Abstract
In terrestrial vertebrates such as birds and mammals, neurotrophin receptor expression is considered fundamental for the specification of distinct somatosensory neuron types where TrkA, TrkB and TrkC specify nociceptors, mechanoceptors and proprioceptors/mechanoceptors, respectively. In turn, Runx transcription factors promote neuronal fate specification by regulating neurotrophin receptor and sensory receptor expression where Runx1 mediates TrkA+ nociceptor diversification while Runx3 promotes a TrkC+ proprioceptive/mechanoceptive fate. Here, we report in zebrafish larvae that orthologs of the neurotrophin receptors in contrast to terrestrial vertebrates mark overlapping and distinct subsets of nociceptors suggesting that TrkA, TrkB and TrkC do not intrinsically promote nociceptor, mechanoceptor and proprioceptor/mechanoceptor neuronal fates, respectively. While we find that zebrafish Runx3 regulates nociceptors in contrast to terrestrial vertebrates, it shares a conserved regulatory mechanism found in terrestrial vertebrate proprioceptors/mechanoceptors in which it promotes TrkC expression and suppresses TrkB expression. We find that Cbfβ, which enhances Runx protein stability and affinity for DNA, serves as an obligate cofactor for Runx in neuronal fate determination. High levels of Runx can compensate for the loss of Cbfβ, indicating that in this context Cbfβ serves solely as a signal amplifier of Runx activity. Our data suggests an alteration/expansion of the neurotrophin receptor code of sensory neurons between larval teleost fish and terrestrial vertebrates, while the essential roles of Runx/Cbfβ in sensory neuron cell fate determination while also expanded are conserved.
Author summary
Our perception of the external world comes from our senses. Often overlooked the skin is our largest sensory organ. Specialized neurons located in the dorsal root ganglion (DRG), which innervate the body, and trigeminal ganglion (TG), which innervate the face, sense the somatosensory perceptions: light touch, temperature, pain (nociceptors) and muscle/limb position (proprioception) via nerve endings that project to the skin. These neurons receive and relay information from these diverse stimuli through distinct subclasses of neurons. Since these neurons arise from common lineages, they provide an excellent system to study how neurons develop and diversify into different subtypes. Runx transcription factors have been shown in terrestrial vertebrates (birds and mammals) to be instrumental in specifying nociceptor and proprioceptor populations by regulating the expression of a class of genes that code for the neurotrophin receptors, which are thought to be essential for specifying these neuronal fates. In our study we show that mechanisms by which Runx transcription factors regulate neurotrophin receptor expression are conserved between zebrafish and terrestrial vertebrates, yet the type of neuron specified by these genes are different such that in zebrafish the neurotrophin receptor TrkC is expressed in a nociceptor lineage instead of the proprioceptor/mechanoreceptor lineage as in terrestrial vertebrates. These data demonstrate that the specification of neuronal lineages is not fundamental to a given neurotrophin receptor but has adapted and evolved from the time fish and terrestrial vertebrates diverged 350 million years ago. Furthermore we show in fish that zebrafish Runx3 has properties that are divided between Runx1 and Runx3 in terrestrial vertebrates. Finally we show that the Runx co-factor Cbfβ is essential for its function, but the high level of Runx3 expression can overcome the loss of Cbfβ, demonstrating that Cbfβ in this context serves solely as a signal amplifier of Runx3 activity.
Introduction
Sensory neurons of the dorsal root ganglia (DRG) and trigeminal ganglia (TG) of terrestrial vertebrates convey somatosensory information from the body and face, respectively. Distinct but overlapping sensations, including touch, proprioception (body position), and nociception (pain) are perceived by different sensory neuron populations [1]. How these distinct neurons acquire their functional properties during development is an important question in understanding the assembly of the somatosensory circuits and may shed light on how these properties change in pathogenic states such as chronic pain.
A consensus model has emerged for how distinct sensory neuron populations develop in mammalian embryos. After their initial establishment from placodal and neural crest origins, sensory neurons become specialized into distinct populations. The development and survival of these populations are controlled by tropomyosin-receptor kinase (Trk) proteins that act as receptors for the neurotrophin family of growth factors. In the DRG, the three primary classes of sensory neurons are marked by distinct expression of neurotrophin receptors: nociceptors including thermoceptors and pruriceptors express TrkA, mechanoreceptors express TrkB and proprioceptors express TrkC [1]. In the TG, TrkC is primarily associated with mechanoreceptors that do not coexpress TrkB [2]. However, it appears that in early zebrafish development neurotrophin receptors may label different subset of somatosensory neurons [3].
The specification of sensory neuron subtypes is under transcription factor (TF) control. In particular, the Runt domain (Runx) TFs have been shown to be key regulators of somatosensory cell fate in terrestrial vertebrates. In the DRG, Runx3 is critical for the development of proprioceptors where it acts to suppress TrkB expression and promote TrkC expression, while in the TG Runx3 is required for the specification of TrkC-expressing mechanoceptors that innervate Merkel cells [2,4–6]. Runx1, which is expressed in a largely distinct population of somatosensory neurons from Runx3, is required for the specification of nonpeptidergic nociceptors from TrkA+ precursors [7,8]. Runx1 is also required for the expression of numerous somatosensory receptors including TRPV1, TRPA1 and Piezo2 [7,8]. The transcription cofactor Core binding factor-beta (CBFβ) is required for Runx-mediated signaling where it functions to enhance Runx binding to DNA 5 to-10-fold and protect Runx from ubiquitin-mediated degradation [9–11]. Whether or not CBFβ plays a modular or obligate role in Runx-mediated somatosensory neuron specification is unknown.
In this study we characterize the Trk receptor code in developing zebrafish and examine the roles of Runx TFs in regulating Trk expression and sensory neuron specification. While it is difficult to make comparisons across species that develop along different timescales and environmental constraints/pressures (terrestrial vertebrates develop in utero or in ovo, while zebrafish develop as free-swimming larvae that are capable of perceiving and responding to external stimuli), we found striking conservation of function as well as substantial differences between these animals. In contrast to terrestrial vertebrates, we find that in larval zebrafish, TrkA, TrkB and TrkC label distinct and overlapping classes of nociceptors. By generating loss of function mutations in zebrafish, we find that Runx3 supports a hybrid of the independent functions Runx1 and Runx3 play in terrestrial vertebrates. We also illuminate the role Cbfβ plays in Runx mediated neuronal specification, showing that Cbfβ acts as a signal amplifier of Runx3 signaling whose loss can be overcome by excess Runx3 expression. These observations argue that while fish diverged from terrestrial vertebrates over 350 million years ago, and may have substantially different somatosensory requirements, core aspects of the molecular program regulating somatosensory neuron specification even in early larval zebrafish remain largely intact.
Results
Neurotrophin receptors mark nociceptors in larval zebrafish
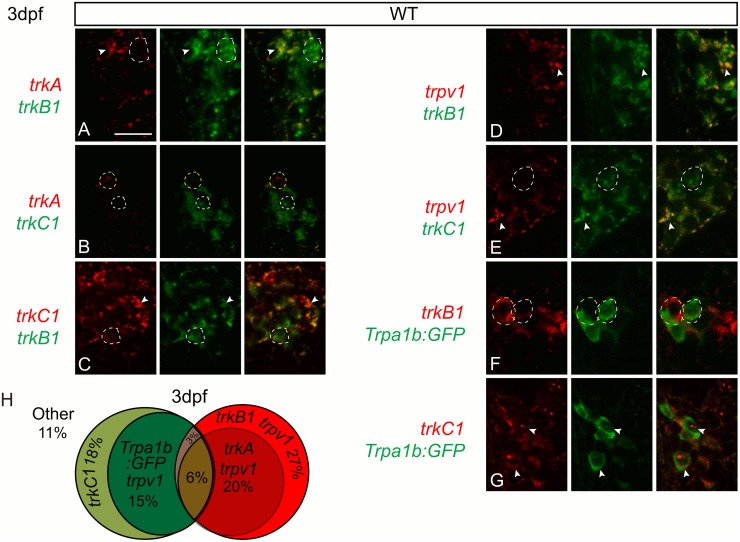
In the TG of larval zebrafish, we characterized the overlap of the neurotrophin receptor populations with each other and with the trpv1 and trpa1b nociceptive ion channels, which respectively are required for larval zebrafish responses to noxious heat and the noxious chemicals, such as allyl isothiocyanate (AITC) [12]. We have previously shown as in mammals that neurons that express trpa1b are completely within the set that expresses trpv1 [13]. In zebrafish at 3dpf, trkC1 expression shows little overlap with trkA or trkB1, while trkA is expressed in a subset of trkB1 neurons (Fig 1A–1C and 1H, Table 1). Expression of trkB1 coincides with trpv1 while trkC1 partially overlaps with trpv1 (Fig 1D–1E, Table 1). This expression pattern is different than that described for the mouse DRG, where during early development trpv1 is broadly coexpressed in TrkA neurons which specify nociceptors, but not in TrkB- or TrkC-expressing neurons which give rise mechanoreceptors and proprioceptors [14,15]. To confirm that in zebrafish trkB1 and trkC1 are indeed expressed in nociceptors, we compared their expression to trpa1. Expression of the zebrafish trpa1b:GFP transgene completely coincides with trkC1 expression and is largely independent of trkB1 expression. We also observed a small population of neurons that coexpressed a combination of trkC1, trkB1, trpv1 and trpa1b:GFP. As in situ hybridization labeling has imperfect cellular resolution, we sought to confirm this small population expressing multiple markers by scatter labeling using CRSPR mediated insertion of the fluorescent reporter mRuby3 into the trkB1 promoter region. Our in situ hybridization results predicted that ~19% (~6/~31) of trkB1-expressing TG neurons should coexpress trpa1b:GFP at 3dpf (Table 1). Similarly we found that 20% (15/74, n = 11 animals) of trkB1:mRuby3-expressing TG neurons co-expressed trpa1b:GFP (S1 Fig). Taken together these results demonstrate that trkA, trkB1 or trkC1 are largely expressed by all or the majority of nociceptive neurons as determined by trpa1b:GFP or trpv1 expression during early larval development. This suggests that while the roles of neurotrophin receptors in somatosensory neuron survival and specification may be conserved, the distinct neuronal populations that these receptors represent are different between zebrafish and tetrapods.
Fig 1. The zebrafish trigeminal ganglion contains many somatosensory subpopulations defined by the neurotrophin receptors and trpv1 and trpa1b nociceptive ion channels.
A-E, Optical sections of double fluorescent in situ hybridization for trkA (red) and trkB1 (green) (A), trkA (red) and trkB1 (green) (B), trkA1 (red) and trkC1 (green) (C), trkC (red) and trkB1 (green) (D), trpv1 (red) and trkB1 (green) (E) and trpv1 (red) and trkC1 (green in wild-type fish at 3dpf. F-G, Optical sections of antibody staining of GFP (green) in conjunction with fluorescent in situ hybridization for trkB1 (F) and trkC1 (G) in trpa1b:GFP fish at 3dpf. H, Representations of 3dpf wild-type somatosensory subpopulations as a percent of the whole TG. Dashed white line outline green cells; Dashed yellow lines outline red cells; Arrowhead indicates double positive cells. Scale bar: 20μm. Embryos per condition (n = 3–4).
Table 1. Trigeminal neuron counts.
| Double positive cells | WT |
|---|---|
| trkA+ trkB1+ | 16.3±1.1 (3) |
| trkA+ trkC1+ | 3.7±0.4 (3) |
| trkB1+ trkC1+ | 5.5±0.8 (4) |
| trkB1+ trpv1+ | 30.7±1.5 (3) |
| trkC1+ trpv1+ | 11.3±1.5 (3) |
| trkB1+ trpa1b:GFP+ | 6.0±1.6 (4) |
| trkC1+ trpa1b:GFP+ | 13.0±1.9 (3) |
Average number of double positive TG neurons for trkA+ trkB1+, trkA+ trkC1+, trkB1+ trkC1+, trkB1+ trpa1b:GFP+, and trkC1+ trpa1b:GFP+ at 3dpf. Data represents mean±SEM (n).
