Abstract
Background
Doppler indices form an integral component of noninvasive evaluation of fetal well-being. There is paucity of information about normal obstetric Doppler indices, particularly from the Indian subcontinent. The aim of the study was to find the values of pulsatility index (PI), resistive index (RI) of umbilical artery (UA), and fetal middle cerebral artery (MCA) and calculate cerebro-placental ratio (CP ratio) for 18–40 weeks of normal gestation so that a reference range of these Doppler values can be postulated.
Methods
200 patients were enrolled in the study for color Doppler study of UA and MCA and were serially followed up at 4–6 weeks interval for Doppler indices. Angle-independent Doppler indices like PI and RI for MCA and UA were obtained during each examination. CP ratio was calculated in each case. All the cases were followed up till delivery and the perinatal outcome was recorded.
Results & Conclusion
The fetal MCA PI and RI showed a parabolic curve with plateau at 28–30 weeks of gestation. A significant correlation was noted between MCA PI and RI with gestational age. UA PI and RI showed a gradual fall over the gestational age with a strong negative correlation. There was a significant correlation between MCA PI and UA PI with their respective RI values. CP ratio has also shown a parabolic curve with turning point at 31–32 weeks of gestation. A significant correlation was noted between CP ratio and gestational age. CP ratio also showed a minimal positive correlation with MCA PI and a strong negative correlation with UA PI.
Keywords: Doppler indices, Middle cerebral artery, Umbilical artery, Cerebroplacental ratio
Introduction
Doppler ultrasonography (DU) velocimetry of fetal and uterine vessels is a well-established method for antenatal monitoring. Certain Doppler waveforms indicating circulatory changes can be used to predict adverse perinatal outcomes.1 DU was successfully introduced in obstetric imaging and fetal monitoring way back in 1977.2 Fitzgerald et al. were the first to report noninvasive demonstration of the umbilical cord (UC) blood flow pattern and suggested that the umbilical artery (UA) waveforms could be abnormal in fetuses with intrauterine growth-restriction (IUGR).3 This breakthrough concept of studying waveforms also resulted in several important clinical applications. Doppler assessment of the UA has now become standard of care in antenatal surveillence.4 Doppler assessment of the fetal middle cerebral artery (MCA) had also been widely used for the diagnosis of fetal anaemia.5
DU waveforms not only reflect blood velocity but also provide information on various aspects of blood flow like presence and direction of flow, velocity profile, flow volume, and impedance. Angle-independent Doppler indices mentioned below were developed for flow velocimetry to avoid inter- and intraobserver variation, and these are widely in use today.6
Among all vessels studied in DU, the UA and MCA are relatively easier to access and evaluate and are reported to be more reproducible. MCA of fetuses had been extensively studied for evaluation of placental compromise and foetalanaemia.7 Combining the Doppler indices of the MCA with that of the UA by the ratio of their pulsatility indices, also known as cerebro-placental ratio (CP ratio), is a useful tool for monitoring fetal health. A low CP ratio indicates relative redistribution of the blood flow to the cerebral circulation and is found to increase accuracy in envisaging complications and adverse outcomes as compared to MCA or UA Doppler indices alone.8 This ratio has now been increasingly used in the surveillance of the fetus at risk by repeating the Doppler studies at regular intervals.
Although reference ranges for these Doppler indices are available in Western literature, there is paucity of such studies in the Indian population.9, 10 The present study has attempted to calculate reference values of pulsatility index (PI), resistive index (RI) of MCA and UA, and calculate CP ratio in a study population of normal gestation with an idea to form a baseline for obstetric Doppler indices in the Indian context for evaluation, monitoring, and appropriate management of high-risk pregnancies.
The aim of the present study was to find the value of PI, RI of UA, and fetal MCA and then calculate CP ratio at 18–40 weeks of normal gestation and the objectives were
-
1.
To assess PI and RI of UA at 18–40 weeks of normal gestation.
-
2.
To assess PI and RI of MCA at 18–40 weeks of normal gestation.
-
3.
To calculate CP ratio for all these patients.
Material and methods
This was a prospective observational study carried out at the Department of Radiodiagnosis, Obstetrics and Gynecology and Pediatrics of a tertiary care and teaching hospital from December 2013 to August 2015. The study has approval of institutional ethical committee. Informed consent was obtained from each patient as per World Health Organization (WHO) format. All ultrasound/Doppler examinations were carried out adhering to the existing Pre-Conception and Pre-Natal Diagnostic Technique guidelines. The study population comprised of all antenatal cases registered during the study period at the Obstetrics and Gynecology and Radiodiagnosis departments of the tertiary care and teaching hospital.
Case definition
Pregnant women in 18–23 weeks of gestation with singleton fetus reporting to the tertiary care hospital.
Inclusion criteria
Normal second trimester pregnancies from 18 weeks onwards.
Exclusion criteria
Diagnosed fetal anomaly before recruitment, multi-fetal pregnancy, history of maternal smoking, known complications in the current pregnancy before recruitment, history of any pre-existing maternal medical condition (such as hypertension, diabetes mellitus, renal disease) likely to affect the fetus, cases which develop complication during the present pregnancy during the study period, and inability to obtain perinatal data.
Conduct of study
All consecutive subjects from the study population meeting the inclusion criteria were included in the study.
