Abstract
Timely treatment of depression and behavioral dysfunction after moderate-to-severe traumatic brain injury (TBI) could improve health, function, and quality of life. We hypothesized 6-month depression would be the stronger contributor to later depression and behavioral dysfunction in a sample of n=88 adults with moderate-to-severe TBI. A structural equation modeling cross-lagged panel analysis, adjusting for all 6-month predictors, revealed 6-month depression had a stronger relationship to 12-month depression (βstand=.55, p=.002) and behavioral dysfunction (βstand=.41, p=.004) than did 6-month behavior behavioral dysfunction (βstand=.17, p=.270, βstand=.30, p=.035). Depression may be in the developmental pathway to behavioral dysfunction, triggering a cycle of reciprocal causality.
Keywords: brain injuries, behavioral symptoms, depression
Introduction
Over 2 million individuals in the United States sustain a new traumatic brain injury (TBI) each year, and over 5 million live with TBI-related disabilities [1]. The long-term consequences of TBI include physical, cognitive, emotional, and behavioral symptoms that can persist for decades and negatively affect community participation, health, and quality of life {2; 3}. Most notably, depression and behavioral dysfunction, including disinhibition, poor decision-making, and apathy {4; 5}, account for the majority of re-hospitalizations beyond the first year post-injury {6}, result in increased medical costs, and strongly contribute to the high suicide risk after TBI {7; 8}. However, the majority of individuals with TBI are not receiving adequate mental health care {9}. Timely treatment of these behavioral and emotional problems could reduce public healthcare burden and save lives.
Behavioral dysfunction occurs frequently (>50%) and persists after severe TBI {3}; it is one of the greatest contributing factors to poor outcomes (e.g. disability, suicidality, quality of life) in this population {10}. Depression has a prevalence of ~50% in the first year after injury {9}, and there is strong association between depression and behavioral dysfunction after TBI {3}. One study reported that irritability and anger, components of behavioral dysfunction {11}, were present in 54.5% of individuals in the general population with depression {12}. Neuroanatomical evidence for the mechanisms of antidepressant treatment points to shared neuroanatomy between depression and behavioral dysfunction {13; 14}, particularly in the prefrontal and orbitofrontal cortexes,{15} which are associated with aggression{16}, impulsivity{17}, and executive dysfunction {18}. There is also evidence that behavioral dysfunction after TBI is more likely to occur in the context of a psychiatric disorder {19–22}, suggesting that depression may be in the developmental pathway to behavioral dysfunction {23}. Miller (2013), in discussing two recent studies that characterize depression {12; 15}, goes so far as to state that “Irritability, anger, anhedonia, or disruptive behavior may be equally defining [as poor mood] of the illness that we call depression” (pg. 1131){24}.
Clearly, the nature and temporality of the relationship between depression and behavioral dysfunction remain elusive, and no study has examined their temporal relationships after TBI. We have recently developed and published a conceptual model that situates behavioral dysfunction after TBI at the intersection of an individual’s cognitive ability (e.g. executive function), emotional state (e.g. depression), and personal factors (e.g. genetics, coping skills){22}. Based on this model, we would hypothesize that changes in emotional state, like depression, would be among the factors contributing to dysfunctional behaviors after TBI. To test this hypothesis, the purpose of this study was to examine temporal relationships between depression and behavioral dysfunction at 6- and 12-months post-TBI using a cross-lagged panel analysis structural equation model (SEM), which examines the structural relationships of repeatedly measured constructs {25}. We hypothesized that: 1) depression and behavioral dysfunction would be strongly associated throughout the first year post-TBI; and 2) 6-month depression would be the stronger contributor to 12-month depression and behavior.
Methods
Participants
Participants in this secondary analysis (n=88) were recruited as part of two IRB-approved cohort studies under an umbrella protocol at the University of XX and were recruited from both acute care and inpatient rehabilitation centers. Enrollment criteria for the parent studies were: 1) non-penetrating TBI-related ICD-9 diagnosis and/or sufficient medical documentation on day of injury [e.g. admission Glasgow Coma Scale (GCS) ≤ 12, CT scan with evidence of intracranial injury, focal neurologic signs] and 2) 16–79 years old. Exclusion criteria were: 1) evidence of prolonged hypoxia (>30 min) occurring prior to admission, 2) untreated endocrine disorder, 3) autoimmune disorder, 3) history of neurological or neurodegenerative disease, and 4) documented history of previous TBI or stroke. An additional inclusion criterion for the present analysis was to have complete behavioral and depression assessments at both 6- and 12- months post-injury (see Figure 1). Ability to complete self-reported assessments was a prerequisite for completing the depression assessment. There were no demographic or clinical characteristic differences between included and excluded individuals with regard to sex, age, or education.