Zebrafish somatosensory neurons express runx and cbfb
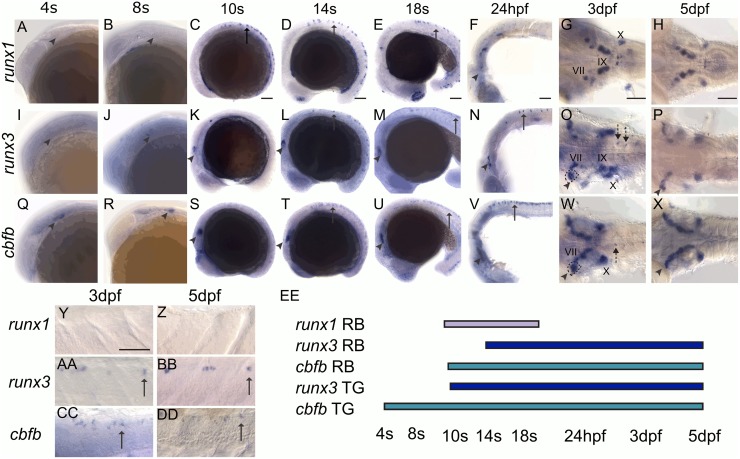
To determine if transcriptional regulators of Trk expression and somatosensory neuron diversification are conserved between fish and tetrapods, we investigated the role of zebrafish Runx TFs and their cofactor Cbfβ in neuronal fate specification. We first characterized the expression of runx1, runx3, and cbfb using colormetric RNA in situ hybridization across multiple timepoints. runx1 is only expressed in Rohon-Beard (RB) sensory neurons, which innervate the body, while runx3 and cbfb are expressed in all three somatosensory neuron populations: RB, DRG, and TG neurons (Fig 2). All three genes are also expressed in other cranial ganglion, such as the facial (VII), glossopharyngeal (IX), and vagal (X) ganglia (Fig 2). In RB neurons, runx1 is transiently expressed with expression observed at the 4 somite (s) stage, but absent by 24hpf (hours post fertilization) (Fig 2A–2D, 2S, 2T and 2Y). In contrast, runx3 and cbfb RB expression begins later at 14s and continues to at least 5dpf (days post fertilization) (Fig 2G–2R and 2U–2Y). In the TG, cbfb expression was detected as early as 4s, while definitive runx3 expression was observed at 10s. Both continued to be expressed till at least 5dpf, while we found no evidence of runx1-expression even at the earliest timepoints measured (Fig 2A-X and 2EE). These data suggest that Runx TFs are in a position to regulate zebrafish somatosensory neuron diversification. However the temporal expression of runx1 in RB neurons and the lack of expression of runx1 in the TG suggest that individual Runx TFs may have specialized roles in tetrapods that are not required in larval teleost fish.
Fig 2. runx1, runx3, and cbfb are expressed in the zebrafish somatosensory system.
A-DD, Colormetric in situ hybridization for runx1 (A-H, Y-Z), runx3 (I-P, AA-BB), and cbfb (Q-X, CC-DD) from 4s-5dpf. A-H, Y-Z, runx1 is expressed in RBs (arrow) and can be detected in facial (VII), glossopharyngeal (IX), and vagal (X) cranial ganglion. I-P, AA-BB, runx3 is expressed in RBs, in the DRG (dashed arrows), the TG (arrowhead) and in the VII, IX, X ganglion. I-P, AA-BB cbfb is expressed in RBs, in the DRG (dashed arrows), the TG (arrowhead) and in the VII, IX, X ganglion. EE, Rohon Beard and trigeminal expression timeline for runx1, runx3, and cbfb. Arrow indicates RBs; Arrowhead, TG; dashed arrow, DRG; VII, facial ganglion; IX, glossopharyngeal ganglion; X, vagal ganglion. Scale bar: 100 μm.
runx1 and runx3 act in the same pathway and are regulated differently from cbfb
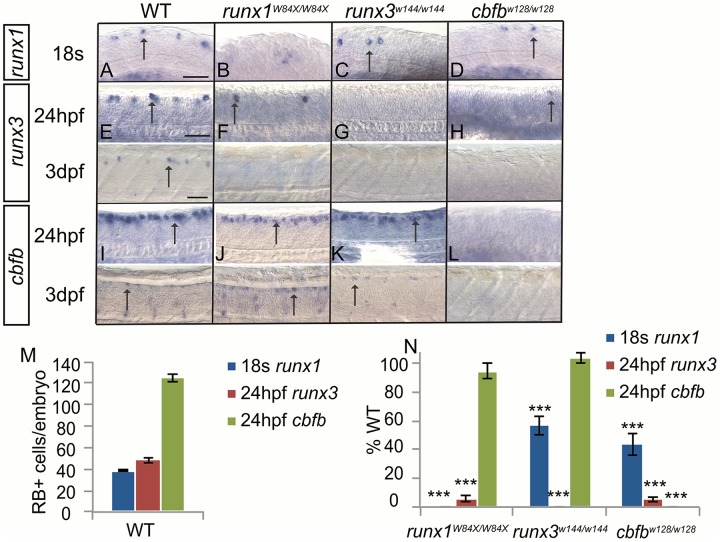
To examine the effect of impaired Runx function in somatosensory neuron development, we obtained a runx1W84X truncation mutant and generated mutations in runx3 and cbfb using CRISPRs and TALENs, respectively [16–18]. We created two nonsense mutations, runx3w144 (1bp del) and cbfbw128 (4bp del); all three mutations predict an early truncation (S2 Fig). In situ hybridization verified the lack of the gene expression in homozygous mutants, most likely caused by nonsense-mediated decay of the RNA transcript (Figs 3B, 3G and 3L and 4B and 4F). All mutants were viable and showed no obvious deformities during the time course of the experiments conducted. However all mutations induced lethality 8-10dpf. In the RBs of wild-type (WT) fish, we found that cbfb was expressed in a large proportion (~76%) of RB cells, while runx3 and runx1 expression was more tightly restricted (Fig 3A, 3E, 3I and 3M; Table 2). Mutations in runx3 or cbfb resulted in a reduction to about half of the runx1+ cells found in WT at 18s stage (Fig 3A, 3C, 3D and 3N; Table 2). Similarly, mutations of runx1 or cbfb resulted in an almost complete loss of runx3 expression in RBs at 24hpf and complete absence by 3dpf (Fig 3F, 3H and 3N; Table 2). As a result in relation to RB neurons the runx1 mutants could be considered a de facto double null mutant for runx1 and runx3. By contrast there was no change in cbfb expression in RB cells at 24hpf or 3dpf in cbfb, runx1 or runx3 mutants (Fig 3J, 3K and 3N; Table 2). Animals heterozygous for mutations in both runx1 and runx3 had significantly fewer runx1- and runx3-expressing RB neurons suggesting that in this neuronal population, Runx1 and Runx3 are functioning in the same pathway (S3 Fig). Animals heterozygous for mutations in cbfb and either runx1 or runx3 however had no effect on runx1, runx3 or cbfb expression (S3 Fig). We can therefore conclude that in the RB population, Runx and Cbfβ help maintain runx1 and runx3 expression and that a cumulative loss of Runx1 and/or Runx3 inhibits the ability of Runx proteins to facilitate runx1 and runx3 expression. Loss of either runx gene, however, does not impact cbfb expression, indicating that cbfb expression is regulated by a Runx independent mechanism.
Fig 3. Runx/Cbfβ signaling is required to maintain runx expression in Rohon Beard neurons.
A-L, Colormetric in situ hybridization for runx1 (A-D) at 18s, runx3 (E-H) at 24hpf and 3dpf, and cbfb (I-L) at 24hpf and 3dpf in the runx1, runx3, and cbfb mutants focusing on the RBs. M-N, Quantification of marker gene expression as total number of RB neurons/embryo and as % WT at 18s or 24hpf. Arrow, RBs; Scale bar: 100 μm. Embryos per condition (n = 3–14). ***p<0.001. All error bars represent S.E.M.
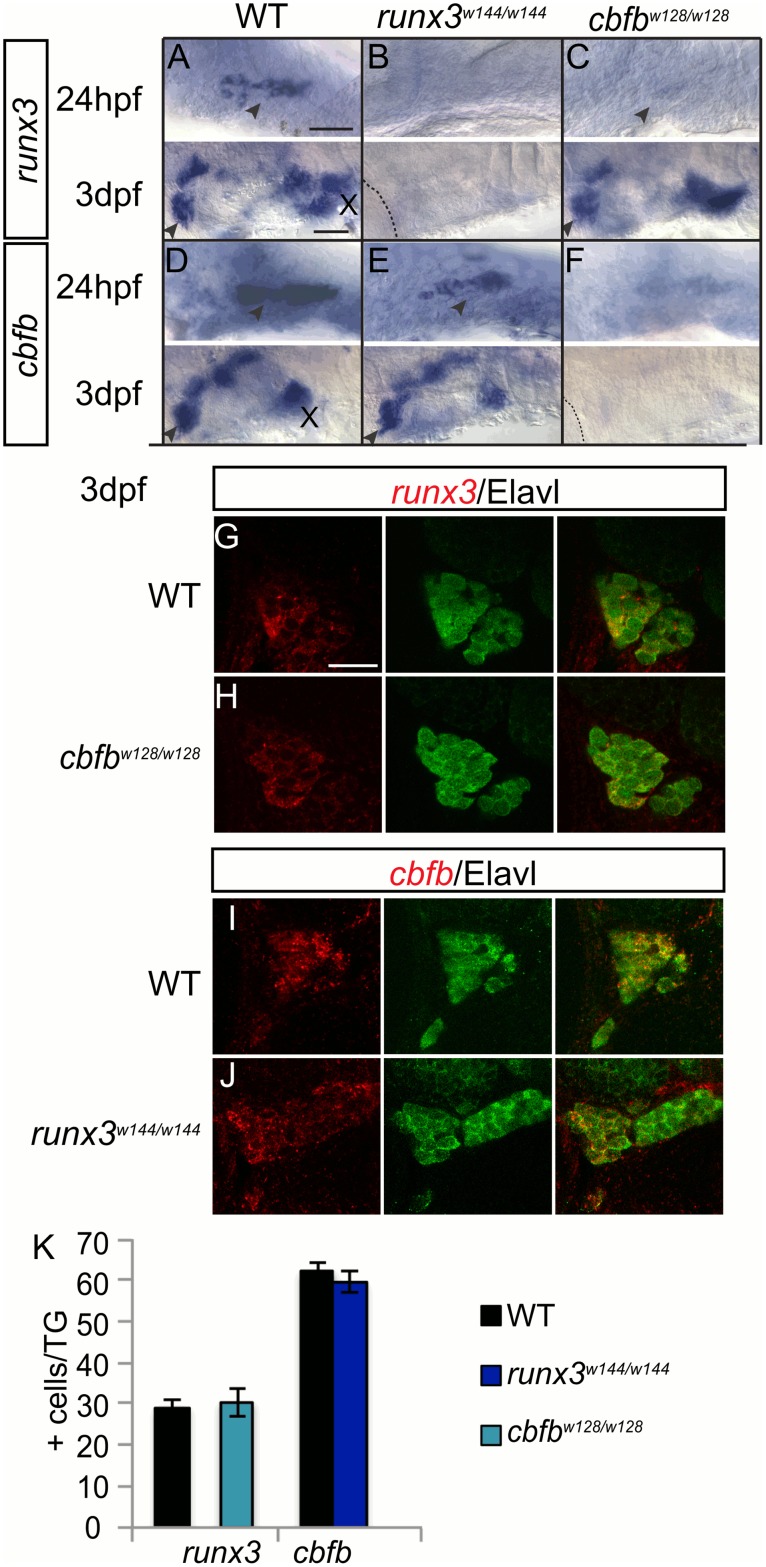
Fig 4. Cbfβ expression does not affect runx expression in the TG.
A-F, Colormetric in situ hybridization for runx3 (A-C) and cbfb (D-F) in the runx3w144/w144 and cbfbw128/w128 mutants focusing on the TG at 24hpf and 3dpf. G-J, Antibody staining of Elavl (green) to label the whole trigeminal in conjunction fluorescent in situ hybridization for runx3 (G-H) and cbfb (I-J) in runx3w144/w144 and cbfbw128/w128 mutants at 3dpf. K, Quantification of marker gene expression as total number of TG neurons/ganglion at 3dpf. Dashed line outlines the eye; Arrowhead, TG; X, vagal ganglion. Scale bar: A-F 100 μm, G-J 20μm. Embryos per condition (n = 3–7). All error bars represent S.E.M.
Table 2. Rohon Beard neuron counts.
| Counts | WT | runx1W84X/W84X | runx3w144/w144 | cbfbw128/w128 |
|---|---|---|---|---|
| 18s runx1 | 38.8±1.1 (14) | 0±0 (6) | 22.0±2.4 (7) | 17.0±3.0 (3) |
| 24hpf runx3 | 48.2±2.9 (5) | 2.9±1.0 (3) | 0±0 (7) | 2.6±0.9 (4) |
| 24hpf cbfb | 124.8±3.4 (10) | 118.3±6.8 (4) | 129.8±5.1 (8) | 0±0 (5) |
Average number of runx1, runx3 or cbfb positive Rohon Beard neurons at 18s or 24hpf. Data represents mean±SEM (n).
We found different regulatory patterns in the TG, where runx3, but not runx1 is expressed. TG somatosensory neurons were identified based on their location and their expression of Elavl, a pan-neuronal marker [19]. In 3dpf wild-type fish, cbfb was expressed in all TG neurons while runx3+ neurons comprised of less than half of the TG population (Fig 4G, 4I and 4K, Table 3). Although at 24hpf, there is a transient reduction in runx3 expression in the cbfb mutant, by 3dpf the number of runx3-expressing neurons is no different than WT (Fig 4A–4C, 4G, 4H and 4K; Table 3). cbfb expression also appeared unchanged in the runx3 mutant at 24hpf and 3dpf in the TG and the total number of cbfb+ cells at 3dpf was unchanged (~96% of WT) (Fig 4D–F, 4I and 4J; Table 3). Consistent with our findings that runx1 is not expressed in the TG, we saw no gross effect on runx3 expression in runx1 mutants at 3 dpf (S4A and S4B Fig). These data suggest that cbfb is not required for the survival of runx3-expressing TG neurons and vice versa. These results are similar to what has been reported in mouse DRG, where runx expression is independent of Runx activity [2].
