Abstract
INTRODUCTION
CD40 is a promising therapeutic target for cancer immunotherapy. In patients with advanced solid malignancies, CD40 agonists have demonstrated some anti-tumor activity and a manageable toxicity profile. A 2nd generation of CD40 agonists has now been designed with optimized Fc receptor (FcR) binding based on preclinical evidence suggesting a critical role for FcR engagement in defining the potency of CD40 agonists in vivo.
AREAS COVERED
We provide a comprehensive review using PubMed and Google Patent databases on the current clinical status of CD40 agonists, strategies for applying CD40 agonists in cancer therapy, and the preclinical data that supports and is guiding the future development of CD40 agonists.
EXPERT COMMENTARY
There is a wealth of preclinical data that provide rationale on several distinct approaches for using CD40 agonists in cancer immunotherapy. This data illustrates the need to strategically combine CD40 agonists with other clinically active treatment regimens in order to realize the full potential of activating CD40 in vivo. Thus, critical to the success of this class of immune-oncology drugs, which have the potential to restore both innate and adaptive immunosurveillance, will be the identification of biomarkers for monitoring and predicting responses as well as informing mechanisms of treatment resistance.
Keywords: CD40 agonists, macrophages, T cells, chemotherapy, cancer, clinical trials
1. Introduction
Strategies designed to harness the immune system for the treatment of cancer have recently demonstrated significant benefit for some patients across a wide-range of malignancies. For example, immune checkpoint inhibitors designed to disrupt inhibitory signals received by T cells through CTLA-4 and PD-1 molecules can improve overall survival by producing durable remissions in cancer patients [1,2]. However, across many cancers, the vast majority of patients still do not respond to immune checkpoint inhibition using blocking antibodies to CTLA-4 and PD-1/PD-L1. Thus, ongoing efforts in cancer immunotherapy are now focused on patient selection (i.e. identifying patients who are most likely to benefit from a particular immunotherapeutic strategy) and understanding mechanisms of resistance (i.e. defining the mechanisms that underlie treatment resistance in order to inform the optimization and selection of an immunotherapeutic approach for a patient) [3].
Successful cancer immunotherapy relies on an alignment of the innate and adaptive arms of the immune system. A key molecule involved in bridging innate and adaptive immunity is CD40, a member of the TNF receptor superfamily. Within the innate immune system, CD40 is expressed on antigen presenting cells (APCs) including subsets of monocytes, macrophages, and dendritic cells. CD40 is also expressed by B cells and platelets as well as some non-hematopoeitic cell types such as fibroblasts, endothelial cells, and smooth muscle cells. Even some tumor cells express CD40 [4–6]. Further, ligation of CD40 in vivo has the potential to elicit an array of outcomes from activation of APCs to induction of tumor cell death [6–9].
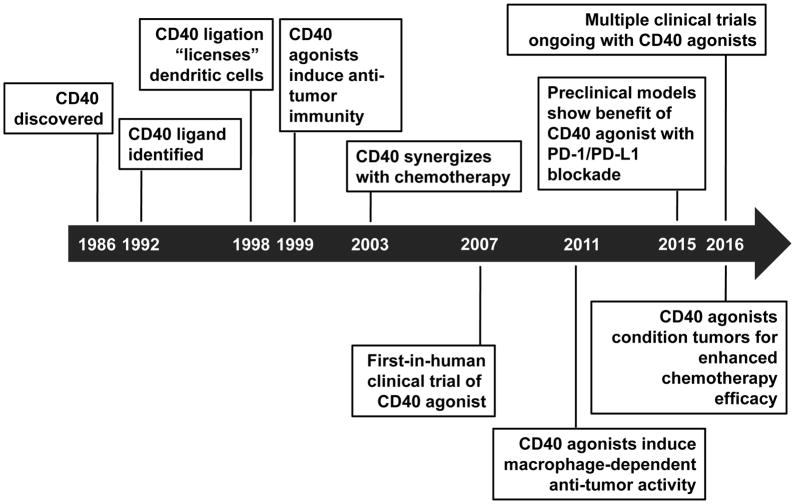
The ligand for CD40 is CD40 ligand (i.e. CD154) which is expressed on a variety of cell types, including activated CD4 T cells [7–9], activated B cells [10], memory CD8 T cells [11], activated natural killer cells [12], granulocytes [13], endothelial cells [14], smooth muscle cells [14], macrophages [14], and activated platelets [15]. In the late 1990s, the interaction between CD40 on dendritic cells (DCs) and CD40 ligand on activated CD4 T cells was found to be a critical step in “licensing” DCs with the capacity to effectively present antigen and activate antigen-specific CD8 T cells [7–9,16]. Specifically, ligation of CD40 on DCs enhanced the expression of co-stimulatory (e.g. CD80 and CD86) and major histocompatibility (MHC) molecules, induced the release of immunostimulatory cytokines, and activated antigen presentation machinery. The importance of CD40 in tumor immunity was subsequently demonstrated in several landmark studies where administration of an agonistic antibody directed against CD40 produced protective T cell immunity in murine models of cancer [17–19]. This early biology laid the foundation for the development of clinical grade CD40 agonists that are now under active investigation in the clinic (Figure 1).
Figure 1. Milestones in the history of CD40 as a target for cancer immunotherapy.
The timeline depicts some of the pivotal milestones in the study of CD40 as a target for cancer immunotherapy. 1990 – CD40 discovered [93]. 1992 – CD40 ligand identified [94,95]. 1998 – CD40 ligation “licenses” dendritic cells [7–9,16]. 1999 – CD40 agonists induce anti-tumor immunity [17–19]. 2003 – CD40 synergizes with chemotherapy [77]. 2007 – First-in-human clinical trial of CD40 agonist [23]. 2011 – CD40 agonists induce macrophage-dependent anti-tumor immunity [25]. 2015 – Preclinical models show benefit of CD40 agonist with PD-1/PD-L1 blockade [87]. 2016 – CD40 agonists “condition” tumors for enhanced chemotherapy efficacy [26]. 2016 –Multiple clinical trials ongoing with CD40 agonists.
2. Designing a potent CD40 agonist
Several approaches have been investigated to activate the CD40 pathway in humans: (i) recombinant human CD40 ligand, (ii) CD40 ligand gene therapy, and (iii) agonistic CD40 antibodies. Each strategy has produced promising clinical activity in early phase studies. For recombinant human CD40 ligand (rhuCD40L), a Phase I study in patients with advanced solid tumors and non-Hodgkin lymphoma investigated subcutaneous dosing of rhuCD40L for five consecutive days repeated every 4–6 weeks in the absence of progressive disease or organ toxicity [20]. Of 32 patients treated, two (6%) developed a partial response on study with four additional patients (16%) demonstrating stable disease lasting at least four months. For CD40 ligand gene therapy (AdCD40L) using adenoviral vectors expressing CD40 ligand, one study reported on eight patients with bladder carcinoma undergoing cystectomy for invasive disease who were treated with AdCD40L instillation into the bladder [21]. Treatment was found to be generally well-tolerated and produced evidence of immune activation, as detected on biopsy and seen by increased infiltration of T cells and expression of IFN-gamma. A second study evaluated intratumoral administration of AdCD40L in 15 patients with metastatic malignant melanoma who were treated with four weekly injections [22]. Nine of the patients also received treatment in combination with low dose cyclophosphamide. While no objective responses were seen on radiographic imaging, metabolic responses detected on FDG-PET imaging were observed in local and distant lesions with evidence of increased T cell infiltration seen on post-treatment biopsies compared to baseline. Together, these clinical studies demonstrate the prospect of using CD40 ligand-based strategies to activate CD40 in patients for cancer therapy.