Figure: 1.
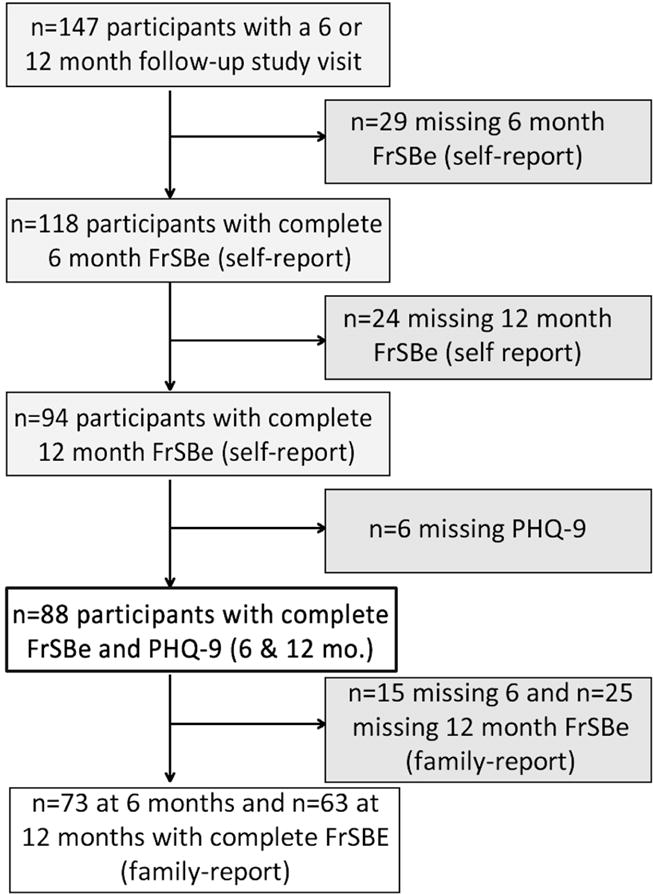
Flow-chart of participants included in the current analysis from a larger population-based study of adults with moderate-to-severe traumatic brain injury
Demographic and clinical characteristics
Demographic (age, sex, education) and clinical characteristics (injury severity, premorbid mental health condition, antidepressant or other psychotropic medication use) were collected through a combination of participant or family member report and medical chart abstraction. Injury severity was measured through the best documented GCS score in the first 24-hours post-injury. Participants (or family members) were asked about any pre-injury history of psychiatric disorder (e.g. depression, anxiety, bipolar disorder). Current medication lists were collected at both 6- and 12-months, from which antidepressants (fluoxetine, citalopram, sertraline, escitalopram, paroxetine, trazodone, duloxetine, venlafaxine, buproprion, mirtazapine, and amitriptyline) and other psychotropic medications (divalproex sodium, aripiprazole, risperidone, and ziprasidone) were extracted.
Depression
The Patient- Health Questionnaire-9 (PHQ9) is a validated self-report assessment of depressive symptoms based on the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. criteria for major depression. The PHQ9 is validated for use after TBI {26} and can reliably discriminate between chronic TBI and depression symptoms {27}. Due to potential differences in the development of depression after TBI (e.g. adjustment to disability {28} vs inflammatory-induced depression{29}), we dichotomized the scale into somatic (sleep, energy, appetite, concentration, and psychomotor slowing/agitation) and mood (little interest/pleasure, feeling down/depressed, feeling bad about self, suicidal ideation) symptoms to serve as indicators for the latent construct of depression in our SEM. For the purposes of descriptive analysis, we categorized participants as depressed or not depressed based on previously established criteria found to be optimal after TBI {26}. Briefly, participants had to endorse at least 5 of the 9 questions on the PHQ9 (≥1), one of which had to be question 1 (depressed mood) or 2 (loss of interest).
Behavioral Dysfunction
The Frontal Systems Behavior Scale (FrSBe) is a validated assessment of behaviors associated with frontal lobe damage {30}. The FrSBe comprises three subscales – apathy, disinhibition, and executive dysfunction – which are assessed via self- or family-report. For the present study, self-reported assessments were used for the primary model, to be consistent with self-reported depressive symptoms. Family-reported assessments were used as a validity check to address potential limitations in self-reporting of behavior. The FrSBe produces norm-based T-scores which are adjusted for age, sex, and education. A higher t-score indicates greater behavioral dysfunction. The subscales served as indicators for the latent construct of behavioral dysfunction in our SEM. The FrSBe has been found to be predictive of community integration {31}, indicating it is a good measure of behavioral dysfunctions likely to impact disability and participation. For descriptive analysis, participants were categorized as having overall behavioral dysfunction when FrSBe total t-scores were >65 per recommendation of scale developers {32}. CITE.