Table 3. Trigeminal ganglion neuron counts.
| neurons/ganglion | WT | runx3w144/w144 | cbfbw128/w128 |
|---|---|---|---|
| runx3 | 29.3±1.8 (7) | 30.3±3.3 (3) | |
| cbfb | 62.25±1.9 (4) | 59.6±2.5 (5) |
Average number of runx3 or cbfb positive TG neurons as identified by Elavl staining at 3dpf in the runx3w144/w144 and cbfbw128/w128 mutants. Data represents mean±SEM (n).
Loss of Runx function does not affect the total number of neurons or apoptosis in the TG or RBs
In some contexts, loss of mammalian Runx function results in sensory neuron death [4,20,21]. We tested whether mutation of zebrafish runx or cbfb genes affected neuronal number in the TG and the RBs. None of the mutants showed any change in the number of RB neurons at 24hpf (Table 4; S5A–S5D and S5H Fig). There was also no change in the number of cells in the TG at 24 hpf or 3dpf (Table 4; S5E–S5G and S5I Fig). We next investigated if loss of Runx activity affected caspase-3 dependent apoptosis. Wildtype, runx1, runx3 and cbfb mutants were stained for activated caspase-3 at 24, 36, 48 and 72 hpf. We observed little activated caspase-3+ apoptotic cells in the TG at any of the timepoints and no difference among the genotypes (Table 5; S6 Fig). Within the time frame observed, these data demonstrate that loss of Runx signaling in zebrafish does not lead to an increase in neuronal cell death or changes in neuron number, though we cannot rule out changes at later timepoints.
Table 4. Elavl neuron counts.
| WT | runx1W84X/W84X | runx3w144/w144 | cbfbw128/w128 | |
|---|---|---|---|---|
| 24hpf RB+ cells | 165.1±4.1 (5) | 162.3±8.2 (3) | 159.0±5.6(3) | 159.5±4.0 (4) |
| 24hpf TG+ cells | 26.0±3.2 (3) | 29.3±2.7 (3) | 24.5±2.0 (4) | |
| 3dpf TG+ cells | 63.0±0.9 (26) | 64.1±3.4 (6) | 62±5.0 (3) |
Average number of Elavl+ 24hpf RB, 24hpf TG, and 3dpf TG cells in the runx1W84X/W84X, runx3w144/w144, and cbfbw128/w128 mutants. Data represents mean±SEM (n).
Table 5. Caspase 3 positive neurons counts.
| Age | WT | runx1W84X/W84X | runx3w144/w144 | cbfbw128/w128 |
|---|---|---|---|---|
| 1dpf | 1.40± .46 (5) | 0.67± .54 (3) | 1.25± .21 (4) | 1.75± .37 (4) |
| 1.5dpf | 0.85± .32 (7) | 1 ± 0.49 (5) | 1 ± 0.40 (6) | 0.66 ± 0.30(6) |
| 2dpf | 0.20± .18 (5) | 0.5 ± 0.25 (4) | 0.00± 0.0 (5) | 0.40± .22 (5) |
| 3dpf | 0.60± .36 (5) | 0.2± .22 (4) | 0.80± .33 (5) | 0.75± .21 (5) |
Average number of Caspase3 positive TG neurons at 24, 36, 48, 72 hours post fertilization in the runx1W84X/W84X, runx3w144/w144, and cbfbw128/w128 mutants. Data represents mean±SEM (n).
runx and cbfb are required for proper Trk receptor expression
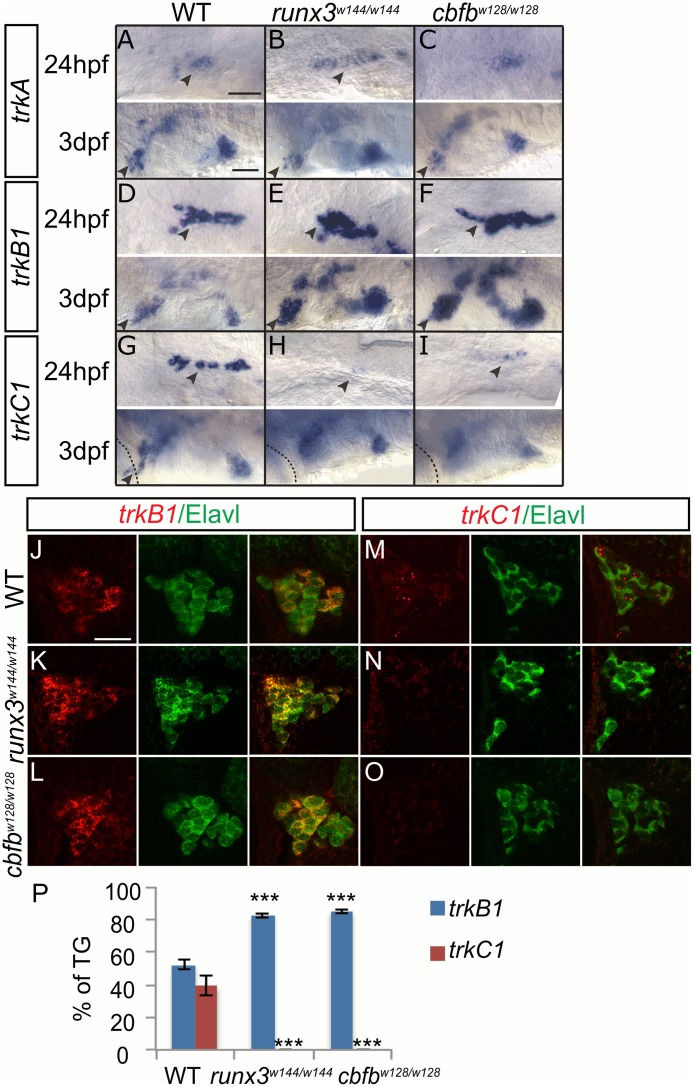
Although there is some discrepancy between different studies, it is clear that Runx regulates Trk receptor expression in mammals. Conditionally knocking out Runx1 in the DRG neurons of mice results in the expansion of TrkA-expressing neurons [7]. In Runx3 knockouts, some studies found an increase in TrkB-expressing neurons in the DRG and TG although other studies reported no change in the DRG [4,6]. Runx3 knockouts also showed either a loss of TrkC+ neurons in the DRG or a decrease in TrkC expression in the DRG and TG [4,6,22]. We performed in situ hybridization for trk receptors in each zebrafish mutant to determine whether Runx could play similar roles in regulating trk receptor expression. Analysis of the TG is simplified as only runx3 but not runx1 is expressed. trkA expression was unchanged in the TG at 24hpf and 3dpf in the runx3 or cbfb mutant (Fig 5A–5C). trkB1 expression increased at 24hpf and 3dpf in the runx3 and cbfb mutant while trkC1 expression was reduced in the TG at 24hpf and absent at 3dpf (Fig 5D–5I). To quantify these changes, we performed fluorescent in situ hybridization and counterstained for Elavl. In WT, we found that ~55% of the TG expressed trkB1 and ~42% of the TG expressed trkC1 (Fig 5P). In both runx3 and cbfb mutants we observed a significant increase in the number of trkB1+ neurons to ~89% of total and a complete loss in the number of trkC1+ neurons (Fig 5J–5P; Table 6). Surprisingly, there was no apparent change in trkC1 expression in other cranial ganglia, such as the facial and vagal ganglia that also express runx and cbfb, suggesting that neurotrophin receptor expression is regulated by an alternative mechanism in these structures.
Fig 5. Loss of runx or cbfb expression affects trk receptor expression in the TG.
A-I, Colormetric in situ hybridization for trkA (A-C), trkB1 (D-F), and trkC1 (G-I) in the runx3w144/w144 and cbfbw128/w128 mutants focusing on the TG at 24hpf and 3dpf. J-O, Antibody staining of Elavl (green) in conjunction with fluorescent in situ hybridization for trkB1 (J-L) and trkC1 (M-O) in the runx3w144/w144 and cbfbw128/w128 mutants at 3dpf. P, Quantification of marker gene expression as total number of TG neurons/ganglion and as % TG at 3dpf. Arrowhead, TG; X, vagal ganglion. Scale bar: A-I 100 μm, J-O 20μm. Embryos per condition (n = 3–5). ***p<0.001. All error bars represent S.E.M.
Table 6. Trigeminal ganglion neuron counts.
| WT | runx3w144/w144 | cbfbw128/w128 | |
|---|---|---|---|
| trkB1 | 35.0±0.5 (4) | 55.4±2.8(5) | 57.0±5.8 (3) |
| trkC1 | 26.4±4.4 (3) | 0.0±0.0 (3) | 0.0±0.0 (3) |
Average number of trkB1 or trkC1 positive TG neurons as identified by Elavl at 3dpf in the runx3w144/w144 and cbfbw128/w128 mutants. Data represents mean±SEM (n).
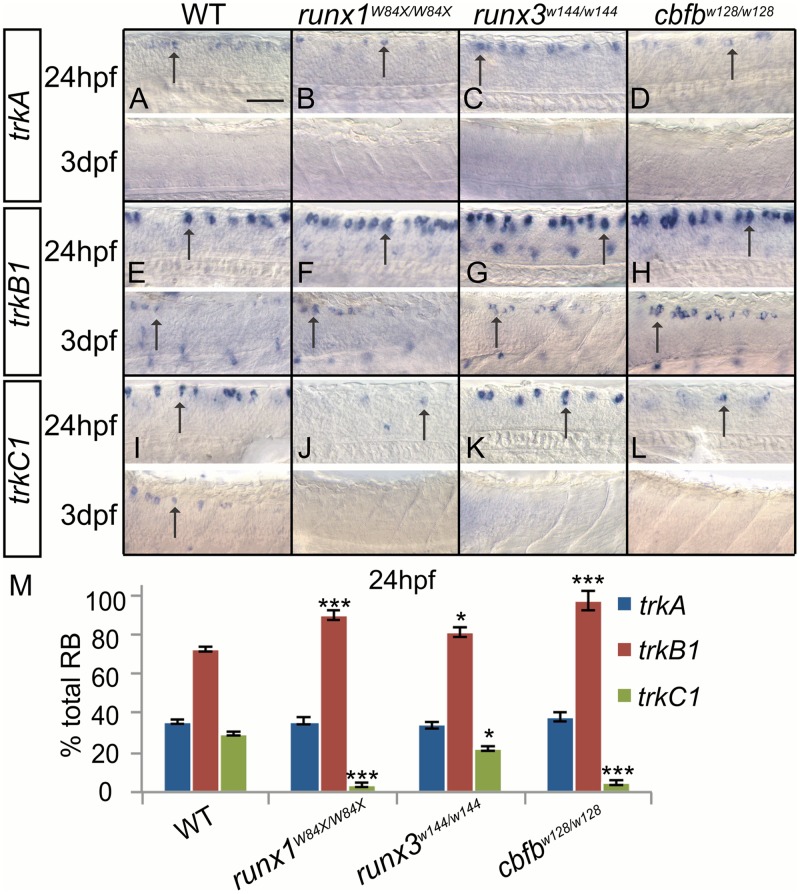
trkA expression was also unchanged in the RBs at 24hpf in the runx1, runx3 and cbfb mutants (Fig 6A–6D; Table 7); we were unable to detect trkA expression in RBs in 3dpf larvae. The number of trkB1+ RB cells was increased at 24hpf and 3dpf in all three mutants while trkC1 expression was reduced at 24hpf and absent at 3dpf (Fig 6E–6N; Table 7). At 24hpf, trkB1+ neurons were about 70% and trkC1+ neurons were about 30% of the total RB neurons (Fig 6M; Table 7). In the runx1 and cbfb mutant, there was a significant increase of trkB1 expression, where almost all of the RB neurons expressed trkB1, and a corresponding decrease such that almost no RB neurons expressed trkC1 (Fig 6M; Table 7). The runx3 mutant showed a less dramatic, but still significant increase in trkB1+ neurons and decrease trkC1+ neurons at 24hpf, potentially due to compensation by early runx1+ expression; however by 3dpf, the distribution of expression in the runx3 mutant was indistinguishable from the other mutants (Fig 6M; Table 7). Furthermore runx1/runx3 heterozygous animals showed a significant increase in trkB1+ neurons and decrease trkC1+ neurons at 24hpf (S3 Fig).
Fig 6. Loss of runx or cbfb expression affects trk receptor expression in the RBs.
A-M, Colormetric in situ hybridization for trkA (A-D), trkB1 (E-H), and trkC1 (I-L) in runx1W84X/W84X, runx3w144/w144 and cbfbw128/w128 mutants focusing on the RBs at 24hpf and 3dpf. M, Quantification of marker gene expression as % total RBs at 24hpf. Arrow, RBs. Scale bar: 100 μm. Embryos per condition (n = 3–12). *p<0.05, ***p<0.001. All error bars represent S.E.M.