The most advanced clinical approach to date for activating CD40 in vivo has involved the use of agonistic CD40 monoclonal antibodies. The first report of an agonistic CD40 antibody in patients with advanced solid malignancies investigated CP-870,893, a fully human and selective CD40 agonist monoclonal antibody (mAb) that was designed with minimal Fc receptor binding activity based on its IgG2 isotype [23]. The most common adverse event with CP-870,893 treatment was grade 1–2 cytokine release syndrome manifested by chills, fever and rigors within minutes to hours after infusion. Treatment was also associated with transient decreases in monocytes, B cells, and platelets as well as increases in liver function tests and D-dimer levels. These pharmacodynamics effects of a CD40 agonist, including hepatic injury and leukocyte trafficking from the peripheral blood, have also been reproduced in preclinical models [24–26], although the precise mechanisms underlying this biology remain ill-defined. Nonetheless, this first-in-human study of a CD40 agonist in patients with advanced cancer demonstrated safety as well as promising clinical activity with a response rate of 14%.
2.1. Fc modification
Since the first clinical report of an anti-CD40 agonist, several other agonists have now been developed and are under active investigation in patients with advanced malignancies (Table 1). These 2nd generation CD40 agonists have been engineered with an IgG1 Fc domain to facilitate enhanced Fc gamma receptor (FcγR) interactions based on findings that increased Fc binding affinity to FcγRIIB enhances the potency of a CD40 agonist in murine models through crosslinking [27,28]. These agonists contrast CP-870,893, which has an IgG2 Fc domain, and thus a low binding affinity to human FcγRs. Recently, antibodies with an IgG2 Fc domain have been found to mediate FcγR-independent agonistic activity that is conferred by the unique hinge properties of this isotype [29]. However, for CP-870,893, the Fc domain of the antibody and FcR crosslinking are not be required for CD40 stimulation [30]. The precise mechanism underlying this finding is unclear but could be explained by binding of CP-870,893 to a unique epitope on human CD40 that produces potent signaling activity.
Table 1.
CD40 agonists and current clinical status
| CD40 agonist | Isotype | Active Clinical Studies |
|---|---|---|
| RO7009789 (formerly CP-870,893) | Fully human IgG2 agonist |
|
| APX005M | Humanized rabbit IgG1 agonist |
|
| ADC-1013 | Fully human IgG1 agonist |
|
| Chi Lob 7/4 | Chimeric IgG1 agonist |
|
| SEA-CD40 | Non-fucosylated humanized IgG1 agonist |
|
Recent work has shown that engineering CP-870,893 with an IgG1 Fc domain, to enhance binding to FcγRIIB, can improve the potency of CP-870,893 as measured by its ability to invoke antigen-specific T cell immunity in a novel humanized mouse model [31]. FcγRIIB is the only inhibitory Fc receptor and variations in the gene encoding this protein have long been associated with susceptibility to autoimmune disease [32]. While FcγRIIB polymorphisms are known [32], whether these variations will have therapeutic implications for the translation of IgG1-modified CD40 agonists is presently unclear. In addition, the enhanced potency of IgG1 CD40 agonists seen in murine models is associated with an increased capacity to produce transient thrombocytopenia[31]. Reducing the dose of the Fc-modified CD40 agonist, though, was found to diminish the level of thrombocytopenia while still maintaining improved agonist activity compared to the IgG2 isotype [31].
The requirement for Fc receptor engagement in vivo for anti-CD40 efficacy is not absolute, as suggested by the activity of CP-870,893 which displays poor FcR binding. Alternative strategies beyond Fc engineering for enhancing anti-CD40 efficacy, such as chemical crosslinking of CD40 antibodies, have also shown Fc-independent activity in murine models [33]. In addition, the requirement for the inhibitory FcγRIIB for in vivo activity of IgG1 anti-CD40 antibodies is not definite. For example, activatory FcRs induced by a TLR3 agonist can restore the in vivo activity of an IgG1 CD40 agonist that is otherwise lost in hosts lacking FcγRIIB [33]. This finding suggests that the role of the FcR for anti-CD40 activity is to provide in vivo cross-linking. Clinical studies investigating IgG1 CD40 agonists are underway and are expected to provide further insight into the role of Fc modification on CD40 agonist-induced toxicity and efficacy in patients with advanced malignancies.
2.2. Clinical grade antibodies targeting CD40
Over the past decade, several clinical grade antibodies that target CD40 have been developed. Each of these agents is distinct with unique properties defined by (i) binding affinity to CD40, (ii) isotype, (iii) requirement for cross-linking for activity, and (iv) ability to block CD40 ligand binding. These clinical grade antibodies include:
CP-870,893 is an anti-CD40 IgG2 antibody with poor FcR binding that does not block CD40 ligand interaction with CD40; can mediate CD40 stimulation in the absence of cross-linking [30]; and has a binding affinity (Kd) of 3.48×10−10M [34].
APX005 is an IgG1 antibody recognizing CD40 that blocks CD40 ligand binding; shows enhanced activity in vitro with cross-linking; and has a Kd of 9.6×10−10M [35].
ADC-1013 is an IgG1 antibody that recognizes CD40 with high binding affinity (Kd 1×10−11M) even under acidic conditions (pH 5.4) with activity dependent on FcR binding and cross-linking [36].
Dacetuzumab (also called SGN-40 and formerly SGN-14) is a humanized IgG1 antibody recognizing CD40 with a Kd of 1×10−9M. Dacetuzumab is a partial agonist that shows weak activity in stimulating B cell proliferation; displays potent anti-proliferative and pro-apoptotic properties against B cell lymphoma lines; and enhances CD40 ligand binding to CD40 [37,38].
SEA-CD40 is a non-fucosylated humanized IgG1 anti-CD40 antibody derived from dacetuzumab (SGN-40) with a Kd of 1×10−9M. SEA-CD40 shows improved agonist activity due to enhanced binding to FcγRIIIa [39,40].
ChiLob 7/4 is a chimeric IgG1 antibody with a Kd of 2×10−10M [41] that requires cross-linking for CD40 stimulation in antigen-presenting cells [42].
Lucatumumab (HCD122) is a fully humanized IgG1 anti-CD40 antagonist that blocks CD40 ligand engagement with CD40. Lucatumumab has a Kd of 5×10−10M and does not display agonistic activity but induces tumor cell death via antibody-dependent cell-mediated cytotoxicity and opsonization [43].
2.3. Toxicity
The use of CD40 agonists in patients with cancer has been associated with several toxicities. For example, treatment with CP-870,893 produces a cytokine release syndrome (CRS) in the majority of patients that is characterized by fever, rigors, and chills. This CRS occurs within minutes to hours after treatment and was the dose-limiting toxicity for CP-870,893 in its early phase dose-finding study [23]. Evidence of hepatotoxicity is also commonly observed with CD40 agonists. Transient elevations in transaminases can be detected within 24 hours of treatment and persist for several weeks before resolution. In preclinical models, this hepatotoxicity has been found to be dependent on CD40-expressing hematopoietic cells, specifically CD11b+ Gr-1+ myeloid cells [24]. In addition, the liver injury induced by an agonistic CD40 antibody is dependent, at least in part, on NADPH oxidase 2 and reactive oxygen species [24]. Preclinical models have suggested that the degree of toxicity associated with systemic immune activation may also be influenced by age. For example, when a CD40 agonist is combined with IL-2 immunotherapy, lethal hepatotoxicity is observed with increasing age [44]. This mortality and the associated pathology seen in the liver, as well as lung and gut, were associated with macrophage-dependent induction of proinflammatory cytokines including IL-6, TNF-α, and IFN-γ. In particular, TNF-α was a major mediator of liver injury and mortality produced with anti-CD40/IL-2 treatment [44]. This finding illustrates the potential role of aging in defining toxicity to CD40 immunotherapy.