Data Analysis
All statistical analyses were performed using IBM SPSS Statistics and AMOS (version 24). Descriptive analyses were conducted for demographic and clinical data to characterize the sample. A correlation matrix was run to assess relationships between all indicator variables (depression and behavioral dysfunction at 6- and 12-months post-TBI) prior to model creation. A cross-lagged panel SEM was implemented to assess the temporal relationships between depression and behavioral dysfunction at 6- and 12-months post-injury. SEM allows for multiple relationships to be analyzed simultaneously, allowing the user to build more complex statistical models rather than running several linear regressions. The relative strengths of longitudinal relationships can be determined through comparison of standardized betas. Both depression and behavioral dysfunction were modeled as constructs (latent variables), which allowed for simultaneous inclusion of multiple observed measures (indicators) and error. Indicators of the latent variable of behavioral dysfunction include the three subscales of the FrSBe: apathy, disinhibition, and executive dysfunction. Indicators for the latent variable depression include PHQ9 somatic symptoms and mood symptoms. Error terms were included for all indicators and disturbance terms were included for latent variables to correct for external factors and other error that may contribute to observed effects. Based on recommended guidelines, model fit indices were assessed, with values in parentheses indicating good fit: χ2 (p>.05), root-mean-square error of approximation (RMSEA ≤.05), Non-normed Fit Index (NNFI ≥.90; also known as the Tucker Lewis Index), and comparative fit index (CFI ≥.95){33}.
Results
Demographic and clinical characteristics of the 88 participants are summarized in Table 1. On average, participants at both time points reported depressive symptoms on the low end of the mild depression range and behavioral symptoms on the high end of the normal range, though there was a very wide range within the cohort as evidenced by the large standard deviations. These means and standard deviations are similar to those reported in a recent study in a large, multisite national database cohort represented by the TBI Model Systems {34}. Premorbid mental health conditions were reported by 18 (20.5%) participants.
Table 1.
Participant Characteristics
| Mean (SD) n=88 |
|
|---|---|
| Demographics | 70 (79.5%) |
| Sex [male, n (%)] | 13.1 (2.1) |
| Education (years) | 37.0 (15.8) |
| Age (years) | |
| GCS [best in 24 hours, median (IQR)] | 8 (6, 10) |
| Premorbid mental health condition | 18 (20.5%) |
| [Yes, n (%)] | |
| Antidepressant use [Yes, n (%)] | |
| 6-Months post-injury | 34 (38.6%) |
| 12-Months post-injury | 30 (34.1%) |
| Other psychotropic medication [Yes, n (%)] | |
| 6-Months post-injury | 4 (4.5%) |
| 12-Months post-injury | 3 (3.4%) |
| Depression (PHQ9) | |
| 6-Months post-injury | |
| Somatic symptoms | 3.4 (3.9) |
| Mood symptoms | 1.9 (2.6) |
| Total score | 5.3 (5.9) |
| Depression [Yes, n (%)] | 28 (41.8%) |
| 12-Months post-injury | |
| Somatic symptoms | 3.0 (3.5) |
| Mood symptoms | 1.8 (2.5) |
| Total score | 4.8 (5.3) |
| Depression [Yes, n (%)] | 23 (26.1%) |
| Behavioral Dysfunction (FrSBe) | |
| 6-Months post-injury | |
| Apathy | 59.0 (20.4) |
| Disinhibition | 54.9 (17.7) |
| Executive Dysfunction | 59.9 (20.1) |
| Behavioral Dysfunction [Yes, n (%)] | 39 (44.3%) |
| 12-Months post-injury | |
| Apathy | 58.1 (18.8) |
| Disinhibition | 56.4 (19.0) |
| Executive Dysfunction | 61.2 (21.6) |
| Behavioral Dysfunction [Yes, n (%)] | 42 (47.7%) |
| Family-Reported Behavioral Dysfunction (FrSBe Family) | |
| 6-Months post-injury (n=73) | |
| Apathy | 72.7 (23.8) |
| Disinhibition | 57.8 (17.0) |
| Executive Dysfunction | 65.4 (17.4) |
| 12-Months post-injury (n=63) | |
| Apathy | 69.1 (21.8) |
| Disinhibition | 59.1 (17.6) |
| Executive Dysfunction | 64.4 (17.8) |
PHQ9=Patient Health Questionnaire 9;
FrSBe=Frontal Systems Behavior Scale;
GCS=Glasgow Coma Scale; IQR=Interquartile Range
At 6-months, 28 participants (31.8%) were depressed, 39 (44.3%) had behavioral dysfunction, and 21 (23.9%) had both depression and behavioral dysfunction; 34 (38.6%) were on an antidepressant medication and 4 (4.5%) were on another psychotropic medication. Of the 28 participants who were depressed, 16 (57.1%) were on an antidepressant. At 12-months, 23 (26.1%) were depressed, 42 (47.7%) had behavioral dysfunction, and 18 (20.5%) had both; 30 (34.1%) were on an antidepressant medication and 3 (3.4%) were on another psychotropic medication. Of the 23 participants who were depressed, 14(60.9%) were on an antidepressant. Table 2 reports the cross-tabulation for 6 and 12-month depression and/or behavioral dysfunction.