Table 7. Rohon Beard neuron counts.
| WT | runx1W84X/W84X | runx3w144/w144 | cbfbw128/w128 | |
|---|---|---|---|---|
| trkA | 58.7±1.6 (12) | 59.33±3.2 (3) | 56.0±2.8 (3) | 62.6±3.5 (5) |
| trkB1 | 119.2±2.4 (6) | 148.0±3.9 (5) | 133.3±4.3 (3) | 160.0±7.9 (3) |
| trkC1 | 48.7±1.3 (8) | 5.8±1.5 (4) | 36.0±1.5 (7) | 7.8±2.2 (4) |
Average number of trkA, trkB1 or trkC1 positive RB neurons at 24hpf in the runx1W84X/W84X, runx3w144/w144, and cbfbw128/w128 mutants. Data represents mean±SEM (n).
The RB and TG data are very similar and support a role for runx and cbfb in suppressing trkB1 expression and promoting trkC1 expression. Since the increase in trkB1+ neurons is mirrored with a decrease in trkC1+ neurons and there is no change in neuronal number or apoptosis, we suggest that Runx/Cbfβ activity suppresses trkB1 expression and promotes trkC1 expression within the same subset of neurons. While apparently having no role in regulating trkA expression, a role Runx1 plays in mammals, the ability of Runx3 to regulate trkB1 and trkC1 expression is conserved between larval fish and terrestrial vertebrates even though these receptors label different neuronal subtypes in these species.
Loss of runx3 leads to the loss of some nociceptive receptors
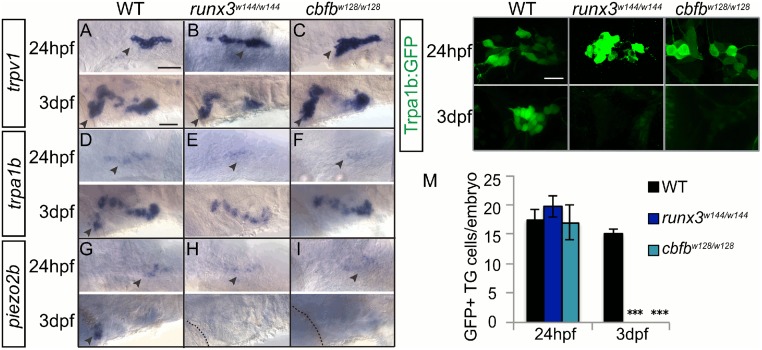
In mammals, loss of Runx1 leads to decreased or complete loss of expression of sensory ion channels including the noxious heat receptor TRPV1, the noxious chemosensor TRPA1, the light touch receptor Piezo2 and nociceptor specific voltage gated sodium channel NaV1.9 in the DRG, with corresponding somatosensory behavioral deficits [7,8]. In the TG, we examined expression of the zebrafish orthologs trpv1, trpa1b, piezo2b, and scn1α (NaV1.7 ortholog) in runx3 and cbfb mutants. The phenotypes of the runx3 and cbfb mutants were identical. trpv1 and scn1a expression appeared normal at 24hpf and 3dpf (Fig 7A–7C). By contrast, trpa1b expression by in situ and by GFP expression in the trpa1b:GFP transgenic reporter line was present at 24hpf, but absent at 3dpf (Fig 7D–7F and 7J–7M; Table 8). Loss of trpa1b expression was highly specific to the TG as expression was normal in other cranial ganglia, again suggesting that the effects of Runx/Cbfβ signaling are specific to somatosensory neurons. piezo2b expression was also present at 24hpf, but was completely gone from the TG by 3dpf (Fig 7G–7I). Consistent with Runx1 not having a role in TG neuronal specification, we observed no change in the number of GFP expressing neurons in runx1 mutants with the trpa1b:GFP transgenic reporter (Table 8; S4C and S4D Fig)
Fig 7. Loss of runx or cbfb expression affects ion channel expression in the TG.
A-I, Colormetric in situ hybridization for trpv1 (A-C), trpa1b (D-F), and piezo2b (G-I) in the runx3w144/w144 and cbfbw128/w128 mutants focusing on the TG at 24hpf and 3dpf. J-L, Antibody staining of GFP (green) in runx3w144/w144; trpa1b:GFP and cbfbw128/w128; trpa1b:GFP mutants. M, Quantification of GFP+ cells/ganglion in the TG at 24hpf and 3dpf. Dashed line outlines the eye; Arrowhead, TG. Scale bar: A-I 100 μm, J-L 20μm. Embryos per condition (n = 3–7). ***p<0.001. All error bars represent S.E.M.
Table 8. Trigeminal ganglion neuron counts.
| WT | runx1W84X | runx3w144/w144 | cbfbw128/w128 | |
|---|---|---|---|---|
| 24hpf trpa1b:GFP+ | 17.5±1.8 (3) | 19.7±1.7(3) | 17.0±3.1 (3) | |
| 3dpf trpa1b:GFP+ | 15.1±0.9 (7) | 14.2±0.7 (13) | 0.0±0.0 (5) | 0.0±0.0 (5) |
| 24hpf Isl1SS:Kaede+ | 17.2±1.3 (5) | 17.0±3.1 (3) | ||
| 3dpf Isl1SS:Kaede+ | 11.1±1.2 (7) | 11.0±1.6 (3) | ||
| 24hpf p2x3b:eGFP+ | 16.2±2.3 (6) | 15.6±2.1 (3) | ||
| 3dpf p2x3b:eGFP+ | 24.5±3.5 (5) | 26.0±2.6 (3) |
Average number of GFP or Kaede positive TG neurons at 3dpf in the runx3w144/w144 and cbfbw128/w128 mutants in the trpa1b:GFP, Isl1SS:Kaede and p2x3b:eGFP transgenic fish. Data represents mean±SEM (n).
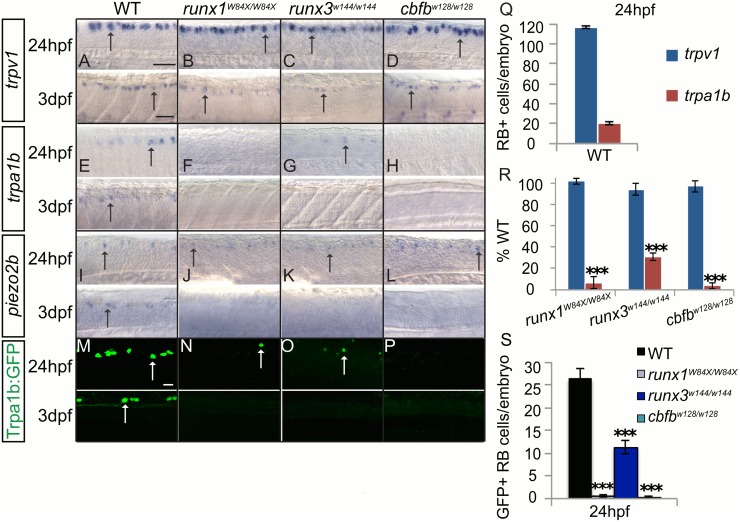
The phenotype in the RB neurons mirrored the TG phenotype. The expression of trpv1 and scn1a was unchanged at 24hpf and 3dpf (Fig 8A–8D and 8Q–8R; Table 9; S7P–S7T Fig). The expression of trpa1b as measured by in situ and GFP expression in the trpa1b:GFP transgenic was significantly reduced at 24hpf and absent at 3dpf in all three mutant lines (Fig 8E–8H and 8M–8P; Table 9). piezo2b expression was normal at 24hpf, but was absent from RB neurons at 3dpf (Fig 8I–8L). Similar to trkC1 expression, Runx/Cbfβ is required for maintenance of trpa1 and piezo2b expression but not for initiation. These data suggests that in larval zebrafish only some aspects of mammalian Runx function in regulating the expression of different sensory ion channels and other nociceptor markers were present. However, these functions are dependent on Runx3 and not Runx1 signaling.
Fig 8. Loss of runx or cbfb expression affects ion channel expression in the RBs.
A-L, Colormetric in situ hybridization for trpv1 (A-D), trpa1b (E-H), and piezo2b (I-L) in the runx1W84X/W84X, runx3w144/w144 and cbfbw128/w128 mutants focusing on the RBs at 24hpf and 3dpf. M-P, Antibody staining of GFP (green) in runx3w144/w144; trpa1b:GFP and cbfbw128/w128; trpa1b:GFP mutants. Q-R, Quantification of marker gene expression as total number of RB neurons/embryo and as % WT at 24hpf. S, Quantification of GFP+ cells in the RBs at 24hpf. Dashed line outlines the eye; Arrow, RBs; Arrowhead, TG; X, vagal ganglion. Scale bar: A-L 100 μm, J-L, M-N 20μm. Embryos per condition (n = 3–12). ***p<0.001. All error bars represent S.E.M.
Table 9. Rohon Beard neuron counts.
| WT | runx1W84X/W84X | runx3w144/w144 | cbfbw128/w128 | |
|---|---|---|---|---|
| trpv1 | 116.3±1.0 (12) | 118.7±3.5 (6) | 110.0±6.7 (3) | 113.0±6.2 (4) |
| trpa1b | 20.1±1.2 (12) | 1.3±1.1 (3) | 6.3±0.7 (4) | 0.8±0.6 (4) |
| trpa1b:GFP | 26.2±2.4 (6) | 0.5±0.3 (4) | 11.3±1.5 (3) | 0.3±0.3 (4) |
| scn1α | 128.3±3.4 (11) | 121.0±3.7 (3) | 120.7±4.3 (3) | 135.7±8.2 (3) |
Average number of trpv1, trpa1b, trpa1b:GFP, and scn1α positive RB neurons at 24hpf in the runx1W84X/W84X, runx3w144/w144, and cbfbw128/w128 mutants. Data represents mean±SEM (n).
We also asked whether loss of zebrafish Runx function changed expression of markers that define nociceptive neuron subtypes in the mouse, such as CGRP, Ret and the ATP receptor P2X3 [7,8]. We examined mRNA expression of cgrp and ret, as well as GFP expression in transgenic reporter lines Isl1SS:Kaede, which labels a subpopulation of peptidergic nociceptors, and P2x3b:eGFP in the runx3 and cbfb mutants [3,23]. In the TG, we saw no change in any of these markers at any of the timepoints we examined (Table 8; S7A–S7F and S7J–S7O Fig). Unfortunately we were unable to document clear and consistent expression of cgrp and ret in RB neurons. We can conclude however that in zebrafish TG, Runx3/Cbfβ signaling is not involved in regulating the expression of markers of peptidergic and nonpeptidergic nociceptors. These functions were likely acquired in terrestrial vertebrates alongside Runx1 specialization in specification of nociceptor cell fate.
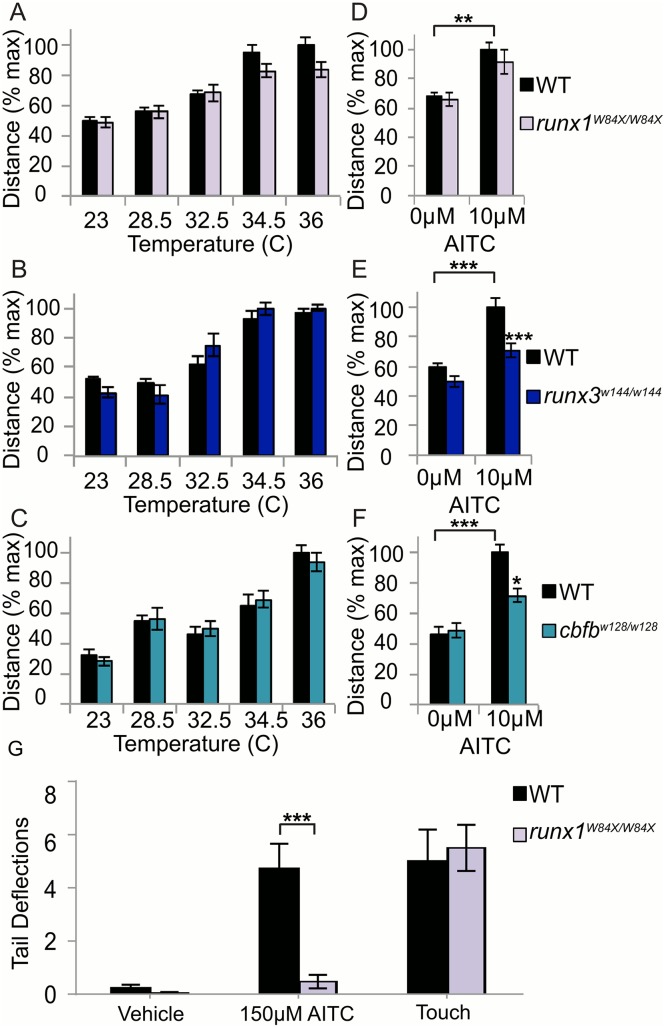
To determine whether changes in trpa1b ion channel expression had functional consequences, we used a locomotor assay at 5 dpf to test whether Runx mutants showed defects in their responses to heat and AITC [13]. runx1, runx3 and cbfb mutants showed no behavioral change in response to heat compared to WT fish (Fig 9A–9C), reflecting our earlier findings showing no change in trpv1 ion channel expression. In contrast, the locomotor responses of 5dpf runx3 and cbfb mutants to AITC were abolished, which reflects the loss of trpa1b expression specifically in the TG and RB of these mutants (Fig 9E and 9F). However, runx1 mutants, which have normal trpa1b expression in the TG but a loss of trpa1b expression in RBs, responded normally to AITC (Fig 9D). To determine if the loss of Trpa1b in RB neurons could affect localized behavioral responses in runx1 mutants, we performed a tail-deflection assay in which AITC was puffed onto the tails of head immobilized 5dpf larvae and tail flick responses were monitored. Tail-deflection to AITC was almost completely abolished in runx1 mutants compared to WT controls while responses to a mechanical stimulus were unaffected (Fig 9G; S1–S6 Videos). This result indicates that while trpa1b expression in RB neurons is required for localized sensitivity to AITC in the body/tail, expression in the TG is sufficient to evoke WT levels of locomotion in response to bath applied AITC.