Thromboembolic events have also been seen with CP-870,893 in several patients, but the relationship of these events to anti-CD40 treatment has been confounded by the increased risk of this adverse event with cancer burden. Autoimmune events, including dermatitis, colitis, hypophysitis and thyroiditis, have not been seen with CD40 antibodies. However, additional toxicities reported with CD40 antibodies do include infusion reactions, noninfectious inflammatory eye disorders (seen specifically with dacetuzumab [45]), anemia, thrombocytopenia, and pleural effusion [23,45–48].
Strategies to ameliorate toxicity associated with a CD40 agonist have been investigated by several groups. One approach involves peritumoral injection. In an immunogenic model of bladder cancer, local peritumoral injection of an agonistic CD40 antibody was found to effectively elicit a tumor-specific T cell response at reduced doses compared to intravenous injection [49]. In addition, biodistribution of CD40 antibodies to the liver was decreased with local compared to systemic injection [49]. Slow-release delivery has also been studied as a strategy to induce immune activation without systemic toxicity. For example, administration of an agonistic CD40 antibody locally in mineral oil Montanide ISA 51 can induce local DC activation leading to tumor-specific T cell immune responses with decreased systemic toxicity compared to systemic administration of an anti-CD40 antibody [50]. However, tumor-specific T cell responses induced with this local injection approach were restricted to antigens presented in the tumor-draining area [50]. Finally, administration of TNF blocking antibodies in combination with anti-CD40/IL-2 immunotherapy has also been shown to lessen treatment-induced hepatotoxicity observed in aged mice while maintaining T cell-dependent anti-tumor activity [44].
In addition to toxicity, CD40 agonists may also induce undesired biology. For example, triggering of CD40 on endothelial cells has been shown to stimulate the angiogenic process and to promote tumor growth [44], thus identifying a potentially undesired site of CD40 activation. CD40 activation has also been implicated in the transformation of primary B cells [51] as well as lymphomagenesis [52]. However, evidence in patients treated with CD40 agonists for these potential pro-tumorigenic events has not been reported to date. Finally, administering chemotherapy within 48 hours after a CD40 agonist can produce lethal toxicity in mouse models of cancer [26]. This toxicity, though, can be avoided by delaying administration of chemotherapy to five days after a CD40 agonist [26].
3. Preclinical modeling of CD40 agonists
Over the past two decades, CD40 agonists have been investigated pre-clinically with studies revealing several approaches for their incorporation into cancer immunotherapy. Early work demonstrated the potential of a CD40 agonist to “license” antigen-presenting cells with the capacity to stimulate potent anti-tumor T cell immunity in several mouse models of cancer [17–19]. Subsequent studies then revealed that toll-like receptor (TLR) agonists could significantly improve the T cell stimulatory capacity of CD40 antibodies. For TLR3, 4, 7, and 9, this effect was dependent on type I IFN signaling [53]. It was then shown that co-administration of a CD40 antibody with a TLR7 agonist and peptide antigen could induce a marked expansion of antigen-specific CD8+ T cells with enhanced cytolytic activity capable of delaying the progression of melanoma in a lung metastasis model [54]. TLR7 agonism was also found to reverse hepatotoxicity seen with anti-CD40 treatment [54].
The ability to enhance T cell priming versus boosting memory T cell responses with CD40 stimuli can be influenced by TLR agonists. For example, combining CD40 antibodies with a TLR3 (Poly I:C) or TLR9 (CpG) agonist significantly enhances the priming effect of CD40 stimuli in combination with a peptide vaccine [55]. However, only the TLR3 agonist with peptide and anti-CD40 effectively boosted tumor-specific CD8 T cell immunity which occurred independently of CD4+ T cells [55,56]. In nonhuman primates, the combination of a human anti-CD40 antibody and a TLR3 agonist (Poly IC:LC) with a peptide antigen also produced potent antigen-specific T cell activation particularly within the lung [57].
The mechanism by which a TLR agonist synergizes with anti-CD40 is due at least in part to the induction of CD70 on DCs which is critical for CD8 T cell priming [58,59]. This induction of CD70 on DCs can also be achieved by combining type I interferons or natural killer (NK) T cell ligands, including α-galactosylceramide (αGalCer) or αC-GalCer, with a CD40 agonist [60]. Together, these findings illustrate the potential of combining CD40 stimuli with TLR agonists, type I interferons, or NKT ligands to elicit potent antigen-specific anti-tumor T cell immunity.
CD40 agonists have also been combined with IL-2 immunotherapy as a strategy to modulate immunosuppression within the tumor microenvironment and to induce tumor-specific T cell immunity. In an orthotopic mouse model of metastatic renal cell carcinoma, this combinatorial treatment approach induced complete regression of metastatic tumors and potent T cell dependent immunity [61]. This anti-tumor activity was associated with increased infiltration of CD8 T cells and NK cells with a concomitant IFN-gamma and Fas-dependent reduction of CD4+ Foxp3+ regulatory T cells as well as suppressive myeloid cells within the tumor microenvironment [62,63]. In addition to this effect on T cells and immunoregulatory cell populations, IL-2/anti-CD40 immunotherapy can stimulate macrophages to inhibit lung metastasis. This effect requires macrophage-dependent nitric oxide synthase expression and subsequent production of nitric oxide to downregulate within primary tumors the expression of matrix metalloproteinases (MMP) known to be key mediators of the metastatic process, specifically MMP-2 and MMP-9 [5]. Thus, combining CD40 stimuli with IL-2 immunotherapy can promote enhanced innate and adaptive immunosurveillance in cancer and in doing so, impact both primary and metastatic disease.
CD40 agonists might also be used to improve the efficacy of adoptive T cell therapies. In a mouse model of melanoma, the combination of IL-2/anti-CD40 with adoptive cell transfer (ACT) of tumor-specific activated T cells recognizing gp100 produced strong anti-tumor activity that was more effective than either ACT of activated T cells or IL-2/anti-CD40 alone [64]. This effect was associated with enhanced in vivo expansion of adoptively transferred CD8+ T cells and dependent on CD40 expression by bone marrow derived cells, IL-12 production, and CD80/86 expression but not CD4 T cells, B cells or CD11c+ cells. In addition, the expansion of adoptively transferred T cells was not dependent on antigen cross-presentation. This finding is consistent with the potential of a CD40 agonist to induce an antigen-nonspecific expansion of memory CD8+ T cells [65]. Thus, this data supports a potential role for CD40 agonists in conditioning the host for enhancing the activity of adoptively transferred activated T cells.
4. Clinical application of CD40 agonists to cancer therapy
The use of CD40 agonists for inducing anti-tumor immune responses has primarily focused on strategies that ignite T cell-dependent anti-tumor immunity as described above. However, CD40 agonists have also been found to directly induce malignant cell apoptosis and to modulate innate immune surveillance with significant therapeutic implications. Thus, three major approaches for using CD40 agonists in cancer have emerged: (i) direct anti-tumor activity, (ii) induction of T cell immunity, and (iii) activation of innate immunosurveillance). Here, we discuss the clinical status of each of these strategies and the preclinical data that supports the rationale of ongoing investigations.
