living nonhuman primate and modern human brains by using nih image software. The cerebellum was segregated by intensity thresholding, and its area was measured in serial slices where it appeared. Cerebellum volume was calculated by multiplying total area by slice thickness. The boundaries of the PCF were determined by using selected endocranial landmarks in serial slices; again, volume was calculated by multiplying total area by slice thickness. A least-squares linear regression permitted estimation of cerebellum volume from PCF volume: CBLM = 3.25 + 1.22 PCR (r2 = 0.89). The regression formula was applied to the PCF volumes measured in the fossil endocasts and CT scans.
Results
Interordinal Comparisons. Regression of log CBLM on log NetBrain established that cerebellar volume is highly correlated with NetBrain volume in the mammalian sample: log CBLM = -0.82 + 0.99 log NetBrain (r2 = 0.99).
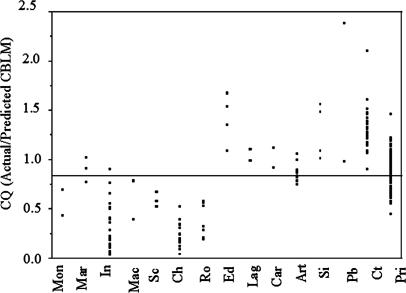
Mean CQ (Fig. 1) for the entire mammalian sample (14 orders) is 0.87 ± 0.12, ranging from 0.23 ± 0.03 in Chiroptera (n = 15) to 2.38 and 0.98 in the Proboscidea (n = 2). Primates, with a CQ of 0.87 ± 0.02 (n = 109), scatter around the overall mean. Differences in CQ among mammalian orders are significant (Wilcoxon/Kruskal–Wallis, P < 0.0001).
Fig. 1.
CQ comparison across mammalian orders. Mon, Monotremata (16); Mar, Marsupalia (16, 17); In, Insectivora (18); Mac, Macroscelidae (18); Sc, Scandentia (18); Ch, Chiroptera (19) (points represent family means); Ro, Rodentia (17); Ed, Edentata (20); Lag, Lagomorpha (21); Car, Carnivora (17, 21); Art, Artiodactyla (26); Si, Sirenia (25); Pb, Proboscidea (24); Ct, Cetacea (22, 23); Pri, Primates (12–14, 18, 21, 27, 28).
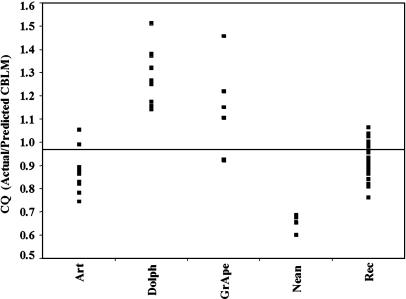
Even when extreme outliers (elephants and whales) are excluded from the analysis, log CQ is only moderately correlated with log body mass (r2 = 0.65). CQ is taxonomically arbitrary as well. The independence of CQ from body mass and taxon is emphasized by a comparison of medium-bodied taxa (43–100 kg). As shown in Fig. 2, great apes (Pan, Gorilla, and Pongo) and the common dolphin (Delphinis delphis) are more similar to each other than recent humans are to great apes or Neandertals. On the other hand, humans are more similar to sheep and pigs than to the other taxa represented.
Fig. 2.
CQ in medium-bodied taxa. (Art, Suidae, Bovidae; Dolph, D. delphis; GrApe, Pan, Pongo, and Gorilla; Nean, Neandertals; Rec, contemporary modern humans). Estimates of body mass for Neandertals are from Ruff et al. (31).
Intraordinal Comparisons. CQ also differs significantly within orders. For example, among bats, CQ ranges from 0.02 in the Craseonycteridae (n = 1) to 0.52 ± 1.03 in the Pteropodidae (n = 49). These families differ significantly from each other and from the Molossidae (CQ = 0.22 ± 0.04; n = 19), who fall close to the Chiropteran mean of 0.23.