Fig 9. Loss of runx or cbfb expression affects locomotor responses of zebrafish to AITC, but not heat.
A-C, Heat behavior in the runx1W84X/W84X (A), runx3w144/w144 (B), and cbfbw128/w128 (C) mutants. D-F, AITC behavior in the runx1 (D), runx3 (E), and cbfb (F) mutants (G) AITC and touch tail deflection behavior of 3dpf runx1 larvae. Embryos per condition (n = 10–25). *p<0.05, **p<0.01, ***p<0.001. All error bars represent S.E.M.
Runx can rescue loss of Cbfβ
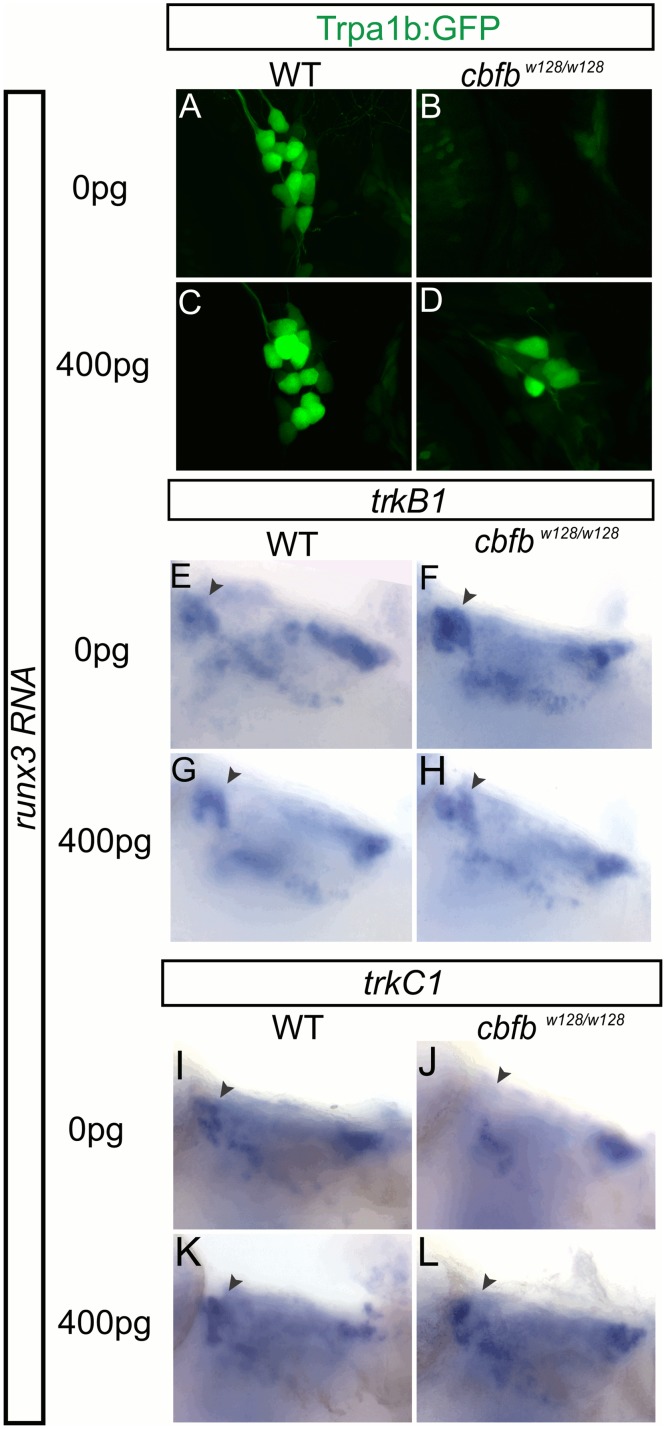
We next examined whether overexpression of runx and/or cbfb could change the fates of somatosensory neurons resulting in ectopic Runx dependent gene expression in normally Runx-negative neurons. We monitored trpa1b:GFP expression in WT, cbfb or runx3 mutant embryos after introduction of runx1, runx3 or cbfb mRNA. In either the TG or in RBs, injection of runx1, runx3, or cbfb into WT embryos resulted in little change the number of neurons expressing GFP (Fig 10A and 10C; Table 10). Chromatin inaccessibility may prevent exogenous Runx/Cbfβ complex from promoting GFP expression in neurons that do not normally express it. To test this idea, we incubated embryos with the histone deacetylase inhibitor valproic acid in combination with runx3 overexpression. Again we saw no increase in GFP-expressing neurons over WT levels (Table 10). This data indicates that Runx/Cbfβ signaling alone is not sufficient to drive trpa1:GFP expression in TG neurons.
Fig 10. Runx3 overexpression rescues the cbfb mutant phenotypes in TG.
A-D, Antibody staining for GFP of TG in cbfb-/- trpa1b:GFP mutants injected with 0 or 400pg of runx3 RNA at 3dpf. E-F TrkB1 colormetric in situ hybridization staining for GFP in cbfbw128/w128; trpa1b:GFP mutants injected with 0 or 400pg of runx3 RNA at 3dpf. I-L TrkC1 colormetric in situ hybridization staining for GFP in cbfbw128/x128; trpa1b:GFP mutants injected with 0 or 400pg of runx3 RNA at 3dpf.
Table 10. Rescue neuron counts.
| TG | WT | runx3w144/w144 | cbfbw128/w128 | ||
|---|---|---|---|---|---|
| % rescued | Avg rescue | % rescued | Avg rescue | ||
| 0pg | 10.8±1.7 (6) | 0% (10) | 0% (9) | ||
| 50pg runx1 RNA | 10.5±0.8 (9) | 37.5% (8) | 2.0±0.7 (3) | 0% (7) | |
| 400pg runx1 RNA | 12.3±1.6 (4) | 66.7% (3) | 4.5±0.7 (2) | 0% (4) | |
| 50pg runx3 RNA | 11.7±1.0 (9) | 71.4% (7) | 9.2±2.7 (5) | 0% (6) | |
| 400pg runx3 RNA | 12.3±0.9 (11) | 36.4% (11) | 4.3±1.2 (4) | 36.4% (11) | 3.0±0.5 (4) |
| 50pg cbfb RNA | 12.8±1.6 (6) | 0% (8) | 20% (10) | 3.0±0.0 (2) | |
| 250pg cbfb RNA | 11.8±1.5 (8) | 0% (8) | 33.3% (9) | 6.0±4.6 (3) | |
| 50pg runx3 RNA 50pg cbfb RNA |
13.6±0.6 (16) | ||||
| 500uM VPA | 8.4±0.8 (10) | ||||
| 500uM VPA 50pg runx3 RNA |
8.5±0.5 (10) | ||||
| RB | |||||
| 0pg | 27.3±2.1 (6) | 0% (10) | 0% (9) | ||
| 50pg runx1 RNA | 37.8±1.3 (9) | 75.0% (8) | 6.2±1.7 (6) | 0% (7) | |
| 400pg runx1 RNA | 36.0±2.8 (4) | 100% (3) | 12.3±4.8 (3) | 0% (4) | |
| 50pg runx3 RNA | 33.8±2.9 (9) | 85.7% (7) | 16.2±2.4 (6) | 0% (6) | |
| 400pg runx3 RNA | 33.5±1.7 (11) | 54.5% (11) | 7.5±2.3 (6) | 27.3% (11) | 7.7±1.8 (3) |
| 50pg cbfb RNA | 26.5±2.6 (6) | 12.5% (8) | 5.0 (1) | 0% (10) | |
| 250pg cbfb RNA | 29.1±2.4 (8) | 25% (8) | 7.0±1.4 (2) | 33.3% (9) | 26.3±5.5 (3) |
| 50pg runx3 RNA 50pg cbfb RNA |
31.5±1.8 (16) | ||||
% of fish with rescue and average number of trpa1b:GFP positive neurons in rescued fish at 3dpf in the runx3w144/w144 and cbfbw128/w128 mutants injected with runx1, runx3 or cbfb RNA and/or incubated with valproic acid (VPA). Data represents mean±SEM (n).
We also tested whether Runx1 could compensate for loss of Runx3 in the TG, and whether overexpression of runx3/runx1 could overcome the loss of cbfb. Each mRNA was able to partially rescue the loss of trpa1:GFP expression in the TG and RBs in corresponding mutants (Table 10). We saw little difference in the ability of runx1 or runx3 to rescue the runx3 null mutant (Table 10), suggesting that they are functionally interchangeable and that differences in phenotype are due to spatial and temporal differences in expression. High concentrations (250pg) of cbfb RNA were unable to rescue trpa1:GFP expression in the TG of the runx3 mutant (Table 10). Excess Cbfβ was able to partially rescue GFP expression in the RB population, likely due to its ability to interact with remaining Runx1 activity in these neurons (Table 10). We next sought to test whether Cbfβ is an obligate cofactor for Runx activity, given the similarities in phenotypes after cbfb and runx loss of function, by injecting runx mRNA into cbfb null mutants. High (400pg) but not low (50pg) concentrations of runx3 RNA rescued GFP expression in cbfb null mutants in both TG and RB neurons (Fig 10B and 10D; Table 10). In cbfb null mutants that showed rescue of trpa1:GFP TG expression after injection of runx3 RNA (400pg), we found rescue of trkC1 expression in the TG of 78% of larvae (7/9) and suppression of trkB1 expression in the TG of 38% of larvae (3/8) (Fig 10E–10L). Together these data indicate that Cbfβ is acting solely through its complex with Runx TFs to facilitate sensory neuron differentiation and that excess Runx can compensate for the loss of Cbfβ enhancement of Runx DNA binding affinity and/or Runx protein stability.
trkC1+ neurons switch fate to become trkB1+ neurons
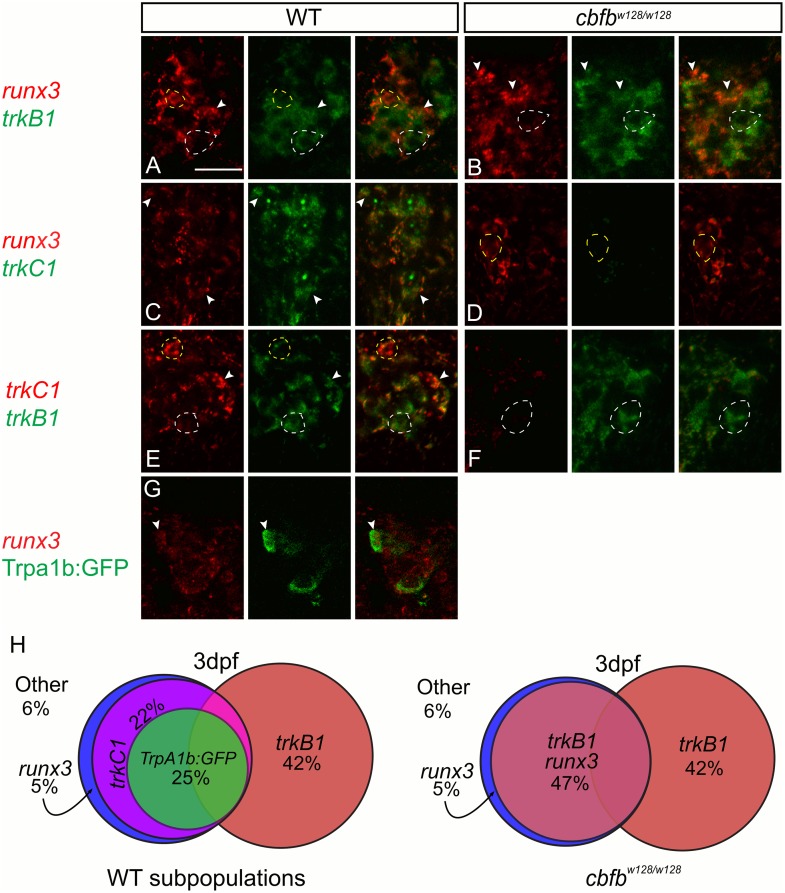
We hypothesized that trkC1-expressing neurons adopted a trkB1+ cell fate in the absence of Runx function. Since expression of runx3 is unchanged in cbfb mutants, we could examine the fates of runx3+ cells in these animals. In WT fish, we found that the runx3 population contained almost all cells that expressed the trpa1b:GFP transgene and trkC1 mRNA (~96% of trpa1b:GFP+ cells express runx3; ~93% of trkC1+ cells express runx3; Fig 11C and 11G; Table 11). By contrast the trkB1 population is largely separate from the runx3+/trkC1+/trpA1b:GFP+ population (~16% and ~21% of trkB1+ cells express trkC1 and runx3 respectively; Fig 11A and 11C; Table 11). In cbfb mutants, we found in nearly all runx3+ TG cells (86%) co-expressed trkB1 (Fig 11A–11F; Table 11). These results, together with our earlier observations that showed no overall changes in TG neuron number or neuronal cell death, suggest that loss of Runx signaling results in trkC1-expressing neurons assuming a trkB1-expressing fate.