4.1. Direct anti-tumor activity
CD40 is expressed on most B cell malignancies, including multiple myeloma, non-Hodgkin’s lymphoma, and chronic lymphocytic leukemia [66,67]. In addition, CD40 has been detected on many solid malignancies, including melanoma, ovarian, bladder, breast, and cervical cancer among others [6,68]. Thus, CD40 expression on malignant cells makes it a potential therapeutic target.
Although CD40 activation has been implicated in lymphomagenesis and the transformation of primary B cells [51,52], CD40 signaling in malignant cells, including many B cell lymphomas and solid malignancies, can produce growth arrest and apoptosis [6]. This effect has been seen with both CD40 ligand [69] and anti-CD40 antibodies [70], and is due at least in part to upregulation of pro-apoptotic proteins such as Bcl-2-associated X protein (BAX) [71].
The potential of CD40 to serve as a therapeutic target has been seen in B cell lymphomas. For example, lucatumumab, a CD40 antagonistic monoclonal antibody, produced regressions in a subset of patients with advanced Hodgkin lymphoma and non-Hodgkin lymphoma [72]. Similarly, in high grade B cell lymphoma, dacetuzumab (SGN-40), which is a weak anti-CD40 agonist, showed potent anti-proliferative and pro-apoptotic activity against malignant cells in vitro and in vivo [70]. This direct cytotoxic effect of dacetuzumab may be due at least in part to antibody-dependent cellular phagocytosis mediated by macrophages [73]. In patients with refractory or recurrent B-cell lymphoma, dacetuzumab produced a response rate of 12% with one complete response among 50 treated patients [45]. Subsequent studies investigating the sensitivity of non-Hodgkin lymphoma cell lines to CD40 stimulation with dacetuzumab have identified a correlation with intrinsic DNA damage, increased proliferative rate, and expression of BCL6 that predict treatment response [37]. In addition, a pre-existing activation of the CD40 pathway in malignant cells was found to strongly correlate with resistance to the direct cytotoxic effects of CD40 stimulation and was associated with clinical response in patients with diffuse large B cell lymphoma treated with dacetuzumab where tumor shrinkage was seen in 21 of 57 (37%) patients [37]. Fc-engineering to enhance binding of CD40 antibodies to FcγRs may also enhance the direct cytotoxic activity of this class of drugs by improving their ability to stimulate antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis by macrophages [74]. In addition to producing direct anti-tumor activity, ligation of CD40 on malignant cells can induce upregulation of MHC molecules, the secretion of multiple soluble factors (e.g. IL-6, IL-8, TNF-α and GM-CSF), and also enhance malignant cell susceptibility to T cell lysis, as seen in melanoma [75]. Thus, CD40 expression on malignant cells is a promising therapeutic target.
4.2. Activating adaptive immunity
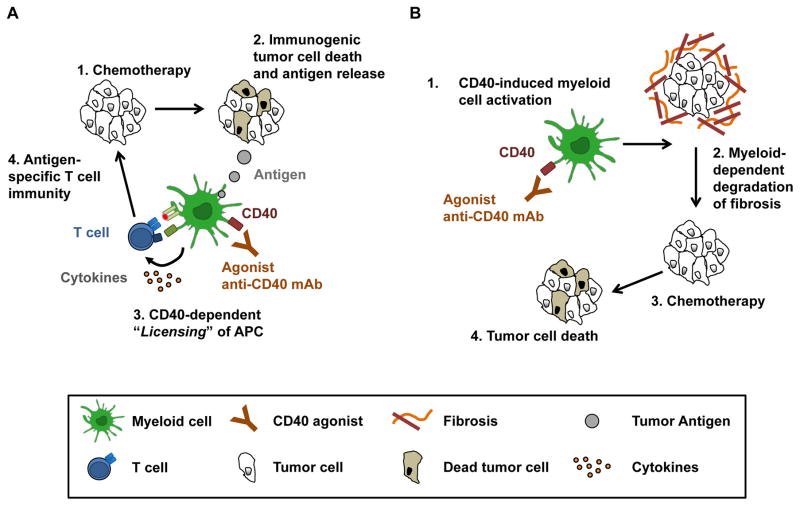
Studies investigating CD40 agonists were initially conducted in immunogenic tumor models and showed that a CD40 agonist could, even by itself, invoke potent T cell dependent anti-tumor immunity leading to cures [17]. However, a critical component to the success of this approach was the presence of tumor antigen, which is necessary for CD40-activated APCs to induce antigen-specific T cell immunity in vivo [18,19]. This biology underlies the hypothesis for ongoing investigations that are combining CD40 agonists with vaccines and chemotherapy. For example, because chemotherapy can elicit an immunogenic form of tumor cell death [76], chemotherapy may induce the release of tumor antigens that would then be phagocytosed by APCs (Figure 2A). With subsequent ligation of the CD40 molecule on APCs, tumor antigenic peptides would then be presented in the context of MHC molecules for antigen-specific T cell stimulation. Thus, this hypothesis, which is supported by preclinical models [77,78], suggests that the sequence of combining CD40 agonists with chemotherapy for activating T cell immunity is critical such that chemotherapy should be administered prior to a CD40 agonist.
Figure 2. Strategies for applying CD40 agonists in cancer therapy.
A. Stimulating T cell immunity. Treatment with chemotherapy (step 1) induces an immunogenic form of tumor cell death with the release of tumor cell antigens (step 2) that are phagocytosed by APCs, such as macrophages and dendritic cells. APCs are then “licensed” by a CD40 agonist (step 3) to present tumor antigen and stimulate tumor specific T cells (step 4). B. Activating innate immunity. Treatment with a CD40 agonist stimulates tumor-infiltrating myeloid cells (step 1) to induce degradation of tumor-associated fibrosis (step 2). Loss of fibrosis renders the tumor more susceptible to chemotherapy (step 3) resulting in tumor cell death (step 4).
The use of chemotherapy to enhance the T cell-stimulatory capacity of a CD40 agonist has been investigated in three clinical studies (Table 2). In the first study, CP-870,893 was combined with paclitaxel and carboplatin and produced a best overall response rate of 20% across patients with multiple solid tumor malignancies [79]. Patients received chemotherapy on day 0 with CP-870,893 administered on day 2 or 7. However, the timing of CP-870,893 administration after chemotherapy did not appear to impact the response rate. Amongst metastatic melanoma patients, the response rate was 12% with 3 of 28 patients achieving a partial response (PR). This finding, though, is very similar to the best overall response reported for CP-870,893 alone in patients with metastatic melanoma where 27% (4 of 15) of patients achieved a PR [23]. In the second study, CP-870,893 was combined with cisplatin and pemetrexed in patients with malignant pleural mesothelioma [46]. Here, CP-870,893 was administered 7 days after chemotherapy. In this study, treatment was well tolerated, but the objective response rate of 40% (6 of 15 patients) was similar to that expected for chemotherapy alone [80]. In the third study, CP-870,893 was combined with weekly dosing of gemcitabine in patients with chemotherapy naïve advanced pancreatic ductal adenocarcinoma [25,48]. CP-870,893 was administered 2 days after the first dose of chemotherapy during each cycle. This treatment strategy produced a response rate of 24% (5 of 21 patients) which was higher than expected for gemcitabine alone which achieves a historical tumor response rate of 5–10% [81–83]. However, evidence for T cell-dependent anti-tumor activity was not observed. Rather, preclinical findings supported a role for CD40 stimulation in invoking productive innate immunity with macrophages mediating anti-tumor activity [25].