In primates, the African great apes (Gorilla and Pan), with a mean CQ of 1.13 ± 0.08 (n = 6), are significantly different from the Cercopithecidae, with a CQ of 0.77 ± 0.03 (Wilcoxon/Kruskal–Wallis, P = 0.002). The great apes are also significantly different from recent humans (CQ = 0.91 ± 0.01; Wilcoxon/Kruskal–Wallis, P = 0.003).
These observations are consistent with the assessment of Finlay and Darlington (6) that taxonomy and body size alone are inadequate to predicting the size of one brain structure from another.
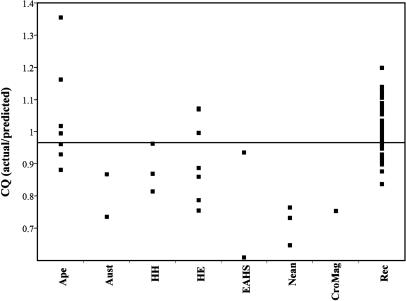
Interhuman Comparisons. To minimize the problem of phylogenetic inertia, which might potentially affect results for the closely related human fossils (32), the interhuman comparisons were based on a formula derived from a reduced major axis regression of CBLM on NetBrain for the recent human sample only. This sample is indisputably monophyletic and relatively large, permitting a fine-grained analysis of CQ patterns while avoiding inappropriate phylogenetic assumptions. CQ values among fossil humans embody the pattern of diversity observed for mammals in general (Fig. 3). The Early to Middle Pleistocene specimens (australopithecines, Homo habilis, and H. erectus), do not differ significantly from each other (Wilcoxon/Kruskal–Wallis, P = 0.042); nor does the Early to Middle Pleistocene group differ significantly from the great apes (Pongo, Gorilla, and Pan), although the difference approaches significance (Wilcoxon/Kruskal–Wallis, P = 0.047). The Early to Middle Pleistocene group does, however, differ from the Late Pleistocene group (Neandertals and Cro-Magnon 1; P = 0.013). Kabwe/Broken Hill (CQ = 0.93) and Swanscombe (CQ = 0.60) bridge the gap between the Early/Middle and Late Pleistocene humans. Cro-Magnon 1, with a CQ of 0.75 (based on the recent human regression formula), falls near the Neandertal mean of 0.71. On the other hand, the Late Pleistocene group is significantly different from recent human mean (CQ = 1.0; P = 0.001).
Fig. 3.
CQ in humans. GrApe, Pan, Pongo, and Gorilla; Aust, australopithecines; HH, H. habilis; HE, H. erectus; EAHS, early archaic H. sapiens (Kabwe/Broken Hill and Swanscombe); Nean, Neandertals; CroMag, Cro-Magnon 1; Rec, contemporary modern humans. Regression formula for CQ calculations derived from recent human sample, excluding fossil humans.
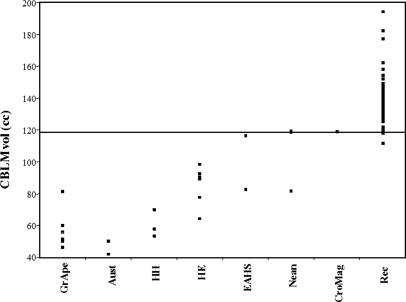
A decrease in a ratio can be achieved in two ways: (i) by decreasing the denominator (CBLM volume) or (ii) by increasing the numerator (NetBrain volume). The data indicate that the decrease in CQ seen in the early archaic H. sapiens and Late Pleistocene humans is due to an increase in the NetBrain. On the other hand, a slight decrease in NetBrain volume in recent humans is accompanied by a significant increase in cerebellum volume (Fig. 4).
Fig. 4.
CBLM in humans. GrApe, Pan, Pongo, and Gorilla; Aust, australopithecines; HH, H. habilis; HE, H. erectus; EAHS, early archaic H. sapiens (Kabwe/Broken Hill and Swanscombe); Nean, Neandertals; CroMag, Cro-Magnon 1; Rec, contemporary modern humans.