Fig 11. trkC1+ neurons switch fate to become trkB1+ neurons in the cbfb mutant zebrafish.
A-F, Optical sections of double fluorescent in situ hybridization for runx3 (red) and trkB1 (green) (A-B), runx3 (red) and trkC1 (green) (C-D), and trkC1 (red) and trkB1 (green) (E-F) in wild-type and cbfb mutants. G-I, Optical sections of antibody staining of GFP (green) in conjunction with fluorescent in situ hybridization for runx3 (G) in trpa1b:GFP fish at 3dpf. H, Representations of 3dpf wild-type and cbfb mutant somatosensory subpopulations as a percent of the whole TG. Dashed white line outline green cells; Dashed yellow lines outline red cells; Arrowhead indicates double positive cells. Scale bar: 20μm. Embryos per condition (n = 3–4). All error bars represent S.E.M.
Table 11. Trigeminal neuron counts.
| Double positive cells | WT | cbfbw128/w128 |
|---|---|---|
| runx3+ trkB1+ | 7.3±0.4 (3) | 25.3±1.8 (3) |
| runx3+ trkC1+ | 24.5±1.1 (4) | 0.0±0.0 (3) |
| trkB1+ trkC1+ | 5.5±0.8 (4) | 0.0±0.0 (3) |
| runx3+ trpa1b:GFP+ | 11.8±1.1 (4) |
Average number of double positive TG cells for runx3+ trkB1+, runx3+ trkC1+, trkB1+ trkC1+, runx3+ trpa1b:GFP+, trkB1+ trpa1b:GFP+, and trkC1+ trpa1b:GFP+ at 3dpf in wild-type and cbfbw128 mutants. Data represents mean±SEM (n).
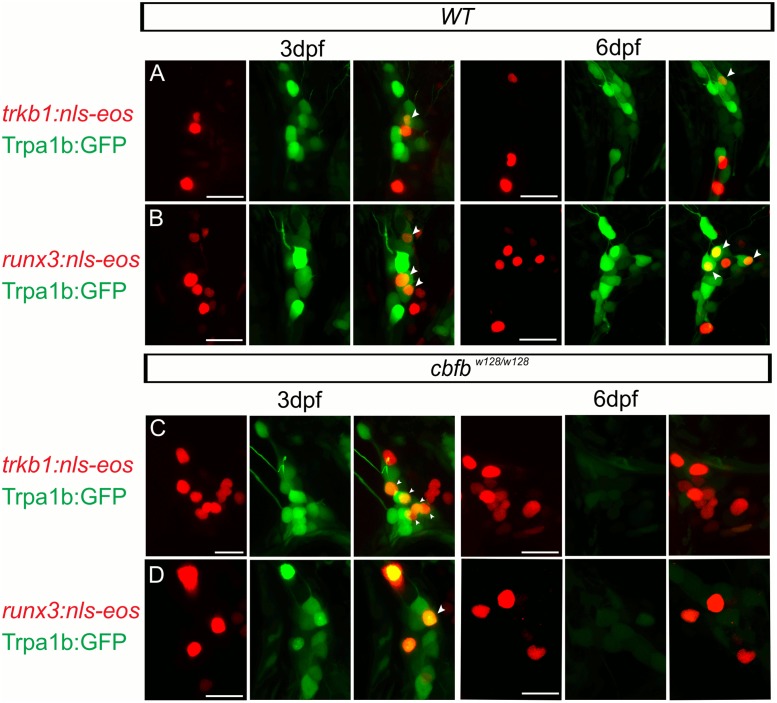
To test directly whether cells changed fate, we labeled cells with the photoconvertible protein Eos. We first examined the time course of expression loss of trpa1b:GFP using high-resolution confocal microscopy. With this methodology, we observed GFP expression in cbfb mutants at 3dpf, when trkC1 and trpa1b expression are absent as measured by in situ hybridization (see Figs 5 and 6) However, GFP was absent by 6dpf (S8 Fig). The ability to visualize these neurons at 3dpf indicated that these neurons were still present and had not been replaced in cbfb mutants with a new runx3+ neuronal subtype. We then performed scatter-labeling of TG neurons by CRISPR-mediated insertion of nls-Eos into the trkB1 or runx3 promoter region of WT and cbfb mutants transgenic for trpa1b:GFP. At 3dpf we photoconverted nls-Eos and imaged the TG to observe residual GFP to identify double positive neurons (Fig 12). We then re-imaged the same animals at 6 dpf when GFP was absent. In all animals imaged, all photoconverted runx3:nls-Eos (WT, 15/15 TG neurons, n = 4 animals; cbfb mutants, 12/12 TG neurons, n = 4 animals) and trkB:nls-Eos (WT, 7/8 TG neurons, n = 2 animals; cbfb mutants, 11/11 TG neurons, n = 2 animals) neurons were still present at 6dpf including those that were formally double positive neurons in cbfb mutants. While these results are consistent with survival at the time points measured, we cannot rule out that these neurons may be lost at later stages. In sum, these data support our conclusion that runx3+ neurons that would normally express trkC1 in WT animals assume a trkB1-expressing cell fate in Runx signaling mutants.
Fig 12. Scatter labeled trkB1 and runx3 neurons persist in cbfbw128/w128; trpa1b:GFP embryos.
(A-D) Maximum intensity projections of transiently expressed nls-Eos in trpA1b:GFP fish at 3dpf and 6dpf. (A) trkb1:nls-Eos in a WT trpa1b:GFP embryo, (B) runx3:nls-Eos in a WT trpa1b:GFP embryo, (C) trkB1:nls-Eos in a in cbfbw128/w128; trpa1b:GFP embryo, (D) runx3:nls-Eos in a in cbfbw128/w128; trpa1b:GFP embryo. Arrowhead indicates double positive nls-Eos/GFP TG neurons. Scale bar: 20μm.
Discussion
In this study, we set out to define the roles that zebrafish Runx/Cbfβ complexes play in refining early larval somatosensory cell fate specification. We first examined expression of neurotrophin receptors that in mammals define distinct somatosensory populations. It is inherently difficult to compare potentially dynamic developmental expression patterns across species due to the temporal differences in development and differences in the time points examined. In most terrestrial vertebrates the neurotrophin receptors TrkA, TrkB and TrkC are required respectively for the survival and specification of distinct classes of nociceptive, mechanoceptive and proprioceptive (DRG) or mechanoceptive (TG) somatosenosory neurons. In contrast, we found in larval zebrafish that the neurotrophin receptor orthologs trkA, trkB1, and trkC1 were expressed in distinct and overlapping patterns in nociceptive neurons as determined by the coexpression of the nociceptive ion channels trpv1 and trpa1b. Collectively these neurons account for nearly 90% of the TG at 3dpf with the remaining neurons not expressing any of these markers. These findings are in line with our previous work that showed that all early born TG neurons expressed nociceptive markers [13]. It is possible that zebrafish neurotrophin receptors segregate into different populations of somatosensory neurons or take on additional roles similar to terrestrial vertebrates at later developmental stages.
To test the roles of Runx TFs in regulating somatosensory neuron specification, we obtained a runx1 mutant and generated loss of function mutations in runx3 and cbfb by genome editing. In contrast to mammals in which Runx1 and Runx3 have distinct functions regulating nociceptors and proprioceptors, we found that zebrafish runx1 and runx3 both regulate nociceptors development. Both runx1 and runx3 play an overlapping role to influence RB nociceptor development in the body, while in the TG, only runx3 has this function. Analogous to mammalian Runx1, zebrafish Runx3 controls the expression of the sensory receptors trpA1b and piezo2b. However while mammalian Runx1 additionally acts to suppress trkA and cgrp expression and promote ret, p2x3 and trpv1 expression, loss of zebrafish runx3 in the TG has no apparent effect on these markers at the time points examined.
As for mammals, we found that loss of zebrafish runx3 results in loss of trkC1 expression and an increase in trkB1 expression, despite the fact that all these genes are expressed in nociceptors in early larval zebrafish and not mechanoreceptors/proprioceptors as in mammals. Taken together these results demonstrate that the core Runx regulatory program is conserved amongst larval zebrafish and mammals. Future experiments examining loss of function of zebrafish neurotrophin genes will be necessary to determine to what degree the roles of neurotrophin receptors in the specification of somatosensory cell fate have diverged.
Regulation of expression of runx genes differed depending on what population of somatosensory neurons they were expressed. Runx/Cbfβ signaling in RB neurons was required for maintaining runx expression as runx1 and runx3 expression was lost in cbfb null mutants and loss of runx1 function led to loss of runx3 expression and to a lesser extent vice versa. This suggests that a primary role of Runx1 in RB neurons is to facilitate runx3 expression. These results are consistent with findings that Runx proteins have been shown to regulate their own promoters [24]. In the TG, loss of cbfb did not alter runx3 expression at the timepoints examined, suggesting that either Runx3 can maintain its own expression in the absence of cbfb or that an idependent mechanism controls runx3 expression. Loss of runx had no effect on cbfb expression in either the TG or RB neurons. This result is consistent with studies in mouse DRG where Runx and Cbfb gene expression were shown to be regulated independently [25].
Cbfβ can act as an obligate cofactor for Runx activity in some tissues while acting to enhance Runx function in others. Cbfβ enhances Runx binding to DNA 5 to-10-fold as well as promotes Runx protein stability. For example, in mouse, a conditional deletion of Runx1 and a conditional rescue of Cbfβ indicated that the formation of hematopoietic stem cells (HSCs) and erythroid/myeloid progenitors required both Runx1 and Cbfβ [26,27]. By contrast in zebrafish, Cbfβ mutants are able to form Runx1-dependent HSCs [28]. In addition, Runx2-dependent intramembranous and endochondral ossification can develop further in the absence of Cbfβ function than they do in Runx2-/- mice [29]. This suggests that in some cases Cbfβ may act to refine Runx function but is not essential for all Runx-dependent activity. A recent study in mice found that conditional knockouts of Runx1 and Cbfβ in the DRG, had identical somatosensory neuron cell fate specification defects, suggesting that in this context Cbfβ serves as an obligate cofactor of Runx1 [25]. Howerver, loss of Cbfβ in the DRG resulted in the loss of Runx1 protein but not runx1 mRNA, so it was not possible to measure the result of Runx transcriptional activity in the absence of Cbfβ [25].
We found that in zebrafish Cbfβ serves as an obligate cofactor of Runx3 such that all somatosensory defects caused by runx3 deletion were phenocopied by cbfb deletion. However expression of high (but not low) levels of runx3 mRNA was able to rescue trpa1b:GFP expression and trkC1 expression while suppressing trkB1 expression in cbfb mutants, suggesting that Runx can function in the absence of Cbfβ. In wild-type fish, neither runx3 and/or cbfb RNA could drive etopic expression of trpa1b:GFP. Additionally, although runx and cbfb are also expressed in other cranial ganglia in addition to the TG, they do not affect the expression of trkC1 or trpa1b in these structures. These results suggest that the role of the Runx/Cbfβ interaction in managing the expression of these genes is highly specific to somatosensory neurons and may require the coexpression of other factors to mediate its effect.
Our data supports the investigation of the genetic determinants of somatososensory neuron development/function in early born larval zebrafish. We have revealed a neurotrophin receptor code in larval zebrafish that is substantially divergent from that of terrestrial vertebrates, yet shown that the developmental program that gives rise to somatosensory neuron diversity remains largely intact.
Our results suggest that in terrestrial vertebrates Runx1 and Runx3 gained additional distinct roles by subfunctionalization, with Runx1 taking over most functions in nociceptors and Runx3 acquiring additional prominent roles in proprioceptors (DRG) or mechanoreceptor (TG) specification. Terrestrial vertebrates have a much larger and well-defined set of proprioceptive neurons, presumably gained with the need for postural somatosensory feedback that occurred as a consequence of the acquisition of tetrapod limbs and the move to land. It is not clear however if teleost fish require the light touch mechanoreceptors and proprioceptors associated respectively with TrkB and TrkC in terrestrial vertebrates [30]. The need for these sensations in terrestrial vertebrates may have necessitated the ceding of nociceptor specification to TrkA and the repurposing of TrkB and TrkC to promote mechanosensory and proprioceptive neuronal fates. The dominance of nociceptive markers in larval zebrafish suggests that early nociceptive development is critical for survival of the free-swimming larvae and may therefore take precedence over other somatosensory modalities.
Materials and methods
Ethics statement
Experiments using zebrafish were performed under the University of Washington Institutional Animal Care and Use Committee protocols #4216–02 (approved on 9/16/2016). The University of Washington Institutional Animal Care and Use Committee (IACUC) follow the guidelines of the Office of Laboratory Animal Welfare and set its policies according to The Guide for the Care and Use of Laboratory Animals. The University of Washington maintains full accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and has letters of assurance on file with OLAW. The IACUC routinely evaluates the University of Washington animal facilities and programs to assure compliance with federal, state, local, and institution laws, regulations, and policies. The OLAW Assurance number is DL16-00292.