Table 2.
History of CD40 agonist clinical trials
| Drugs | Phase | # of patients | Tumor type | Efficacy | ClinicalTrials.gov Identifier | References |
|---|---|---|---|---|---|---|
| CP-870,893 (single dose) | 1 | 29 | Advanced solid tumors | BORR: 14% | NCT02225002 | [15,42] |
| CP-870,893 (weekly dosing) | 1 | 27 | Advanced solid tumors | BORR: 0% | [43] | |
| CP-870,893 + Carboplatin/Paclitaxel | 1 | 30 | Advanced solid tumors | BORR: 20% | NCT00607048 | [26] |
| CP-870,893 + Cisplatin/Pemetrexed | 1 | 15 | Mesothelioma | BORR: 40% | [27] | |
| CP-870,893 + Gemcitabine | 1 | 21 | Pancreas cancer | BORR: 24% | NCT00711191 | [17,29] |
| CP-870,893 + Tremelimumab | 1 | 24 | Melanoma | BORR: 27.3% | NCT01103635 | [34] |
| CP-870,893 + poly IC:LC + peptide vaccine | 1 | Melanoma | Not reported | NCT01008527 | ||
| ChiLob 7/4 (weekly dosing) | 1 | 21 | Advanced solid tumors | BORR: 0% | NCT01561911 | [44] |
The lack of benefit seen clinically with delivering chemotherapy prior to a CD40 agonist may reflect the presence of additional immune suppressive mechanisms orchestrated by developing tumors. Consistent with this idea, in a mouse model of spontaneous pancreatic carcinoma, macrophages residing outside of the tumor microenvironment have been shown to regulate the potential of gemcitabine and a CD40 agonist to elicit productive T cell anti-tumor immunity [78]. This finding implies a role for additional mechanisms beyond CD40 that are critical for T cell priming and infiltration into poorly immunogenic cancers. The mechanism of immune suppression mediated by this population of macrophages, though, is currently unknown.
To unleash the therapeutic potential of a CD40 agonist, combination studies are now being explored with more potent chemotherapeutic regimens under the premise that this will improve antigen release, shift the innate immune reaction to cancer from immunosuppressive to immunostimulatory, or both [84]. An ongoing neoadjuvant clinical study combining CP-870,893 with gemcitabine and nab-paclitaxel in patients with surgically resectable pancreatic carcinoma may provide insight into this prospect of using chemotherapy in combination with a CD40 agonist to induce T cell dependent anti-tumor immunity in a poorly immunogenic tumor (NC02588443). In addition, CD40 agonists are being combined with inhibitors of immune checkpoint molecules such as CTLA-4 (NCT01103635) and PD-1/PD-L1 (NCT02304393, NCT2706353) blocking antibodies. A phase I study investigating CP-870,893 in combination with tremelimumab, a fully human IgG2 mAb targeting CTLA-4, showed clinical activity with a response rate of 27.3% in patients with metastatic melanoma [85]. Further studies, though, are required to discern whether this activity is superior to that previously seen with single agent treatment of CP-870,893 (RR 27%) [23] or tremelimumab (11%) [86] in patients with metastatic melanoma. Certainly, in immunogenic models of cancer, blockade of CTLA-4 and PD-1/PD-L1 has enhanced the therapeutic potential of a CD40 agonist [87]. However, in poorly immunogenic spontaneously arising tumors, thus far this strategy has been met with marked resistance [88]. Nonetheless, the prospect of using a CD40 agonist to stimulate T cell dependent anti-tumor immunity will likely require additional interventions (e.g. TLR agonists or cytokine-based immunotherapy as discussed above) that are aimed at activating key immune pathways and disrupting critical inhibitory pathways that together regulate the T cell stimulatory capacity of a CD40 agonist.
4.3. Activating innate immunity
The development of CD40 agonists for cancer immunotherapy has almost exclusively focused on the potential of CD40 to bridge innate and adaptive immunity for induction of productive anti-tumor T cell immune responses. However, CD40 agonists have also been found to redirect tumor-infiltrating myeloid cells from pro- to anti-tumor [25,89]. This biology has been seen in several murine models of cancer including spontaneous pancreatic carcinoma, neuroblastoma, and melanoma where treatment with a CD40 agonist activates macrophages with tumoricidal activity [25,26,89]. In a model of pancreatic carcinoma, macrophage activation resulted in rapid degradation of collagen-based cancer fibrosis seen as early as 18 hours after treatment [25,26]. This anti-fibrotic effect was dependent on a subset of CCR2+ monocytes that were recruited to tumors via the chemokine CCL2. Tumor-infiltrating monocytes were activated by IFN-γ released systemically in response to CD40 activation and were necessary to shift the profile of matrix metalloproteinases within tumors in favor of fibrosis degradation (Figure 2B). The implications of this anti-fibrotic activity, though, were not initially appreciated as by itself, treatment with a CD40 agonist produced macrophage-dependent tumor regressions albeit transiently [25].
The capacity of a CD40 agonist to modulate fibrosis, which can act as a diffusional barrier to drug delivery [90,91], suggested its potential to also enhance the activity of cytotoxics. To this end, CD40 agonists have been found to condition tumors for enhanced sensitivity to chemotherapy [26]. This finding may explain the anti-tumor activity seen in a Phase I study of patients with advanced pancreatic carcinoma treated with gemcitabine chemotherapy and a CD40 agonist [25,48]. In this study, patients received a first dose of gemcitabine at 48 hours prior to a CD40 agonist, but then also received two additional infusions of gemcitabine at 5 and 12 days after CD40 treatment. The dose of gemcitabine delivered prior to CD40 treatment was intended to facilitate release of tumor antigen for activation of antigen-specific T cells. However, biopsies of regressing tumors showed no evidence of T cell infiltration to explain tumor responses, which were ultimately concluded to be related to macrophage-dependent anti-tumor activity based on preclinical modeling [25]. In retrospect, this initial dose of gemcitabine prior to a CD40 agonist may have been detrimental due to the myelosuppressive effects of gemcitabine [81,83], which could have limited the full potential of macrophage-dependent anti-tumor activity. In contrast, the timing of gemcitabine administration at 5 days after a CD40 agonist has now been shown to produce enhanced cytotoxic activity in preclinical models [26]. This timing has also been found to be safe in both patients and mice, although delivery of chemotherapy within 48–72 hours after a CD40 agonist is associated with severe hepatotoxicity [26,92]. While the precise mechanism of this toxicity is unclear, proper timing of chemotherapy after a CD40 agonist avoids this adverse event while maintaining therapeutic efficacy [26]. Thus, CD40 agonists may offer a novel strategy for improving outcomes with standard of care cytotoxic chemotherapy.
The finding that CD40 agonists could induce productive immunosurveillance independent of T cells was unexpected, but demonstrates the inherent plasticity of the innate immune response to cancer and its potential to be harnessed for therapy. A major difference, though, between macrophage- and T cell-dependent immunity is in the ability to produce memory responses. Whereas T cells can provide long-lived immunosurveillance, macrophages are more likely to be beneficial for tumor debulking. Nonetheless, strong preclinical rationale support further clinical investigation of CD40 agonists for enhancing tumor sensitivity to cytotoxic therapies and invoking macrophage-dependent anti-tumor immunity.