Because the bulk of the NetBrain comprises the Neocortex, it is reasonable to infer that neocortical expansion in Early Archaic and Late Pleistocene humans outpaced cerebellar expansion. For recent humans, on the other hand (and counterintuitively), CQ is high because the cerebellum is both absolutely and relatively larger than it is in earlier humans.
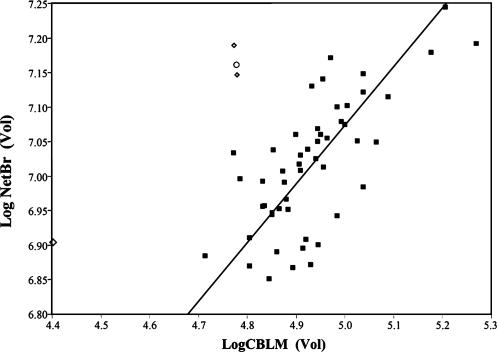
Mean cerebellum volume in Neandertals (106.35 ± 12.32 cm3) is both absolutely and relatively smaller than the mean for recent humans (139.76 ± 2.54 cm3). Additionally, a plot of NetBrain against CBLM (Fig. 5) clarifies that CQ in Neandertals is low also because the rest of the brain (mean NetBrain = 1,197.52 ± 101.66 cm3) is large, compared with the recent human sample (mean NetBrain = 1,116.92 ± 15.72 cm3). Cro-Magnon 1, with a NetBrain volume of 1,289.08 cm3 and a cerebellum volume of 118.92 cm3, embodies the archaic pattern of a relatively large NetBrain and a relatively small cerebellum.
Fig. 5.
Log NetBrain on log CBLM in H. sapiens. The reduced major axis regression line is plotted for recent humans. ⋄, Neandertals; ○, Cro-Magnon 1; ▪, recent humans.
Discussion
Cerebellar Contribution to Cognition. The cerebellum appears to enhance and sharpen precise timing of neural events and to promote the smooth control of rapid, stereotyped neural responses, regardless of whether it is processing sensory, motor, or cognitive signals (33–37). Neocortical regions with reciprocal cerebellar circuitry are summarized in Table 1.
This complementary cognitive interaction of the cerebellum and neocortex has been modeled as a dichotomy between explicit (declarative) and implicit (procedural) cognitive processes (38, 39).
The neocortex plays a role in both explicit and implicit learning, but explicit learning invokes more intense neocortical activity (39). Explicit learning results in “knowing that,” as opposed to “knowing how,” and facilitates the manipulation of data to provide unique, flexible solutions in unforeseen situations. On the other hand, although the cerebellum may be invoked for explicit tasks, e.g., word searches or stem completions (40), its primary processing mode appears to be implicit, algorithmic, and rule-based, resulting in “know-how.”
Given these relationships, the evolution of CQ in humans can be modeled as a three-stage process, where emerging cognitive behaviors are correlated with changes in CQ that reflect functional shifts in the neocortical/cerebellar relationship.
Stage 1: Cultural Intensification (Pliocene, Early to Middle Pleistocene). The living great apes are distinguished from monkeys by their expanded frontal lobes and lateral cerebellar lobes (12, 41). Presumably, this morphology was present in the common ancestor of the African great apes and early humans, along with rudimentary tool-using and symbolic behaviors. By the Late Pliocene, humans were relying on intensified cultural behaviors, particularly the use of stone tool technology in foraging and food processing (42, 43).
Simple stone tool production recruits the cerebellum as well as sensory-motor and superior parietal “association” areas of the neocortex (44). Parietal/cerebellar circuitry also is recruited for differentiation of motor functions (36), enhancement of goal-directed behaviors (45, 46), judgment of the velocity of moving stimuli (47), spatial event processing (37), elementary visuospatial and memory functions (46), and working memory (48).
Behavioral and cognitive innovations in Pliocene humans were correlated with an increase in absolute cerebellar and NetBrain volume, compared with the great apes, with the NetBrain outpacing the cerebellum. Neocortical expansion was accompanied by reorganization involving the parietal lobe in early members of the genus Homo (49–52).