Zebrafish
Zebrafish were maintained at 28.5°C on a 14h/10h light/dark cycle following established methods. Embryos were maintained in E2 medium, and staged according to the standard manual [31]. runx1W84X mutants were obtained from the Liu laboratory (National Institutes of Health, Bethesda, MD, USA) [16]. A subset of the trigeminal was identified using TgBAC(trpa1b:EGFP)a128TG and Tg(isl1:Gal4-VP16,14xUAS:Kaede)a128, referred to as trpa1b:GFP and Isl1SS:Kaede, from the Schier laboratory (Harvard University, Cambridge, MA, USA) [3], and Tg(p2rx3b:EGFP)sl1Tg, referred to as p2x3b:eGFP, from the Voigt laboratory (Saint Louis University School of Medicine, St. Louis, MO, USA) [23].
Generation of the zebrafish cbfb and runx3 truncation mutations
All mutations were generated the AB strain of zebrafish and prior to analysis each genetic mutant was backcrossed for at least two generations into the AB background.
TALEN target site selection and assembly
Transcription activator-like effector nucleases (TALENs) were used to generate a mutation in zebrafish cbfb. TALENs were assembled using the Golden Gate assembly protocol and library [17]. TALE binding sites in exon 2,of the cbfb genomic sequence, 5′- TTAAATACACCGGTTTCCGC-3′ and 5′-TTCTGGAAGCGCGCCTGCCT-3′, were identified using TALE-NT 2.0 [32].
CRISPR target site selection and assembly
sgRNAs were designed using http://crispr.mit.edu. We used a two-oligo PCR method to make the template DNA [33]. A scaffold oligo containing the Cas9 recognition loop and an oligo with a T7 binding site, the runx3 sgRNA sequence, and homology to scaffold oligo were synthesized. The scaffold oligo sequence was 5′-GATCCGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTAT TTTAACTTGCTATTTCTAGCTCTAAAAC-3′. The runx3 sgRNA oligo sequence was 5′-AATTAATACGACTCACTATA(GTGCAACAAAACCCTTCCCG)GTTTTAGAGCTAGAAATAGC-3′; the target in exon 3 is indicated in parentheses.
mRNA synthesis and microinjection of zebrafish embryos
TALEN expression vectors were linearized with SmaI and transcribed in vitro using the mMessage mMachine T7 and Poly(A) Tailing Kit (ThermoFisher). The pT3TS-nCas9n plasmid (Addgene), linearized using XbaI and purified [18], was used in an in vitro transcription reaction (T3 mMessage mMachine, ThermoFisher). The runx3 guide RNA was synthesized using the MegaScript T7 Kit (ThermoFisher). RNA products were cleaned by phenol-chloroform and isopropanol precipitation. The TALEN mixture containing equal amounts of each mRNA (400 pg each) was injected into one-cell stage AB strain zebrafish embryos. The CRISPR/Cas9 injection contained 300pg of Cas9 mRNA and 50ng of runx3 gRNA and was also injected into one-cell stage zebrafish embryos. Injected embryos were raised to adulthood, outcrossed, and gDNA isolated from F1 embryos to identify mutants.
Genomic DNA Isolation
Individual embryos were processed as previously described [34]. Embryos were incubated in 1x base solution from a 50x stock (1.25 M NaOH, 10mM EDTA pH12) at 95°C for 30 min in 25μl and then a 2x neutralization solution from a 50x solution (2M Tris-HCl pH5) was added.
High Resolution Melt (HRM) curve analysis
Primers for identifying the cbfb mutation were 5′- AACACTCTTCTGTGCCTTTTTCATCC -3′ and 5′- TGAGGTGCGTGTACTCACTATCTCTG -3′. Primers for the runx3 mutation were 5′- CCAAACTTTCTCTGCTCGGTCCT -3′ and 5′- GAGCGCGAGTTCTGTTTGTAGC -3′. HRM mix contained 400μl 5x Gotaq buffer (Promega), 40μl 10mM dNTPs, 120μl 25mM Mgcl2, 100μl DMSO (Sigma), 100μl 20x EvaGreen (Biotium), 60μl Taq, and up to 1000μl water. PCR reactions contained 0.5μl of each primer (10μM), 10μl of HRM mix, 1μl of gDNA, and water up to 20μl. PCR was performed in a BioRad CFX Connect, using 96 well plates (BioRad cat. No. HSP9601). PCR reaction protocol was 95°C for 2 min, then 40 cycles of 95°C for 45 sec, 60°C for 30 sec, and 72°C for 30 sec, followed by 95°C for 30 sec and 60°C for 1 min. Melting curves were generated over a 65–95°C range. Curves were analyzed with the BioRad Precision Melt Analysis Software to identify mutations. Sequencing identified cbfbw128 as a 4bp deletion (nt109-112 (CACG)) and runx3w144 as a 1bp deletion (nt247 (C)), which both predict an early truncation (S1 Fig).
DNA constructs
In situ probe constructs
A clone for Ret (Clone ID: 9038324) was purchased from Open Biosystems. scn1a and piezo2b were cloned from total RNA extracted from 72hpf zebrafish embryos. scn1a and piezo2b cDNA was amplified by performing reverse transcription PCR with Superscript II (ThermoFisher) using primers (F: 5’- gctagaattcTTTACTCCGCCAGGACCT-3’; R: 5’- gctagtcgacTAAAGCGTCCCACACAG -3’) and (F: 5’- gatcgaattcGTCTTTCTGATCTGGTCCT -3’; R: 5’- gatagtcgacTGGT GCTCTCCTGTTTG -3’) respectively. scn1a and piezo1b cDNA were cloned into pBluescript SK+ (Stratagene) for in situ hybridization with EcoRI and SalI.
mRNA injection
Full length cbfb, runx1, and runx3 were cloned from total RNA extracted from 72hpf zebrafish embryos. cbfb, runx1, and runx3 cDNA were amplified by performing reverse transcription PCR with Superscript II (Invitrogen) using primers (F: 5’- GATAGAATTCATGCCTCGGGTGGTCC -3’; R: 5’-GATAGTCGACCTAGCGCATCTTGTGATCATCAGT-3’), (F: 5’- taatacgactcactatagggATGGTTTTTCTTTGGGACGCC-3’; R: 5’- TCAGTATGGCCTCCAGACGG -3’), and (F: 5’-GCTAGGTACC ATGCATATTCCCGTAGACC-3’; R: 5’-GCGCGAATTCtttctaaatcttagtacggc-3’) respectively. cbfb and runx3 coding sequences were TA cloned into pCR2.1 (ThermoFisher), linearized with KpnI and transcribed in vitro using the mMessage mMachine T7 and Poly(A) Tailing Kit to generate capped, polyadenylated mRNA. The runx1 coding sequence was purified and transcribed in vitro using the mMessage mMachine T7 and Poly(A) Tailing Kit to generate capped, polyadenylated mRNA. A range of mRNA concentrations was injected into embryos derived from a cross between trpa1b:GFP/runx3+/w144 and Tg(Trpa1b:GFP)/cbfb+/w128 parents. Data were analyzed using two-factor ANOVA.
Whole mount in situ hybridization and antibody staining
Digoxigenin (DIG) labeled riboprobes for runx1, cbfb [28], cgrp [3], trkA, trkB1, trkC1 [35], trpv1, and trpa1b [13] and fluorescein (FLR) labeled riboprobes for trkB1 and trkC1 were generated as previously described. Full length (2.1kb) trkA was amplified using primers (F: 5’- ATGGCTGACCATAGGGTGGCC-3’ and R: 5’- TAATACGACTCACTATAGGG CTACTCCAGGATGTCCAGGTAGAC-3’) and transcribed with T7 polymerase (ThermoFisher) to generate DIG-labeled riboprobe. An 870bp (colormetric) or 450bp (fluorescence) runx3 fragment was amplified using primers (F: 5’- gcatattcccgtagacccga-3’ and R: 5’- gatcTAATACGACTCACTATAGGGCGGA GTATGTGAAGTG-3’; F: 5’-agccacttcacatactccgc-3’ and R: 5’-gatcTAATACGACTCACTAT AGGGttagtacggcctccag-3’) respectively and transcribed with T7 polymerase. ret was linearized with NotI and transcribed with T3 polymerase (ThermoFisher). scn1a and piezo2b were linearized with EcoRI and transcribed with T7 polymerase.
In situ hybridization was carried out as previously described [36,37]. In brief, embryos were hybridized with DIG-labeled or FLR-labeled RNA probes overnight at 55°C followed by stringent washes. Samples were incubated with anti-DIG Biotin-conjugated Fab fragments (Roche, 1:1000 DIG, 1:500 FLR) and then incubated with Cy3- or FITC-tyramide (PerkinElmer). Embryos were stained with mouse anti-Elavl antibody (ThermoFisher HuC+HuD antibody 16A11, 1:1000) to identify trigeminal sensory neurons and/or rabbit anti-GFP (Invitrogen, 1:1000) to identify a subset of trigeminal sensory neurons in trpa1b:GFP fish as previously described and imaged by confocal microscopy [13]. Isl1SS:Kaede fish were stained with rabbit anti-Kaede antibody (MBL International, 1:1000). Active caspase-3 staining was used to identify TG neurons undergoing apoptosis with rabbit anti-active Caspase 3 (ThermoFisher bdb559565). In double in situ hybridization experiments, double positive cells were identified as consistently as possible by shape. Colocalization of Elavl, GFP, or an in situ probe with an in situ probe was analyzed by confocal imaging in single optical planes. Data were analyzed using ANOVA.
Behavioral analysis
Larvae were raised on a 14/10 h light/dark cycle at 28.5°C. At 5dpf, individual larvae were placed in single wells on a 100 M 96-well mesh plate (MANM 100 10; Millipore). The base plate, into which the mesh plate was inserted, was constructed from .002” aluminum Shim in a can (ASTM–B– 209; Shopaid), which had been scrubbed. Base plates were thoroughly washed and soaked in distilled water before use. Two base plates filled with embryo medium were placed on each side of a dual solid-state heat/cool plate (AHP-12000CP; Teca) set to control and test temperatures, with an intervening film of water to facilitate temperature transfer. Larvae were loaded into each well of the mesh plate placed on the control side with a cut pipette. The mesh plate was then transferred from control to test temperature and larval movement videotaped for 4 minutes. To assay chemical responses, larval movement was recorded for 4 min after the mesh plate was placed into the lid of a 24 multiwell tissue culture plate (353047; BD Labware) containing allyl isothiocyanate (AITC; mustard oil; Sigma-Aldrich 377430; diluted in 1% DMSO). Behavioral responses were recorded using a Canon high definition digital video camcorder with a frame rate of 60 fps. The locomotor activity of each larva was analyzed with EthoVision XT locomotion tracking software (Noldus Information Technology, Inc.). Data were analyzed using ANOVA.
Tail deflection behavior
At 3dpf, the larval zebrafish offspring of a runx1+/W84X cross were imbedded in 1.5% agarose at the bottom of individual wells filled with 10mL of E2 medium. The agarose surrounding the tail of each fish was cut away allowing the tail to float freely. These fish were then exposed to either 150μM AITC (Sigma-Aldrich) in 1% DMSO (Sigma-Aldrich), 1% DMSO vehicle, or touch with 0.018cm diameter fishing line (Maxima). All solutions contained phenol red (Sigma-Aldrich) for visualization and were applied in a 50ms pulse by a Picosprizter II microinjection apparatus (General Valve Corporation). The behavioral response of each fish was recorded using a Canon high definition digital video camcorder with a frame rate of 60 fps and were manually scored based on the number of tail deflections. Following behavioral analysis animals were genotyped as described. Statistical significances was determined using ANOVA.
CRISPR/Cas9 mediated scatter labeling of TG neurons
Transient transgenic labeling of trigeminal neurons was performed as previously described [38]. In short, single cell embryos are injected with Cas9 protein, a reporter containing plasmid, a guide RNA (gRNA) targeting the endogenous promoter of trkB1 or runx3, and a gRNA targeting the injected plasmid. mRuby3 (pKanCMV-mClover3-mRuby3 was a gift from Michael Lin (Addgene plasmid # 74252)) and nls-Eos reporter plasmids were generated by cloning into the XbaI/BamHI sites of pbsk-Mbait-Hsp-GFP (gift from Shin-ichi Higashijima)[39]. The following gRNA sequences were used: mBait (GGCTGCTGCGGTTCCAGAGG), runx3 (GGGTTTAAGCGACCAATCAG), and trkB1 (GGTGTGTTTGCTGCTTCGTG).
At 3dpf injected embryos were photoconverted using a DAPI filter and screened for nls-Eos expression. Embryos expressing nls-Eos in the TG were anesthetized with Mesab, mounted in 2% agarose, and imaged on a Zeiss LSM 880 confocal microscope. They were then returned to standard rearing conditions, before imaging at 6dpf.
Supporting information
A-C, Maximum intensity projection of a single TG from a 3dpf TrpA1:GFP (green) embryo transiently expressing mRuby from the trkB1(red) promoter. Arrowhead indicates double positive cells. Scale bar: 20μm.
(TIFF)
A. Location of mutations in exons. B. Nucleotide location of mutations and predicted early termination stop codon. C. Location of predicted early stop in protein structure.