5. Concluding Remarks
The CD40 pathway is involved in several facets of immune regulation. Expressed on multiple cell types of hematopoietic and non-hematopoietic origin, CD40 is a critical regulator of anti-tumor immunity due to its ability to “license” APCs for priming of antigen-specific T cells and its capacity to redirect tumor-infiltrating myeloid cells with anti-tumor and anti-fibrotic activity. Agonists capable of activating the CD40 pathway have been developed and critical elements to their design have been elucidated – in particular the role of crosslinking for unleashing the potency of a CD40 agonist in vivo. Based on this knowledge, several CD40 agonists engineered for enhanced FcR binding have now entered the clinic and are being evaluated. Yet, challenges remain in how to develop CD40 agonists for cancer immunotherapy. In hematological malignancies, CD40 antibodies have been used to induce direct cytotoxic activity and to elicit mechanisms of antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis. In contrast, in solid malignancies the primary focus has been to use CD40 agonists to provoke T cell dependent anti-tumor immunity. While this strategy has produced some efficacy, particularly in patients with metastatic melanoma, many patients do not respond. Preclinical data suggest that this may be due, at least in part, to a subset of macrophages that regulate the capacity of a CD40 agonist to stimulate tumor-specific T cells and the need for additional immune stimuli (e.g. TLR agonists or cytokines including IL-2 and type I interferons) to effectively prime and boost tumor-specific T cell immunity. In contrast, CD40 agonists have recently been shown to also condition tumors for enhanced sensitivity to chemotherapy. This finding may explain encouraging results seen when chemotherapy was combined with a CD40 agonist in patients with pancreatic carcinoma and deserves further clinical investigation to determine the potential of this strategy. Together, CD40 remains a promising target for cancer immunotherapy that has demonstrated some activity in patients. Building on this experience with rational drug combinations and proper sequencing with cytotoxic chemotherapy will be critical for its ultimate incorporation into the immunotherapy armamentarium.
6. Expert Commentary
CD40 agonists can be potent stimulants of the immune system. However, as single agent therapy, clinical activity has been limited and thus, it has become clear that effective translation of CD40 agonists into cancer therapy will hinge on its combination with other treatments. This will require not only an increased understanding of what makes a CD40 agonist effective in vivo but also identification of measurable biomarkers to monitor its activity. Preclinical studies have suggested a role for Fc modification for enhancing the potency of an agonist CD40 mAb. Whether this design strategy will improve the activity of a CD40 agonist in patients, though, still remains to be determined.
CD40 agonists can stimulate both innate and adaptive anti-tumor immunity as well as produce direct cytotoxic activity against CD40 expressing malignant cells. While much attention has focused on how to use CD40 agonists to stimulate T cell immunity, preclinical models have shown that CD40 agonists can also be used to condition tumors for enhanced sensitivity to chemotherapy. Biomarkers to monitor this “conditioning” effect have been identified and can be used to understand the potential of this strategy in patients. In contrast, mechanisms that regulate the capacity of CD40 agonists to induce T cell immunity are still being understood. In both patients and preclinical models, T cell stimulation with CD40 agonists has been most successful in immunogenic tumor models. This finding may suggest a role for CD40 agonists in bolstering existing, but weak, T cell immune responses to cancer and thus, supports a development approach for CD40 agonists that focuses on patient selection to identify those who are most likely to benefit from CD40 immunotherapy. Further, preclinical studies have clearly defined that additional stimuli can enhance the potency of a CD40 agonist for priming and boosting antigen-specific T cell immunity. In addition, CD40 agonists have shown potential in preclinical models for improving the efficacy of adoptive T cell therapies. Moving forward, combining CD40 agonists with additional immune agonists (e.g. TLR agonists) designed to enhance the T cell stimulatory capacity of antigen presenting cells and with adoptive T cell therapy deserves clinical investigation. This combinatorial approach to using a CD40 agonist may also be critical to unleashing T cell immune responses against poorly immunogenic tumors marked by T cell exclusion.
7. Five-year view
Continued investigations into the biology that regulates the capacity of CD40 agonists to invoke T cell immune responses and condition tumors for enhanced responsiveness to cytotoxic therapies will help inform the future development of CD40 agonists. Molecular profiling and multiplex immunohistochemistry will aid in the identification of biomarkers of both response and resistance to CD40 therapy which will guide the positioning of this unique class of immune oncology drugs. Over the next several years, safety and clinical activity of a 2nd generation of CD40 agonists will be unveiled and in doing so, will create opportunities for strategically-designed treatment combinations aimed at broadening the potential of immunotherapy. It is expected that CD40 agonists will be evaluated in combination with cytotoxic agents, novel immune checkpoint inhibitors, and other immune agonists. Overall, CD40 agonists are strong immune stimulants but are also only one piece of a complex puzzle necessary to restore productive innate and adaptive immune surveillance in cancer.
8. Key Issues
The CD40 molecule is a member of the TNF receptor superfamily and is expressed by multiple cell types including monocytes, macrophages, dendritic cells, B cells, platelets, fibroblasts, endothelial cells, smooth muscle cells and some malignant epithelial cells.
The ligand for CD40, CD40 ligand (CD154), is expressed on a variety of cells including activated CD4 T cells, activated B cells, memory CD8 T cells, activated natural killer cells, granulocytes, endothelial cells, smooth muscle cells, and activated platelets. Activation of the CD40 pathway is a critical step in “licensing” antigen presenting cells with the capacity to effectively present antigen and stimulate antigen-specific T cells.
CD40 targeted therapies include recombinant human CD40 ligand, CD40 ligand gene therapy, and CD40 antibodies.
CD40 monoclonal antibodies can stimulate innate and adaptive anti-tumor immunity as well as mediate direct cytotoxic activity against malignant cells.
In early phase clinical studies, CD40 antibodies have been generally well-tolerated and shown promising anti-tumor activity.
Systemic immune activation elicited by CD40 agonists can produce toxicity that limits dosing. Preclinical models have suggested novel strategies for improving toxicity without impacting efficacy.
Fc-engineering or TLR activation to enhance FcR binding in vivo and chemically cross-linking of CD40 agonists can increase the potency of anti-CD40 antibodies through Fc-dependent and -independent mechanisms, respectively.
In some preclinical models, chemotherapy administered prior to a CD40 agonist enhances the development of T cell dependent anti-tumor immunity, although this treatment combination has not yet produced similar results in patients.
Additional immune stimuli (e.g. TLR agonists, IL-2, and type I IFN) can improve the capacity of CD40 agonists to prime and boost antigen-specific T cell immune responses.
CD40 agonists elicit a systemic immune reaction that can induce tumor-infiltrating myeloid cells with anti-tumor and anti-fibrotic properties.
CD40 agonists can be used to condition tumors for enhanced chemotherapy efficacy supporting a role for CD40 agonists delivered prior to chemotherapy.
Several clinical grade CD40 agonists are being evaluated in early phase clinical trials.
Acknowledgments
Funding
This work was supported by a National Institutes of Health grant K08 CA138907 (GL Beatty), by a National Institutes of Health grant R01 CA197916 (GL Beatty), by grant 2013107 from the Doris Duke Charitable Foundation (GL Beatty), and by IRACDA fellowship grant K12GM081295 (KB Long).