Stage 2: Declarative Multiplicity (Middle to Late Pleistocene). Absolute cerebellum volume expanded somewhat in Middle Pleistocene humans, leveling off in the Late Pleistocene. By contrast, the NetBrain continued to expand, reaching its upper limit in Late Archaic and early modern humans. Cultural development during this stage is characterized by an increasing variety of objects, sets of objects, and accompanying complex learned behaviors (e.g., linear and recurrent prepared core techniques, soft hammer retouch, and indirect percussion in stone knapping, burial of the dead, personal ornamentation, intentional graphic productions (and, by ≈32,000 years ago, representational art), sophisticated pyrotechnology, and long-distance transportation of raw materials.
The CQ of the early modern Cro-Magnon 1 falls close to the Neandertal mean (Fig. 5), and it is significantly different from that of recent humans (Wilcoxon/Kruskal–Wallis, P = 0.009). This observation is consistent with accumulating evidence that Neandertals and early modern humans were capable of similar manipulative behaviors (53) and made similar types of artifacts, in both Middle Paleolithic and initial Upper Paleolithic contexts (54–57). In addition, the archeological record for a number of localities in Europe, Africa, and the Middle East shows a behavioral continuum for many of the subsistence activities practiced by Middle and early Upper Paleolithic humans (58–66).
Although archeological indicators are consistent with the similarity in CQ between Neandertals and early anatomically modern humans, the fossil evidence paints a more ambiguous picture. Early modern humans exhibit a mosaic pattern, compared with Neandertals, later Upper Paleolithic, or recent humans with respect to skeletal indicators of manipulative activity. For example, humeral robusticity and bilateral asymmetry are greater in both Neandertals and later Upper Paleolithic modern humans than in early Upper Paleolithic fossils (67–69). By contrast, the functional morphology of the hand is significantly different in Neandertals and early modern humans in the Near East, even though both are associated with generally similar Middle Paleolithic technologies (70). This mosaic picture is further complicated by the fact that skeletal morphology is highly responsive to loading patterns as well as climatic effects (67, 71–75). Thus, it is difficult to determine the relative contributions of developmental plasticity, habitual loading patterns, and heritability in shaping these features.
A similar dilemma arises in considering cerebellar plasticity. Recent investigations by Hutchinson et al. (76) suggest that frequency and intensity of complex cognitive behaviors, such as music practice, correlate with increases in cerebellar volume. Additionally, cerebellar representational topography changes with exposure to new tools and related motor routines (77, 78). As with skeletal morphology, it appears that variation in cerebellar morphology is partitioned between genetic factors and developmental and functional plasticity.
Stage 3: Complexity Management (Terminal Pleistocene and Holocene). The pattern of cerebellar variation in Late Pleistocene humans is consistent with an emerging recognition that the Upper Paleolithic is not a unitary entity but comprises a range of temporally and geographically diverse cultures. For example, the initial or transitional Upper Paleolithic lacks evidence of compound, hierarchically implemented technologies (e.g., bows, armatures, weirs, loom weights, and eyed needles) that are commonly found later at Upper Paleolithic sites (58, 79–82). Although it is impossible to say whether earlier humans were capable of producing these hierarchical, compound technologies, late Upper Paleolithic humans appear to have encountered increasing cognitive demands as social and cultural complexity increased. These new demands may have taxed the information processing capacities of Late Pleistocene Neandertals and early modern humans. Information processing in these humans may have been limited ultimately by the sheer size of their neocortical processing networks (83). The secondary expansion of the cerebellum observed in Holocene humans would have streamlined neocortical networking by providing the infrastructure for rule-based, procedural organization of sequential operations across many cognitive domains in response to cultural pressures.
Summary. The cerebellar contribution to modern human cognition is the result of a complex evolutionary process involving reciprocal connections between the cerebellum and the neocortex in response to intensified cultural demands. The interaction between the cerebellum and the neocortex is analogous to the reciprocal interaction between explicit/procedural and implicit/declarative cognitive operations. The relation between cerebellar volume and the volume of the rest of the brain may be expressed as a CQ, a ratio of actual to expected cerebellar volume.