Table 2.
Cross-tabulation of 6 and 12 month depression and/or behavioral dysfunction
| Month 12 | |||||
|---|---|---|---|---|---|
| Month 6 | 1 | 2 | 3 | 4 | Total |
| 1. None | 37 | 3 | 5 | 1 | 46 |
| 2. Depression only | 4 | 1 | 2 | 4 | 11 |
| 3. Behavioral Dysfunction only | 3 | 1 | 7 | 3 | 14 |
| 4. Both | 1 | 1 | 6 | 9 | 17 |
|
| |||||
| Total | 45 | 6 | 20 | 17 | 88 |
The correlation matrix (Table 3) revealed that our indicator variables were correlated within each latent construct (r=.560–.690, p<.01 for depression and r=.468–.769, p<.01 for behavioral dysfunction) which ensures that the selected indicators are each representative of their respective constructs. Unlike the correlations presented later in the SEM model, these correlations do not adjust for any other factors. When evaluating across constructs, somatic symptoms of depression generally had stronger positive associations with behavioral symptoms than did mood symptoms of depression. However, the moderate-to-large correlations overall suggest that no single indicator variable is driving the relationships between latent variables (i.e. depression and behavioral dysfunction) observed in the SEM models.
Table 3.
Correlation matrix of all endogenous variables at the 6 and 12 months post-injury
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Somatic Symptoms 6 Month | .519a | .690a | .263b | .658a | .550a | .461a | .534a | .574a | .555a |
| 2. Somatic Symptoms 12 Month | .402a | .560a | .409a | .531a | .350a | .428a | .440a | .512a | |
| 3. Mood Symptoms 6 Month | .378a | .465a | .353a | .426a | .437a | .437a | .364a | ||
| 4. Mood Symptoms 12 Month | .214b | .359a | .330a | .304a | .252a | .320a | |||
| 5. Apathy 6 Month | .541a | .468a | .315a | .739a | .458a | ||||
| 6. Apathy 12 Month | .333a | .544a | .465a | .757a | |||||
| 7. Disinhibition 6 Month | .659a | .649a | .466a | ||||||
| 8. Disinhibition 12 Month | .477a | .693a | |||||||
| 9. Executive Dysfunction 6 Month | .569a | ||||||||
| 10. Executive Dysfunction 12 Month |
Correlation is significant at the 0.01 level
Correlation is significant at the 0.05 level
All corresponding self and family subscales were significantly correlated (r=0.299–0.582, p<.02), and discrepancies between self and family report, on average, were small (mean=2.88–12.10 points across subscales) and consistent with previous studies examining self-reported versus family-reported discrepancies on the FrSBe {35}.
SEM Model
Figure 2 shows the theorized model with correlations and standardized path loadings (standardized betas). Latent variables loaded onto all of the indicators, as anticipated (p’s < 0.001). Depression and behavioral dysfunction at 6-months and the disturbance (error) terms at 12-months were also significantly correlated (p’s < 0.001). In addition to the a priori theorized model, the modification indices feature of SPSS Amos also prompted us to add a correlation between somatic depression and apathy to improve model fit. Given that there may be effects of fatigue, psychomotor slowing, and poor concentration (somatic symptoms) on one’s ability to initiate and engage in activities (apathy), this correlation is theoretically justified.
Figure: 2.