Footnotes
Declaration of Interest
GL Beatty has received research funding from Novartis, Incyte, Halozyme and Biothera. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
* Article of interest
** Article of considerable interest
- 1.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posner MR, Cavacini LA, Upton MP, Tillman KC, Gornstein ER, Norris CM., Jr Surface membrane-expressed CD40 is present on tumor cells from squamous cell cancer of the head and neck in vitro and in vivo and regulates cell growth in tumor cell lines. Clin Cancer Res. 1999;5(8):2261–2270. [PubMed] [Google Scholar]
- 5.Weiss JM, Gregory Alvord W, Quinones OA, Stauffer JK, Wiltrout RH. CD40 expression in renal cell carcinoma is associated with tumor apoptosis, CD8(+) T cell frequency and patient survival. Hum Immunol. 2014;75(7):614–620. doi: 10.1016/j.humimm.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. The Journal of experimental medicine. 1996;183(1):159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. This paper demonstrates a key role for CD40 signaling in antigen presenting cells for priming of CD8+ cytolytic T cell responses. [DOI] [PubMed] [Google Scholar]
- **8.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. This paper showed that signaling through CD40 on antigen presenting cells was critical for providing T cell help in the stimulation of antigen-specific cytotoxic T cells. [DOI] [PubMed] [Google Scholar]
- **9.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. This study revealed that dendritic cells activated via CD40 signaling or viral infection could stimulate the generation of cytolytic T cells without the need for CD4 T cell help. [DOI] [PubMed] [Google Scholar]
- 10.Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS, Lipsky PE. The CD40 ligand expressed by human B cells costimulates B cell responses. J Immunol. 1995;154(10):4996–5010. [PubMed] [Google Scholar]
- 11.Frentsch M, Stark R, Matzmohr N, et al. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013;122(3):405–412. doi: 10.1182/blood-2013-02-483586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40-CD40 ligand interaction. The Journal of experimental medicine. 1997;185(12):2053–2060. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365(6444):340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 14.Mach F, Schonbeck U, Sukhova GK, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(5):1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 16.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393(6684):413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- **17.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5(5):548–553. doi: 10.1038/8426. This paper demonstrated that CD40 agonists could be used to overcome T cell tolerance against tumor-derived antigens. [DOI] [PubMed] [Google Scholar]
- **18.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5(7):780–787. doi: 10.1038/10503. This study demonstrated that an agonist CD40 mAb could induce T cell dependent eradication of large established lymphomas in mice without the need for CD4 T cell help. [DOI] [PubMed] [Google Scholar]
- **19.Diehl L, den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5(7):774–779. doi: 10.1038/10495. This paper revealed a role for CD40-CD40 ligand interactions in defining T cell tolerance versus priming to tumor antigens. [DOI] [PubMed] [Google Scholar]
- 20.Vonderheide RH, Dutcher JP, Anderson JE, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19(13):3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 21.Malmstrom PU, Loskog AS, Lindqvist CA, et al. AdCD40L immunogene therapy for bladder carcinoma--the first phase I/IIa trial. Clin Cancer Res. 2010;16(12):3279–3287. doi: 10.1158/1078-0432.CCR-10-0385. [DOI] [PubMed] [Google Scholar]
- 22.Loskog A, Maleka A, Mangsbo S, et al. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. British journal of cancer. 2016;114(8):872–880. doi: 10.1038/bjc.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. The first clinical study of an agonistic CD40 antibody conducted in patients with advanced solid malignancies showed safety and promising clinical activity. [DOI] [PubMed] [Google Scholar]
- 24.Medina-Echeverz J, Ma C, Duffy AG, et al. Systemic Agonistic Anti-CD40 Treatment of Tumor-Bearing Mice Modulates Hepatic Myeloid-Suppressive Cells and Causes Immune-Mediated Liver Damage. Cancer immunology research. 2015;3(5):557–566. doi: 10.1158/2326-6066.CIR-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **25.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. This paper demonstrated in humans and mice that CD40 agonists could invoke productive immunosuveillance in cancer that was dependent on macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. IFNgamma and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016;6(4):400–413. doi: 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333(6045):1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White AL, Chan HT, Roghanian A, et al. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187(4):1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 29.White AL, Chan HT, French RR, et al. Conformation of the human immunoglobulin G2 hinge imparts superagonistic properties to immunostimulatory anticancer antibodies. Cancer Cell. 2015;27(1):138–148. doi: 10.1016/j.ccell.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richman LP, Vonderheide RH. Role of crosslinking for agonistic CD40 monoclonal antibodies as immune therapy of cancer. Cancer immunology research. 2014;2(1):19–26. doi: 10.1158/2326-6066.CIR-13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic Activity of Agonistic, Human Anti-CD40 Monoclonal Antibodies Requires Selective FcgammaR Engagement. Cancer Cell. 2016;29(6):820–831. doi: 10.1016/j.ccell.2016.05.001. This paper describes a novel humanized mouse model for evaluating CD40 agonists and demonstrates a role for the Fc region of an agonist CD40 mAb in defining its T cell stimulatory capacity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10(5):328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.White AL, Dou L, Chan HT, et al. Fcgamma receptor dependency of agonistic CD40 antibody in lymphoma therapy can be overcome through antibody multimerization. J Immunol. 2014;193(4):1828–1835. doi: 10.4049/jimmunol.1303204. This paper demonstrates that crosslinking of CD40 antibodies by either activatory or inhibitory FcRs is critical for its in vivo potency and shows that alternative strategies using chemical crosslinking can produce activity that is Fc independent. [DOI] [PubMed] [Google Scholar]
- 34.Bedian V, Gladue RP, Corvalan J, et al. Methods of treating cancer and enhancing immune responses with antibodies that bind CD40. 7338660. US. 2008
- 35.Zhang Y, Yu G, Zhu W. Anti-cd40 antibodies and methods of use. 2014070934. WO. 2014
- 36.Mangsbo SM, Broos S, Fletcher E, et al. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell-dependent tumor immunity. Clin Cancer Res. 2014;21(5):1115–1126. doi: 10.1158/1078-0432.CCR-14-0913. [DOI] [PubMed] [Google Scholar]
- **37.Burington B, Yue P, Shi X, et al. CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma. Sci Transl Med. 2011;3(74):74ra22. doi: 10.1126/scitranslmed.3001620. This paper demonstrates that pre-existing activation of the CD40 pathway and DNA damage are critical determinants of the direct cytotoxic effects of CD40 antibodies in B cell lymphomas. [DOI] [PubMed] [Google Scholar]
- 38.Francisco JA, Donaldson KL, Chace D, Siegall CB, Wahl AF. Agonistic properties and in vivo antitumor activity of the anti-CD40 antibody SGN-14. Cancer research. 2000;60(12):3225–3231. [PubMed] [Google Scholar]
- 39.Gardai SJ, Epp A, Linares G, et al. Abstract 2472: SEA-CD40, a sugar engineered non-fucosylated anti-CD40 antibody with improved immune activating capabilities. Cancer Res. 2015;75(15 Suppl) Abstract 2472. [Google Scholar]
- 40.Gardai SJ, Epp A, Linares G, et al. A sugar engineered non-fucosylated anti-CD40 antibody, SEA-CD40, with enhanced immune stimulatory activity alone and in combination with immune checkpoint inhibitors. J Clin Oncol. 2015;33(suppl) Abstract 3074. [Google Scholar]
- 41.Johnson PW, Steven NM, Chowdhury F, et al. A Cancer Research UK phase I study evaluating safety, tolerability, and biological effects of chimeric anti-CD40 monoclonal antibody (MAb), Chi Lob 7/4. J Clin Oncol. 2010;28(15s) Abstract 2507. [Google Scholar]