The model presented above suggests that cerebellar/neocortical evolution occurred in three stages. Stage 1: Early encephalization involving expansion of the neocortex as Pliocene and Early to Middle Pleistocene humans developed an adaptive pattern of technologically assisted foraging. Stage 2: Dramatic encephalization involving primarily the neocortex in Middle to Late Pleistocene humans. This neocortical expansion was accompanied by a proliferation of cultural objects and sets of objects, as well an increased frequency of complex behavioral routines. Some of the behaviors that would have relied on increased neocortical processing include sophisticated pyrotechnology, prepared core techniques in stone knapping, and concept-mediated marking (84). This stage of cognitive evolution may be characterized as one of declarative multiplicity, in which the neocortex was taxed to the feasible limit of its networking capacity (83). Stage 3: An increase in cognitive efficiency as a result of expanded cerebellar capacity in late Late Pleistocene and Holocene humans. This stage of cognitive evolution may be characterized as one of complexity management. In terms of their NetBrain volume, recent humans appear to be able to do more with less, thanks to secondary cerebellar expansion that permitted efficient processing of cognitive operations without an increase in NetBrain volume.
Acknowledgments
We thank R. L. Holloway (Columbia University, New York) for providing endocasts; J.-J. Hublin and M. Braun for access to CT scans; K. Semendeferi, J. Rilling, T. Insel, and J. Csernansky for MRIs; A. Bergstrom and L. Wang for technical assistance; J. Clark and the Archeology Technologies Laboratory of North Dakota State University (Fargo) for access to facilities; and R. L. Holloway, C. MacLeod, S. Thompson, and E. Trinkaus for encouragement, insight, and constructive comments. This work was supported by the L. S. B. Leakey and Wenner–Gren Foundations and the University of New Mexico, Albuquerque.
Author contributions: A.H.W. designed research, performed research, analyzed data, and wrote the paper.
Abbreviations: PCF, posterior cranial fossa; CBLM, cerebellum volume; NetBrain, brain value; CQ, cerebellar quotient; CT, computed tomography.
References
- 1.Symington, J. (1916) J. Anat. Physiol. 11, 111-130. [PMC free article] [PubMed] [Google Scholar]
- 2.Holloway, R. L. (1966) Am. Anthropol. 68, 103-121. [Google Scholar]
- 3.Holloway, R. L. (1996) in Handbook of Human Symbolic Evolution, eds. Lock, A. & Peters, C. R. (Clarendon, Oxford), pp. 74-108.
- 4.Clark, C. A., Mitra, P. P. & Wang, S. S.-H. (2001) Nature 411, 189-193. [DOI] [PubMed] [Google Scholar]
- 5.Finlay, B. L. & Darlington, R. B. (1995) Science 268, 1578-1584. [DOI] [PubMed] [Google Scholar]
- 6.Finlay, B. L., Darlington, R. B. & Nicastro, N. (2001) Behav. Brain Sci. 24, 263-308. [PubMed] [Google Scholar]
- 7.Barton, R. A. (2002) Nature 415, 134-135. [DOI] [PubMed] [Google Scholar]