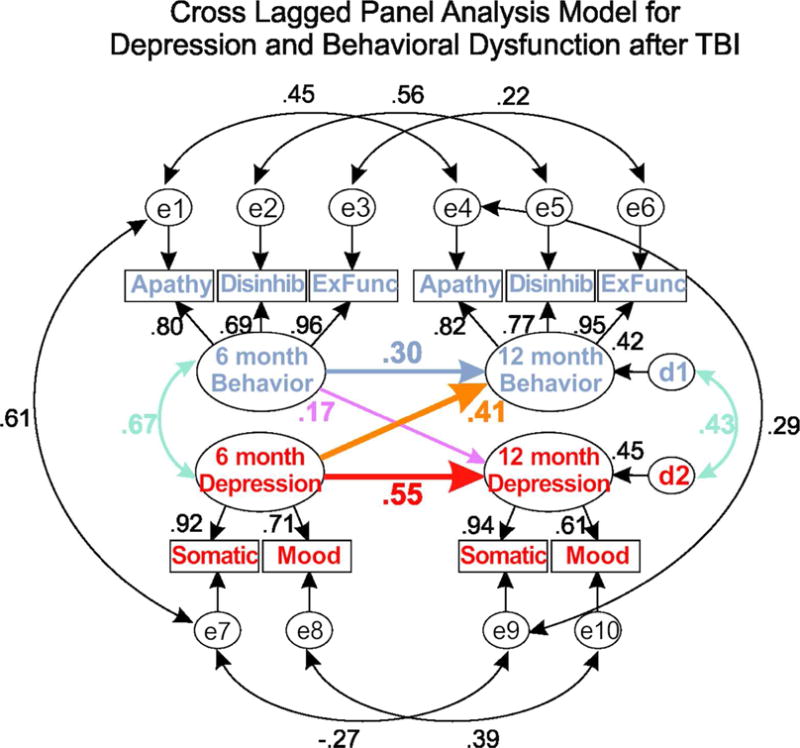
Graphical representation of the cross-lagged panel analysis of depression and behavioral dysfunction in the first year after traumatic brain injury. As per convention, indicators (behavioral dysfunction and depression subscales) are represented as boxes, with latent variables (behavioral dysfunction, depression) presented as ovals. Values along curved lines are correlation coefficients. Straight lines between 6 and 12 month latent variables are standardized betas (ranging from 0-1), and thickness of the lines is indicative of strength of the association. All correlations and standardized betas >.30 are statistically significant (p<.05).
Latent Variable Relationships
Depression at 6-months was a significant predictor of depression (p = 0.002) at 12-months and behavioral dysfunction (p = 0.004) at 12-months post-injury, controlling for 6-month behavioral dysfunction. While 6-month behavioral dysfunction was a significant predictor of behavioral dysfunction at 12-months (p = 0.035), it was not a significant predictor of 12-month depression (p = 0.270), controlling for 6-month depression. The crossed path analysis showed that the strength of the relationship was stronger between 6-month depression and 12-month behavioral dysfunction (betastand=.41) than between 6-month behavioral dysfunction and 12-month depression (betastand=.17). This finding indicates that depression is the strongest contributor to behavioral dysfunction, but there may be some reciprocal causality over time. The total model (6-month depression and 6-month behavioral dysfunction) accounted for 42% of the variance in 12-month behavioral dysfunction and 45% of the variance in 12-month depression.
Indicators
There was a strong correlation in the SEM model between somatic symptoms of depression and behavioral apathy at 6-months (r=.61), which is likely why including this correlation improved the model fit. Correlations between indicators at 6-months and the same indicators at 12-months were generally strong, with the exception of executive function and somatic symptoms, where correlations were not statistically significant. These small and non-statistically significant correlations suggests that executive function and somatic symptoms may change for some participants, but not for others, resulting in a nonlinear relationship from one time point to the next. Betas for all indicators show consistent loading of latent variables over time, which demonstrates reliability of our latent variable constructs.
SEM Fit Indices
Our overall model demonstrated good fit based on fit indices. The χ222 = 26.10 (p = 0.248) and the ratio of the χ2 to the degrees of freedom of 1.19 both indicate a good model fit {36}. The RMSEA was .046, the NNFI was .957, and the CFI was .993, also indicating good fit. When taken together, the fit statistics suggest that even this small sample size (n=88) was sufficient to produce a valid model based on a strong a priori theoretical framework and acceptable reliabilities {33}.
Discussion
A seminal study on the rates of depression in the first year after TBI reported that over 50% of individuals hospitalized for TBI met criteria for depression in the first year after injury, and only 44% of those individuals received either antidepressants or counseling {9}. Notably, most of the new incidence of post-traumatic depression occurred within the first six months {9}. However, little is known about the temporal development of behavioral dysfunction or the temporal relationships between depression and behavioral dysfunction after TBI. Timing of symptom development after injury has implications for clinical management, whether through behavioral or pharmacological intervention. Early screening and effective triage to appropriate intervention or follow-up is needed, to inform resource allocation and provide effective care for those not receiving needed services.