- 42.Chowdhury F, Johnson PW, Glennie MJ, Williams AP. Ex vivo assays of dendritic cell activation and cytokine profiles as predictors of in vivo effects in an anti-human CD40 monoclonal antibody ChiLob 7/4 phase I trial. Cancer immunology research. 2013;2(3):229–240. doi: 10.1158/2326-6066.CIR-13-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byrd JC, Kipps TJ, Flinn IW, et al. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(11):2136–2142. doi: 10.3109/10428194.2012.681655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchlaka MN, Sckisel GD, Chen M, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. The Journal of experimental medicine. 2013;210(11):2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Advani R, Forero-Torres A, Furman RR, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(26):4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 46.Nowak AK, Cook AM, McDonnell AM, et al. A phase 1b clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann Oncol. 2015;26(12):2483–2490. doi: 10.1093/annonc/mdv387. [DOI] [PubMed] [Google Scholar]
- 47.Johnson P, Challis R, Chowdhury F, et al. Clinical and biological effects of an agonist anti-CD40 antibody: a Cancer Research UK phase I study. Clin Cancer Res. 2015;21(6):1321–1328. doi: 10.1158/1078-0432.CCR-14-2355. [DOI] [PubMed] [Google Scholar]
- 48.Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19(22):6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandin LC, Orlova A, Gustafsson E, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer immunology research. 2014;2(1):80–90. doi: 10.1158/2326-6066.CIR-13-0067. [DOI] [PubMed] [Google Scholar]
- 50.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17(8):2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 51.Kusam S, Munugalavadla V, Sawant D, Dent A. BCL6 cooperates with CD40 stimulation and loss of p53 function to rapidly transform primary B cells. International journal of cancer. 2009;125(4):977–981. doi: 10.1002/ijc.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homig-Holzel C, Hojer C, Rastelli J, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. The Journal of experimental medicine. 2008;205(6):1317–1329. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahonen CL, Doxsee CL, McGurran SM, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. The Journal of experimental medicine. 2004;199(6):775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahonen CL, Wasiuk A, Fuse S, et al. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111(6):3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer research. 2008;68(23):9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer research. 2009;69(23):9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson EA, Liang F, Lindgren G, et al. Human Anti-CD40 Antibody and Poly IC:LC Adjuvant Combination Induces Potent T Cell Responses in the Lung of Nonhuman Primates. J Immunol. 2015;195(3):1015–1024. doi: 10.4049/jimmunol.1500078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178(3):1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 59.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174(2):710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 60.McWilliams JA, Sanchez PJ, Haluszczak C, Gapin L, Kedl RM. Multiple innate signaling pathways cooperate with CD40 to induce potent, CD70-dependent cellular immunity. Vaccine. 2010;28(6):1468–1476. doi: 10.1016/j.vaccine.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy WJ, Welniak L, Back T, et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol. 2003;170(5):2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- 62.Weiss JM, Back TC, Scarzello AJ, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(46):19455–19460. doi: 10.1073/pnas.0909474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss JM, Subleski JJ, Back T, et al. Regulatory T cells and myeloid-derived suppressor cells in the tumor microenvironment undergo Fas-dependent cell death during IL-2/alphaCD40 therapy. J Immunol. 2014;192(12):5821–5829. doi: 10.4049/jimmunol.1400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C, Lewis CM, Lou Y, et al. Agonistic antibody to CD40 boosts the antitumor activity of adoptively transferred T cells in vivo. J Immunother. 2012;35(3):276–282. doi: 10.1097/CJI.0b013e31824e7f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tietze JK, Wilkins DE, Sckisel GD, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119(13):3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellat-Deceunynck C, Bataille R, Robillard N, et al. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84(8):2597–2603. [PubMed] [Google Scholar]
- 67.Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85(12):3378–3404. [PubMed] [Google Scholar]
- 68.Young LS, Eliopoulos AG, Gallagher NJ, Dawson CW. CD40 and epithelial cells: across the great divide. Immunology today. 1998;19(11):502–506. doi: 10.1016/s0167-5699(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 69.Elmetwali T, Searle PF, McNeish I, Young LS, Palmer DH. CD40 ligand induced cytotoxicity in carcinoma cells is enhanced by inhibition of metalloproteinase cleavage and delivery via a conditionally-replicating adenovirus. Molecular cancer. 2010;9:52. doi: 10.1186/1476-4598-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Law CL, Gordon KA, Collier J, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer research. 2005;65(18):8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 71.Szocinski JL, Khaled AR, Hixon J, et al. Activation-induced cell death of aggressive histology lymphomas by CD40 stimulation: induction of bax. Blood. 2002;100(1):217–223. doi: 10.1182/blood.v100.1.217. [DOI] [PubMed] [Google Scholar]
- 72.Fanale M, Assouline S, Kuruvilla J, et al. Phase IA/II, multicentre, open-label study of the CD40 antagonistic monoclonal antibody lucatumumab in adult patients with advanced non-Hodgkin or Hodgkin lymphoma. British journal of haematology. 2014;164(2):258–265. doi: 10.1111/bjh.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oflazoglu E, Stone IJ, Brown L, et al. Macrophages and Fc-receptor interactions contribute to the antitumour activities of the anti-CD40 antibody SGN-40. British journal of cancer. 2009;100(1):113–117. doi: 10.1038/sj.bjc.6604812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horton HM, Bernett MJ, Peipp M, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116(16):3004–3012. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- 75.von Leoprechting A, van der Bruggen P, Pahl HL, Aruffo A, Simon JC. Stimulation of CD40 on immunogenic human malignant melanomas augments their cytotoxic T lymphocyte-mediated lysis and induces apoptosis. Cancer research. 1999;59(6):1287–1294. [PubMed] [Google Scholar]
- 76.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 77.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer research. 2003;63(15):4490–4496. [PubMed] [Google Scholar]
- 78.Beatty GL, Winograd R, Evans RA, et al. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology. 2015;149(1):201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vonderheide RH, Burg JM, Mick R, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2(1):e23033. doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 81.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 82.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 83.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byrne KT, Vonderheide RH. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell reports. 2016;15(12):2719–2732. doi: 10.1016/j.celrep.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bajor DL, Mick R, Riese MJ, et al. Abstract CT137: Combination of agonistic CD40 monoclonal antibody CP-870,893 and anti-CTLA-4 antibody tremelimumab in patients with metastatic melanoma. Cancer Res. 2015;75(15 Suppl) Abstract CT137. [Google Scholar]
- 86.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zippelius A, Schreiner J, Herzig P, Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer immunology research. 2015;3(3):236–244. doi: 10.1158/2326-6066.CIR-14-0226. [DOI] [PubMed] [Google Scholar]
- 88.Winograd R, Byrne KT, Evans RA, et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer immunology research. 2015;3(4):399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lum HD, Buhtoiarov IN, Schmidt BE, et al. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006;79(6):1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 90.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Byrne KT, Leisenring NH, Bajor DL, Vonderheide RH. CSF-1R-Dependent Lethal Hepatotoxicity When Agonistic CD40 Antibody Is Given before but Not after Chemotherapy. J Immunol. 2016;197(1):179–187. doi: 10.4049/jimmunol.1600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 95.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]