- 8.Barton, R. A. & Harvey, P. H. (2000) Nature 405, 1055-1058. [DOI] [PubMed] [Google Scholar]
- 9.De Winter, W. & Oxnard, C. E. (2001) Nature 409, 710-714. [DOI] [PubMed] [Google Scholar]
- 10.Matano, S. & Hirasaki, E. (1996) Folia Primatol. 6, 209-226. [DOI] [PubMed] [Google Scholar]
- 11.Matano, S. (2001) Am. J. Phys. Anthropol. 114, 163-165. [DOI] [PubMed] [Google Scholar]
- 12.MacLeod, C. E., Zilles, K., Schleicher, A., Rilling, J. K. & Gibson, K. R. (2003) J. Hum. Evol. 44, 401-429. [DOI] [PubMed] [Google Scholar]
- 13.Rilling, J. K. & Insel, T. R. (1998) Brain Behav. Evol. 52, 308-314. [DOI] [PubMed] [Google Scholar]
- 14.Semendeferi, K. & Damasio, H. (2000) J. Hum. Evol. 26, 317-332. [DOI] [PubMed] [Google Scholar]
- 15.Whiting, B. A. & Barton, R. A. (2003) J. Hum. Evol. 1, 3-10. [DOI] [PubMed] [Google Scholar]
- 16.Pirlot, P. & Nelson, J. (1978) Austr. Zool. 20, 171-179. [Google Scholar]
- 17.Meyer, J. (1981) Brain Behav. Evol. 19, 60-71. [DOI] [PubMed] [Google Scholar]
- 18.Stephan, H., Frahm, H. D. & Baron, G. (1981) Folia Primatol. 35, 1-29. [DOI] [PubMed] [Google Scholar]
- 19.Baron, G., Stephan, H. & Frahm, H. D. (1996) in Comparative Neurobiology in Chiroptera: Macromorphology, Brain Structures, Tables, and Atlases (Birkhaeuser, Basel), Vol. 1, pp. 318-325. [Google Scholar]
- 20.Pirlot, P. & Kamiya, T. (1983) J. Hirnforsch. 21, 1-9. [Google Scholar]
- 21.Blinkov, S. M. & Glezer, I. (1968) The Human Brain in Figures and Tables: A Quantitative Handbook (Plenum, New York).
- 22.Marino, L. (1998) Brain Behav. Evol. 51, 230-238. [DOI] [PubMed] [Google Scholar]
- 23.Marino, L., Rilling, J. K., Lin, S. K. & Ridgeway, S. (2000) Brain Behav. Evol. 56, 204-211. [DOI] [PubMed] [Google Scholar]
- 24.Haug, H. (1970) Ergeb. Anat. Entwicklungsgesch. 43, 1-70. [PubMed] [Google Scholar]
- 25.Reep, R. L. & O'Shea, T. J. (1990) Brain Behav. Evol. 35, 185-194. [DOI] [PubMed] [Google Scholar]
- 26.Kruska, D. & Röhrs, M. (1974) Z. Anat. Entwicklungsgesch. 144, 61-73. [DOI] [PubMed] [Google Scholar]
- 27.Klekamp, J., Riedel, A., Harper, C. & Kretschmann, H.-J. (1987) J. Anat. 150, 191-210. [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder, P. J., Bilder, R. M., Wu, H., Bogerts, B. & Lieberman, J. A. (1995) Neuropsychologia 33, 407-419. [DOI] [PubMed] [Google Scholar]
- 29.Pickering, S. P. (1930) Am. J. Phys. Anthropol. 15, 2-51. [Google Scholar]
- 30.National Institutes of Health (1999) nih image and object image for Macintosh (Natl. Inst. Health, Bethesda), Version 1.62. (http://rsb.info.nih.gov/nih-image/).
- 31.Ruff, C. E., Trinkaus, E. & Holliday, T. W. (1997) Nature 387, 173-176. [DOI] [PubMed] [Google Scholar]
- 32.Felsenstein, J. (1985) Am. Nat. 125, 1-15. [Google Scholar]
- 33.Arriada-Mendicoa, N., Otero-Silceo, E. & Corona-Vazquez, T. (1999) Rev. Neurol. 29, 1075-1082. [PubMed] [Google Scholar]
- 34.Fox, E. A., Sitompul, A. F. & van Schaik, C. P. (1999) in The Mentality of Gorillas and Orangutans, eds. Parker, S. T., Miles, L. & Mitchell, R. (Cambridge Univ. Press, Cambridge, U.K.), pp. 99-116.