Our primary finding was that 6-month depression had strong effects on both 12-month depression and behavioral dysfunction, suggesting that once an individual has clinically significant depressive symptoms after injury, these symptoms can continue to self-perpetuate and lead to behavioral dysfunction. A recently published study suggests that sertraline may be effective for preventing the development of post-TBI depression {37}, which, based on our findings, may also prevent the development of behavioral dysfunction. When depression is present, studies indicate that dopaminergic antidepressants may be effective for addressing concurrent apathy and cognition, while serotonergic antidepressants may be better for addressing concurrent disinhibition {38; 39}. However, efficacy of antidepressants for improving depression after TBI remains unclear {40}, as in the present study, where there were participants who were depressed while currently taking antidepressants. In addition to questionable efficacy, prescribing of antidepressants after TBI is variable and often occurs for reasons other than depression. Prompt depression screening and treatment during and continuing immediately after rehabilitation discharge is critical and could have downstream effects on the development of chronic depression and behavioral dysfunction after injury.
While depression was the strongest predictor in our model, continual evaluation of behavioral dysfunction is important as well. Only a small percentage (~16%) of those with either depression, behavioral dysfunction, or both at 6-months reported no significant symptoms at 12- months, and of those with both depression and behavioral dysfunction at 6-months, ~53% still had both at 12-months. Given the high prevalence of both post-traumatic depression and behavioral dysfunction, and the low percentage of individuals reported in previous studies to be receiving mental health treatment {9}, it is clear that there is substantial room for improvement in community-based clinical management of individuals with TBI that could substantively improve health, participation, and quality of life and decrease healthcare costs and economic burden of TBI.
This is the first study to examine the temporal relationships between depression and behavioral dysfunction across the first year after TBI. What is unique about TBI – or other acquired neurological conditions, like stroke – is a defined time of onset and specific neurobiological pathology. Based on studies like these and the extremely frequent occurrence of depression after TBI compared to the general population, it is clear that TBI is a clinical population that requires specific study to understand the causal pathways between biology, depression, and behavioral dysfunction.
This study was limited by being a secondary analysis of data from two prospective studies with common data elements and time points and by having only two data points (6 and 12-months post-injury). A prospective study design with more frequent assessment over time would provide even better information as to the best timing and targets for intervention. While the FrSBe is an established measure of behavior associated with frontal lobe dysfunction, it does not capture all domains of behavioral dysfunction after TBI, such as anger, irritability, or aggression. A more comprehensive assessment of behavioral dysfunction would increase the generalizability of these data. A general rule of thumb for SEM analysis would suggest a minimum of 200 subjects; however, these rules can be overly conservative and not generalizable to each study in question{41}. Recent work suggests that studies with a well- developed model, high degrees of freedom, and more liberal desired power estimate (.80), allow for SEM models to be reasonably run with sample sizes of around 140 or fewer participants {42}. Even with our a priori theoretical model and strong model fit indices, our sample size does fall below this threshold; thus replication of these data is warranted. Though the sample size was sufficient for the analyses conducted, a larger sample size would allow for better missing data imputation and more comparisons within the SEM model, such as relationships between the individual indicators across latent constructs, and for the addition of other covariates, such as premorbid mood disorder, antidepressant use, or injury type/location. We attempted a Full Information Maximum Likelihood data imputation method with 148 participants, and while beta values remained consistent, the model fit indices were poor, likely due to the large amount of missing data imputed.
Relying on self-report is necessary for assessing emotional and other internal states. However, there are potential limitations related to impaired self-awareness and recall bias for more objective problems like behavioral dysfunction. Despite these potential limitations, relying on self-report is often necessary in both research and clinical settings, and we have demonstrated that self- and family-reported behavioral dysfunction were correlated in our sample. The ability to provide a self-report of these symptoms was required for this study, which meant that those with the most severe cognitive impairment in the first year after TBI who could not provide self-report were excluded. However, those able to provide a self-report are also likely the best candidates for psychological or behavioral intervention, therefore making the study sample still representative of those whose clinical care would likely be informed by these results.
Future Directions
Ecological momentary assessment (EMA), which involves repeated measures of symptoms in real time in an individual’s natural environment, reduces reporting errors that occur as a result of poor recall or impaired self-awareness after TBI {43}. EMA of depressive and behavioral symptoms could be an effective long-term screening approach and could enable the development of temporal and potentially mechanistic pathways from injury and neurobiological changes to depression and behavioral dysfunction. Future work is needed to determine whether long-term screening improves depression treatment after TBI, whether treating depression leads to improved behavioral dysfunction over time, and whether identified risk factors for behavioral dysfunction in the context of depression could inform personalized treatment approaches.