(TIFF)
A-T, Colormetric in situ hybridization for runx1 (A-D), runx3 (E-H), trkB1 (I-L), trkC1 (M-P) trpa1b (Q-T) in RB neurons of WT, runx1+/W84X/runx3+/w144, runx1+/W84X/cbfb+/w128 and runx3+/w144/cbfb+/w128 double heterozygous mutants at 18s (runx1) and 24hpf (runx3, trkB1, trkC1, and trpa1b). U, Quantification of marker gene expression as % WT at 18s or 24hpf. Arrow, RBs; Scale bar: 100 μm. Embryos per condition (4–15). *p<0.05, **p<0.01, ***p<0.001. All error bars represent S.E.M.
(TIFF)
A-B, Colormetric in situ hybridization for runx3 in WT (A) and runx1W84X/W84X null mutants (B). C-D, Trpa1:GFP expression in WT (C) and runx1W84X/W84X null mutants (D).
(TIFF)
A-G, Antibody staining for Elavl at 24hpf and 3dpf in the runx1W84X/W84X, runx3w144/w144, and cbfbw128/w128 mutants. H-I, Quantification of Elavl expression in the RB population at 24hpf and the TG population at 24hpf and 3dpf. Scale bar: 20μm. Embryos per condition (n = 3–26). All error bars represent S.E.M.
(TIFF)
A-P, Maximum intensity projections of antibody staining for HuC (green) and active caspase3 (red) at 1dpf (A-D), 1.5dpf (E-H), 2dpf (I-L), or 3dpf (M-P) Scale bar: 20μm.
(TIFF)
A-I, Colormetric in situ hybridization for cgrp (A-C), ret (D-F), and scn1α (G-I) in the runx3w144/w144 and cbfbw128/w128 mutants focusing on the TG at 24hpf and 3dpf. J-M, Antibody staining of Kaede (red) in cbfbw128/w128; Isl1SS:Kaede mutants and of GFP (green) in cbfbw128/w128; P2X3.2:eGFP mutants at 24hpf and 3dpf. N-O, Quantification of Kaede+ and GFP+ cells in the TG at 24hpf and 3dpf. P- S, Colormetric in situ hybridization for scn1α in the runx1W84X/W84X, runx3w144/w144 and cbfbw128/w128 mutants focusing on the RBs at 24hpf and 3dpf. T, Quantification of marker gene expression as %WT in RBs at 24hpf. Arrow, RBs; Arrowhead, TG. Scale bar: A-M 100 μm, N-Q 20μm. Embryos per condition (n = 3–11). All error bars represent S.E.M.
(TIFF)
A-H. Maximum intensity projections of TrpA1b:GFP expression from the same TG in a WT (A- D) or cbfbw128/w128 (E-H) embryo over five days. Scale bar: 20μm.
(TIFF)
(MP4)
(MP4)
(MP4)
(MP4)
(MP4)
(MP4)
Acknowledgments
We thank members of the Dhaka and Raible labs for helpful discussions and technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: National Institute of Health R01DE23730 (AD, DWR); National Institute of Health GM07270 (PG); and National Insititute of Health T32HD007183. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8: 114–27. doi: 10.1038/nrn2057 [DOI] [PubMed] [Google Scholar]
- 2.Senzaki K, Ozaki S, Yoshikawa M, Ito Y, Shiga T. Runx3 is required for the specification of TrkC-expressing mechanoreceptive trigeminal ganglion neurons. Mol Cell Neurosci. 2010;43: 296–307. doi: 10.1016/j.mcn.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Pan YA, Choy M, Prober DA, Schier AF. Robo2 determines subtype-specific axonal projections of trigeminal sensory neurons. Dev Camb Engl. 2012;139: 591–600. doi: 10.1242/dev.076588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, et al. , others. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21: 3454–3463. doi: 10.1093/emboj/cdf370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AI, de Nooij JC, Jessell TM. Graded Activity of Transcription Factor Runx3 Specifies the Laminar Termination Pattern of Sensory Axons in the Developing Spinal Cord. Neuron. 2006;49: 395–408. doi: 10.1016/j.neuron.2005.12.028 [DOI] [PubMed] [Google Scholar]
- 6.Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A Role for Runx Transcription Factor Signaling in Dorsal Root Ganglion Sensory Neuron Diversification. Neuron. 2006;49: 379–393. doi: 10.1016/j.neuron.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Chen C-L, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, et al. Runx1 Determines Nociceptive Sensory Neuron Phenotype and Is Required for Thermal and Neuropathic Pain. Neuron. 2006;49: 365–377. doi: 10.1016/j.neuron.2005.10.036 [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa M, Senzaki K, Yokomizo T, Takahashi S, Ozaki S, Shiga T. Runx1 selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons. Dev Biol. 2007;303: 663–674. doi: 10.1016/j.ydbio.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 9.Ogawa E, Inuzuka M, Maruyama M, Satake M, Naito-Fujimoto M, Ito Y, et al. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194: 314–331. doi: 10.1006/viro.1993.1262 [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Wang Q, Crute BE, Melnikova IN, Keller SR, Speck NA. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993;13: 3324–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G, Shigesada K, Ito K, Wee HJ, Yokomizo T, Ito Y. Dimerization with PEBP2beta protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J. 2001;20: 723–733. doi: 10.1093/emboj/20.4.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing RJ, Dhaka A. ThermoTRPs and Pain. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2016;22: 171–187. doi: 10.1177/1073858414567884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gau P, Poon J, Ufret-Vincenty C, Snelson CD, Gordon SE, Raible DW, et al. The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J Neurosci Off J Soc Neurosci. 2013;33: 5249–5260. doi: 10.1523/JNEUROSCI.5403-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavanaugh DJ, Chesler AT, Bráz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci Off J Soc Neurosci. 2011;31: 10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30: 582–593. doi: 10.1038/emboj.2010.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sood R, English MA, Belele CL, Jin H, Bishop K, Haskins R, et al. Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation. Blood. 2010;115: 2806–2809. doi: 10.1182/blood-2009-08-236729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, Zhang F. A transcription activator-like effector toolbox for genome engineering. Nat Protoc. 2012;7: 171–192. doi: 10.1038/nprot.2011.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jao L-E, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A. 2013;110: 13904–13909. doi: 10.1073/pnas.1308335110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendergast A, Linbo TH, Swarts T, Ungos JM, McGraw HF, Krispin S, et al. The metalloproteinase inhibitor Reck is essential for zebrafish DRG development. Dev Camb Engl. 2012;139: 1141–1152. doi: 10.1242/dev.072439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theriault FM, Roy P, Stifani S. AML1/Runx1 is important for the development of hindbrain cholinergic branchiovisceral motor neurons and selected cranial sensory neurons. Proc Natl Acad Sci U S A. 2004;101: 10343–10348. doi: 10.1073/pnas.0400768101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A, Senzaki K, Ozaki S, Yoshikawa M, Shiga T. Runx1 promotes neuronal differentiation in dorsal root ganglion. Mol Cell Neurosci. 2012;49: 23–31. doi: 10.1016/j.mcn.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Ito K, Osato M, Lee B, Bae S-C, Ito Y. The Transcription Factor Runx3 Represses the Neurotrophin Receptor TrkB during Lineage Commitment of Dorsal Root Ganglion Neurons. J Biol Chem. 2007;282: 24175–24184. doi: 10.1074/jbc.M703746200 [DOI] [PubMed] [Google Scholar]
- 23.Kucenas S, Soto F, Cox JA, Voigt MM. Selective labeling of central and peripheral sensory neurons in the developing zebrafish using P2X(3) receptor subunit transgenes. Neuroscience. 2006;138: 641–652. doi: 10.1016/j.neuroscience.2005.11.058 [DOI] [PubMed] [Google Scholar]
- 24.Komori T. Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab. 2003;21: 193–197. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, O’Donovan KJ, Turner EE, Zhong J, Ginty DD. Extrinsic and intrinsic signals converge on the Runx1/CBFβ transcription factor for nonpeptidergic nociceptor maturation. eLife. 2015;4 doi: 10.7554/eLife.10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457: 887–891. doi: 10.1038/nature07619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, et al. Erythroid/Myeloid Progenitors and Hematopoietic Stem Cells Originate from Distinct Populations of Endothelial Cells. Cell Stem Cell. 2011;9: 541–552. doi: 10.1016/j.stem.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bresciani E, Carrington B, Wincovitch S, Jones M, Gore AV, Weinstein BM, et al. CBFβ and RUNX1 are required at 2 different steps during the development of hematopoietic stem cells in zebrafish. Blood. 2014;124: 70–78. doi: 10.1182/blood-2013-10-531988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, et al. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet. 2002;32: 633–638. doi: 10.1038/ng1015 [DOI] [PubMed] [Google Scholar]
- 30.Bone Q, Moore R. Biology of Fishes. Taylor & Francis; 2008. [Google Scholar]
- 31.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, others. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203: 253–310. doi: 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- 32.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39: e82 doi: 10.1093/nar/gkr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassett AR, Tibbit C, Ponting CP, Liu J-L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4: 220–228. doi: 10.1016/j.celrep.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker ND, Hutchinson SA, Ho L, Trede NS. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. BioTechniques. 2007;43: 610, 612, 614 [DOI] [PubMed] [Google Scholar]
- 35.Martin SC, Marazzi G, Sandell JH, Heinrich G. Five Trk receptors in the zebrafish. Dev Biol. 1995;169: 745–758. doi: 10.1006/dbio.1995.1184 [DOI] [PubMed] [Google Scholar]
- 36.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3: 59–69. doi: 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman SG, Peters NC, Altaras AE, Berg CA. Optimized RNA ISH, RNA FISH and protein-RNA double labeling (IF/FISH) in Drosophila ovaries. Nat Protoc. 2013;8: 2158–2179. doi: 10.1038/nprot.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura Y, Hisano Y, Kawahara A, Higashijima S. Efficient generation of knocki-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering. Sci Rep. 2014;4: 6545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajar BT, Wang ES, Lam AJ, Kim BB, Jacobs CL, Howe ES, et al. Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Sci Rep. 2016;6: 20889 doi: 10.1038/srep20889 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A-C, Maximum intensity projection of a single TG from a 3dpf TrpA1:GFP (green) embryo transiently expressing mRuby from the trkB1(red) promoter. Arrowhead indicates double positive cells. Scale bar: 20μm.
(TIFF)
A. Location of mutations in exons. B. Nucleotide location of mutations and predicted early termination stop codon. C. Location of predicted early stop in protein structure.
(TIFF)
A-T, Colormetric in situ hybridization for runx1 (A-D), runx3 (E-H), trkB1 (I-L), trkC1 (M-P) trpa1b (Q-T) in RB neurons of WT, runx1+/W84X/runx3+/w144, runx1+/W84X/cbfb+/w128 and runx3+/w144/cbfb+/w128 double heterozygous mutants at 18s (runx1) and 24hpf (runx3, trkB1, trkC1, and trpa1b). U, Quantification of marker gene expression as % WT at 18s or 24hpf. Arrow, RBs; Scale bar: 100 μm. Embryos per condition (4–15). *p<0.05, **p<0.01, ***p<0.001. All error bars represent S.E.M.
(TIFF)
A-B, Colormetric in situ hybridization for runx3 in WT (A) and runx1W84X/W84X null mutants (B). C-D, Trpa1:GFP expression in WT (C) and runx1W84X/W84X null mutants (D).
(TIFF)
A-G, Antibody staining for Elavl at 24hpf and 3dpf in the runx1W84X/W84X, runx3w144/w144, and cbfbw128/w128 mutants. H-I, Quantification of Elavl expression in the RB population at 24hpf and the TG population at 24hpf and 3dpf. Scale bar: 20μm. Embryos per condition (n = 3–26). All error bars represent S.E.M.
(TIFF)
A-P, Maximum intensity projections of antibody staining for HuC (green) and active caspase3 (red) at 1dpf (A-D), 1.5dpf (E-H), 2dpf (I-L), or 3dpf (M-P) Scale bar: 20μm.
(TIFF)
A-I, Colormetric in situ hybridization for cgrp (A-C), ret (D-F), and scn1α (G-I) in the runx3w144/w144 and cbfbw128/w128 mutants focusing on the TG at 24hpf and 3dpf. J-M, Antibody staining of Kaede (red) in cbfbw128/w128; Isl1SS:Kaede mutants and of GFP (green) in cbfbw128/w128; P2X3.2:eGFP mutants at 24hpf and 3dpf. N-O, Quantification of Kaede+ and GFP+ cells in the TG at 24hpf and 3dpf. P- S, Colormetric in situ hybridization for scn1α in the runx1W84X/W84X, runx3w144/w144 and cbfbw128/w128 mutants focusing on the RBs at 24hpf and 3dpf. T, Quantification of marker gene expression as %WT in RBs at 24hpf. Arrow, RBs; Arrowhead, TG. Scale bar: A-M 100 μm, N-Q 20μm. Embryos per condition (n = 3–11). All error bars represent S.E.M.
(TIFF)
A-H. Maximum intensity projections of TrpA1b:GFP expression from the same TG in a WT (A- D) or cbfbw128/w128 (E-H) embryo over five days. Scale bar: 20μm.
(TIFF)
(MP4)
(MP4)
(MP4)
(MP4)
(MP4)
(MP4)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.