- 35.Ito, M. (1993) Trends Neurosci. 16, 448-454. [DOI] [PubMed] [Google Scholar]
- 36.Leiner, H. C., Leiner, A. L. & Dow, R. S. (1986) Behav. Neurosci. 100, 443-454. [DOI] [PubMed] [Google Scholar]
- 37.Petrosini, L., Leggio, M. G. & Molinari, M. (1998) Prog. Neurobiol. 56, 191-210. [DOI] [PubMed] [Google Scholar]
- 38.Exner, C., Koschack, J. & Irle, E. (2002) Learn. Mem. 9, 376-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson, E. M. & Pascual-Leone, A. (2003) Curr. Biol. 13, R65-R6. [DOI] [PubMed] [Google Scholar]
- 40.Desmond, J. E., Gabrieli, J. D. E. & Glover, G. H. (1998) Neuroimage 7, 368-376. [DOI] [PubMed] [Google Scholar]
- 41.MacLeod, C. E., Zilles, K., Schleicher, A. & Gibson, K. R. (2001) in All Apes Great and Small, eds. Galdikas, B. M., Briggs, N., Sheeran, L. K., Shapiro, G. L. & Goodall, J. (Plenum, New York), Vol. 1, pp. 35-53. [Google Scholar]
- 42.Semaw, S., Renne, P., Harris, J. W. K., Feibel, C. S., Bernor, R. L., Fesseha, N. & Mowbray, K. (1997) Nature 385, 333-336. [DOI] [PubMed] [Google Scholar]
- 43.Asfaw, B., White, T. D., Lovejoy, O., Latimer, B., Simpson, S. & Suwa, G. (1999) Science 284, 629-635. [DOI] [PubMed] [Google Scholar]
- 44.Stout, D., Toth, N. & Schick, K. (2000) J. Archaeol. Sci. 27, 1215-1223. [Google Scholar]
- 45.Daum, I., Schugens, M., Reimold, C., Dichgans, J. & Birnbaumer, N. (1993) Behav. Neurosci. 107, 411-419. [DOI] [PubMed] [Google Scholar]
- 46.Brodal, P. & Bjaalie, J. G. (1997) Prog. Brain Res. 114, 227-249. [DOI] [PubMed] [Google Scholar]
- 47.Leiner, H. C., Leiner, A. L. & Dow, R. S. (1993) Trends Neurosci. 16, 444-454. [DOI] [PubMed] [Google Scholar]
- 48.Middleton, F. & Strick, P. (1997) Prog. Brain Res. 114, 553-566. [DOI] [PubMed] [Google Scholar]
- 49.Tobias, P. V. (1967) Olduvai Gorge (Cambridge Univ. Press, Cambridge, U.K.).
- 50.Tobias, P. V. (1975) in Primate Functional Morphology and Evolution, ed. Tuttle, R. (Mouton, The Hague, The Netherlands), pp. 353-392.
- 51.Begun, D. & Walker, A. (1993) in The Nariokotome Homo erectus Skeleton, eds. Walker, A. & Leakey, R. E. (Harvard Univ. Press, Cambridge, MA), pp. 326-358.
- 52.Holloway, R. L. (1975) in Primate Functional Morphology and Evolution, ed. Tuttle, R. (Mouton, The Hague, The Netherlands), pp. 393-416.
- 53.Niewoehner, W. A., Bergstrom, A., Eichele, D., Zuroff, M. & Clark, J. T. (2003) Nature 422, 395. [DOI] [PubMed] [Google Scholar]
- 54.Hublin, J.-J. (1992) Philos. Trans. R. Soc. London B 337, 185-191. [DOI] [PubMed] [Google Scholar]
- 55.Lévêque, F. & Vandermeersch, B. (1980) C. R. Acad. Sci. Paris 291, 187-189. [Google Scholar]
- 56.Vandermeersch, B. (1981) Les Hommes Fossiles deq Afzeh (Centre National de la Recherche Scientifique, Paris).
- 57.Shea, J. (2003) Evol. Anthropol. 12, 161-204. [Google Scholar]
- 58.Straus, L. G. (1988) in The Emergence of Modern Humans: An Archaeological Perspective, ed. Mellars, P. (Edinburgh Univ. Press, Edinburgh), pp. 276-302.