Acknowledgments
The contents of this manuscript were developed under grants from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR: 90DP0041), the Centers for Disease Control (CDC: R49 CCR 323155), the National Science Foundation (NSF-DGE-0549352), and the Department of Defense (DOD: W81XWH-071-0701). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this manuscript do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the Federal Government.
References
- 1.Centers for Disease Control and Prevention (CDC) CDC Grand Rounds: Reducing Severe Traumatic Brain Injury in the United States. MMWR Morb Mortal Wkly Rep. 2013;62:549–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant RA, O’Donnell ML, Creamer M, et al. The psychiatric sequelae of traumatic injury. Am J Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- 3.Sabaz M, Simpson GK, Walker AJ, et al. Prevalence, comorbidities, and correlates of challenging behavior among community-dwelling adults with severe traumatic brain injury: a multicenter study. J Head Trauma Rehabil. 2014;29:E19–30. doi: 10.1097/HTR.0b013e31828dc590. [DOI] [PubMed] [Google Scholar]
- 4.Kelly G, Brown S, Todd J, et al. Challenging behaviour profiles of people with acquired brain injury living in community settings. Brain Inj. 2008;22:457–470. doi: 10.1080/02699050802060647. [DOI] [PubMed] [Google Scholar]
- 5.Arciniegas DB, Wortzel HS. Emotional and Behavioral Dyscontrol After Traumatic Brain Injury. Psychiatr Clin North Am. 2014;37:31–53. doi: 10.1016/j.psc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Marwitz JH, Cifu DX, Englander J, et al. A multi-center analysis of rehospitalizations five years after brain injury. J Head Trauma Rehabil. 2001;16:307–317. doi: 10.1097/00001199-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Reeves RR, Panguluri RL. Neuropsychiatric complications of traumatic brain injury. J Psychosoc Nurs Ment Health Serv. 2011;49:42–50. doi: 10.3928/02793695-20110201-03. [DOI] [PubMed] [Google Scholar]
- 8.The Epidemiology Impact of Traumatic Brain Injury A Bri&. The Journal of Head Trauma Rehabilitation [Internet] doi: 10.1097/00001199-200609000-00001. LWW [cited 2015 Jan 21] Available from: http://journals.lww.com/headtraumarehab/Fulltext/2006/09000/The_Epidemiology_and_Impact_of_Traumatic_Brain.1.aspx. [DOI] [PubMed]
- 9.Bombardier CH, Fann JR, Temkin NR, et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA J Am Med Assoc. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao V, Lyketsos C. Neuropsychiatric Sequelae of Traumatic Brain Injury. Psychosomatics. 2000;41:95–103. doi: 10.1176/appi.psy.41.2.95. [DOI] [PubMed] [Google Scholar]
- 11.Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Psychiatr Clin North Am. 2010;33:807–819. doi: 10.1016/j.psc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Judd LL, Schettler PJ, Coryell W, et al. Overt irritability/anger in unipolar major depressive episodes: past and current characteristics and implications for long-term course. JAMA Psychiatry. 2013;70:1171–1180. doi: 10.1001/jamapsychiatry.2013.1957. [DOI] [PubMed] [Google Scholar]
- 13.Chan K-L, Campayo A, Moser DJ, et al. Aggressive behavior in patients with stroke: association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil. 2006;87:793–798. doi: 10.1016/j.apmr.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Choi-Kwon S, Han SW, Kwon SU, et al. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness a double-blind, placebo-controlled study. Stroke. 2006;37:156–161. doi: 10.1161/01.STR.0000190892.93663.e2. [DOI] [PubMed] [Google Scholar]
- 15.Heller AS, Johnstone T, Peterson MJ, et al. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70:1181–1189. doi: 10.1001/jamapsychiatry.2013.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miczek KA, Fish EW. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- 17.Economidou D, Theobald DE, Robbins TW, et al. Norepinephrine and Dopamine Modulate Impulsivity on the Five-Choice Serial Reaction Time Task Through Opponent Actions in the Shell and Core Sub-Regions of the Nucleus Accumbens. Neuropsychopharmacology. 2012;37:2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins TW, Arnsten AFT. The Neuropsychopharmacology of Fronto-Executive Function: Monoaminergic Modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James LM, Strom TQ, Leskela J. Risk-Taking Behaviors and Impulsivity Among Veterans With and Without PTSD and Mild TBI. Mil Med. 2014;179:357–363. doi: 10.7205/MILMED-D-13-00241. [DOI] [PubMed] [Google Scholar]
- 20.Wisco BE, Marx BP, Holowka DW, et al. Traumatic Brain Injury, PTSD, and Current Suicidal Ideation Among Iraq and Afghanistan U.S. Veterans. J Trauma Stress. 2014 doi: 10.1002/jts.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myrga JM, Juengst SB, Failla MD, et al. COMT and ANKK1 genetics interact with depression to influence behavior following severe TBI. Neurorehabil. Neural Repair. 2015 doi: 10.1177/1545968316648409. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juengst SB, Switzer G, Oh BM, et al. Conceptual model and cluster analysis of behavioral symptoms in two cohorts of adults with traumatic brain injuries. J Clin Exp Neuropsychol. 2016:1–12. doi: 10.1080/13803395.2016.1240758. [DOI] [PubMed] [Google Scholar]
- 23.Juengst SB, Arenth PM, Wagner AK. Biopsychosocial outcomes in the first year after traumatic brain injury: behavior, depressive symptoms, and self-perception. Arch Phys Med Rehabil. 2015 In Press. [Google Scholar]
- 24.Miller MC. When Depression Doesn’t Lead With Depression. JAMA Psychiatry. 2013;70:1131–1132. doi: 10.1001/jamapsychiatry.2013.3493. [DOI] [PubMed] [Google Scholar]
- 25.Autoregressive and cross-lagged panel analysis for longitudinal data. (PDF Download Available) [Internet][cited 2016 Jul 27] Available from: https://www.researchgate.net/publication/259453465_Autoregressive_and_cross-lagged_panel_analysis_for_longitudinal_data.