- 59.Straus, L. G. & Heller, C. (1990) in The Early Upper Paleolithic, British Archaeological Reports International Series, eds. Hoffecker, J. F. & Wolf, C. (Archaeopress, Oxford), Vol. 437, pp. 97-133. [Google Scholar]
- 60.Bar-Yosef, O. (2004) Int. J. Osteoarcheol. 14, 333-342. [Google Scholar]
- 61.Churchill, S. E. & Smith, F. H. (2000) Am. J. Phys. Anthropol. 113, 61-115. [DOI] [PubMed] [Google Scholar]
- 62.Mellars, P. (1999) Curr. Anthropol. 40, 341-364. [Google Scholar]
- 63.Kuhn, S. L. (2002) Evol. Anthropol. 11, 198-210. [Google Scholar]
- 64.Marean, C. W. & Assefa, Z. (1999) Evol. Anthropol. 8, 22-37. [Google Scholar]
- 65.Stiner, M. C. & Kuhn, S. L. (1992) Am. Anthropol. 94, 306-339. [Google Scholar]
- 66.D'Errico, F., Zilhão, J., Julien, M., Baffier, D. & Pelegrin, J. (1998) Curr. Anthropol. 39, S1-S44. [Google Scholar]
- 67.Trinkaus, E., Churchill, S. E. & Ruff, C. B. (1994) Am. J. Phys. Anthropol. 93, 1-34. [DOI] [PubMed] [Google Scholar]
- 68.Churchill, S. E., Weaver, A. H. & Niewoehner, W. A. (1996) Quatern. Nova 6, 413-447. [Google Scholar]
- 69.Trinkaus, E. & Churchill, S. E. (1999) J. Arch. Sci. 26, 173-184. [Google Scholar]
- 70.Niewoehner, W. A. (2001) Proc. Natl. Acad. Sci. USA 98, 2979-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanyon, L. E., Goodship, A. E., Pye, C. J. & MacFie, J. H. (1982) J. Biomech. 15, 141-154. [DOI] [PubMed] [Google Scholar]
- 72.Lanyon, L. E. (1982) in Bone in Clinical Orthopaedics, ed. Sumner-Smith, G. (Saunders, Philadelphia), pp. 273-304.
- 73.Pearson, O. M. (2000) Curr. Anthropol. 41, 569-607. [PubMed] [Google Scholar]
- 74.Holliday, T. W. (1995) J. Hum. Evol. 32, 423-447. [DOI] [PubMed] [Google Scholar]
- 75.Churchill, S. E. & Formicola, V. (1997) Int. J. Osteoarchaeol. 7, 18-38. [Google Scholar]
- 76.Hutchinson, S., Hui-Lin Lee, L., Gaab, N. & Schlaug, G. (2003) Cereb. Cortex 13, 943-949. [DOI] [PubMed] [Google Scholar]
- 77.Imamizu, H. S., Miyauchi, S., Tamada, T., Sasaki, Y., Takino, R., Putz, B., Yoshioka, T. & Kawato, M. (2000) Nature 403, 192-195. [DOI] [PubMed] [Google Scholar]
- 78.Imamizu, H., Kuroda, T., Miyauchi, S., Yoshioka, T. & Kawato, M. (2003) Proc. Natl. Acad. Sci. USA 100, 5461-5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soffer, O., Adovasio, J. M. & Hyland, D. C. (2000) Curr. Anthropol. 41, 511-537. [Google Scholar]
- 80.Soffer, O. (2004) Curr. Anthropol. 45, 407-412. [Google Scholar]
- 81.Straus, L. G., Bischoff, J. L. & Carbonell, E. (1993) Préhist. Eur. 3, 11-27. [Google Scholar]
- 82.Straus, L. G. (1995) Evol. Anthropol. 4, 4-16. [Google Scholar]
- 83.Kien, J. (1991) J. Hum. Evol. 20, 157-165. [Google Scholar]
- 84.Bednarick, R. G. (1995) Curr. Anthropol. 36, 605-634. [Google Scholar]