- 26.Fann JR, Bombardier CH, Dikmen S, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Cook KF, Bombardier CH, Bamer AM, et al. Do somatic and cognitive symptoms of traumatic brain injury confound depression screening? Arch Phys Med Rehabil. 2011;92:818–823. doi: 10.1016/j.apmr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anson K, Ponsford J. Coping and emotional adjustment following traumatic brain injury. J Head Trauma Rehabil. 2006;21:248–259. doi: 10.1097/00001199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Juengst SB, Kumar RG, Failla MD, et al. Acute Inflammatory Biomarker Profiles Predict Depression Risk Following Moderate to Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho JO, Ready RE, Malloy P, et al. Confirmatory factor analysis of the Frontal Systems Behavior Scale (FrSBe) Assessment. 2013;20:632–641. doi: 10.1177/1073191113492845. [DOI] [PubMed] [Google Scholar]
- 31.Reid-Arndt SA, Nehl C, Hinkebein J. The Frontal Systems Behaviour Scale (FrSBe) as a predictor of community integration following a traumatic brain injury. Brain Inj BI. 2007;21:1361–1369. doi: 10.1080/02699050701785062. [DOI] [PubMed] [Google Scholar]
- 32.Grace J, Malloy P. FrSBe, frontal systems behavior scale: Professional manual. Psychological Assessment Resources; 2001. [Google Scholar]
- 33.Structural equations modeling: Fit Indices, sample size, and advanced topics. [Internet][cited 2016 Jul 28] Available from: http://www.sciencedirect.com/science/article/pii/S1057740809001120.
- 34.Juengst SB, Adams LM, Arenth PM, et al. Satisfaction with Life Trajectories and Life Roles up to 5 Years after Injury: A Traumatic Brain Injury Model Systems Project. Rehabil Psychol. In press. [Google Scholar]
- 35.Lengenfelder J, Arjunan A, Chiaravalloti N, et al. Assessing frontal behavioral syndromes and cognitive functions in traumatic brain injury. Appl Neuropsychol Adult. 2015;22:7–15. doi: 10.1080/23279095.2013.816703. [DOI] [PubMed] [Google Scholar]
- 36.CiteULike: Principles and Practice of Structural Equation Modeling, Second Edition (Methodology In The Social Sciences) [Internet][cited 2016 Jul 28] Available from: http://www.citeulike.org/group/3304/article/1579326.
- 37.Jorge RE, Acion L, Burin DI, et al. Sertraline for preventing mood disorders following traumatic brain injury: A randomized clinical trial [Internet] JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.2189. [cited 2016 Sep 20] Available from: http://dx.doi.org/10.1001/jamapsychiatry.2016.2189. [DOI] [PubMed]
- 38.Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12:226–232. doi: 10.1176/jnp.12.2.226. [DOI] [PubMed] [Google Scholar]
- 39.Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42:48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 40.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma. 2009;26:2383–2402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iacobucci D. Structural equations modeling: Fit indices, sample size, and advanced topics. J Consum Psychol. 2010;20:90–98. [Google Scholar]
- 42.Kim KH. The Relation Among Fit Indexes, Power, and Sample Size in Structural Equation Modeling. Struct Equ Model Multidiscip J. 2005;12:368–390. [Google Scholar]
- 43.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]