Measurements were obtained using a duplex Doppler ultrasound machine LOGIQ P5 unit with a curvilinear low frequency transducer. The various technical parameters were: frequency – 2–5 MHz, spectral frequency – 2.5 MHz, sample volume – 4 mm, filter – medium, and PRF – 4–5 MHz.
The entire procedure was completed in these steps:
-
a)
216 cases were selected from the study population.
-
b)A total of 16 cases were excluded from the study for the following reasons:
-
•8 cases: Lost to follow-up.
-
•4 cases: Developed complications during the study period.
-
•4 cases: Perinatal data were not available.
-
•
-
c)
A total of 200 cases were finally included in the study and analyzed.
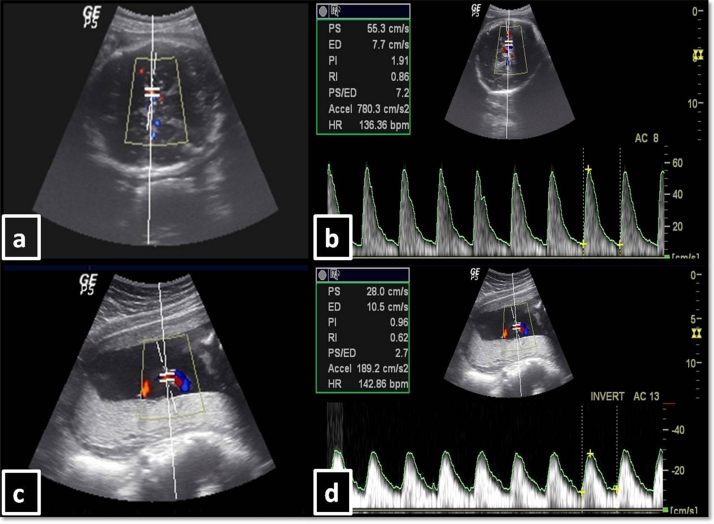
Measurements of MCA PI and RI, UA PI and RI, and CP ratio (Fig. 1)
Fig. 1.
Doppler velocimetry methods for MCA and UA. (a) Identification of MCA, (b) correction of beam angle and calculation of MCA Doppler indices by auto method, (c) identification of free floating segment of umbilical cord, (d) correction of beam angle and calculation of UA Doppler indices by auto method.
All cases were subjected to duplex Doppler examination after the findings of the biometry were confirmed, using a 3.5-MHz curvilinear transducer. Doppler waveforms were recorded from the fetal MCA and UA over three consecutive cardiac cycles. The patients were examined in semirecumbent position with the fetus in a resting and apneic state. Spectral waveforms were obtained with 4 mm sized sample volume using a medium filter.
-
•
MCA PI and RI: The MCA nearer to the probe was identified in each case using color Doppler. Spectral trace was obtained from the MCA immediately after its origin with a sample volume of 4 mm. Angle correction was done in each case and it was ensured that the angle of insonation was between 0 and 60°. PI and RI were measured both manually as well as in auto mode over three consecutive cardiac cycles. The measurements were repeated and two successive readings showing same results were noted for the study.
-
•
UA PI and RI: UA was identified in each case using color Doppler. Spectral trace was obtained with a sample volume of 4 mm from the free loop of the UC. In case of difficulty in obtaining the free loop of the UC, the placental insertion of the cord was tracked along to help localizing the free loop. Angle of insonation was maintained between 0 and 60°. PI and RI were measured both manually and in auto mode over three consecutive cardiac cycles. The measurements were repeated and two successive readings showing same results were finally noted for the study.
-
•
MCA PI/UA PI ratio: After ensuring technically satisfactory examination and measurements, the ratio of the MCA PI to the UA PI (CP ratio) was calculated in each case and noted.
Follow-up studies
Measurements of MCA PI and RI, UA PI and RI, and CP ratio were obtained on minimum of two occasions at least 4–6 weeks apart in each case.
Data collected was entered simultaneously into Microsoft excel worksheets designed appropriately.
Statistical analysis
The data collected was analyzed using software SPSS Version 17.0 for Windows (SPSS, Chicago, Illinois). Scatter plot is done for the compiled data.
Results
Out of the total of final 200 cases, maximum numbers of cases were in the age group of 21–25 years (96, 48% cases) followed by 26–30 years (74, 37% cases) age group. Majority of the cases in study population were primigravida (98, 49% cases) followed by 2nd gravida (83, 41.5% cases) forming the second largest group. Maximum numbers of cases were in 19th week of gestation and minimum numbers of cases were in 40th week of gestation with mean gestational age of 27.4 ± 6.15 weeks (Table 1). Mean and standard deviation (SD) of MCA and UA PI, RI, and CP ratio has been calculated for each gestational age along with percentile values and are presented in tabulated format (Table 2, Table 3, Table 4, Table 5, Table 6). A total of 773 Doppler examinations were carried out for the study population and were taken into consideration for analysis.
Table 1.
Distribution of gestational age in weeks.
| Gestational age (weeks) | Number of patients | Percentage (%) |
|---|---|---|
| 18 | 34 | 4.40 |
| 19 | 66 | 8.54 |
| 20 | 38 | 4.92 |
| 21 | 31 | 4.01 |
| 22 | 23 | 2.98 |
| 23 | 57 | 7.37 |
| 24 | 48 | 6.21 |
| 25 | 38 | 4.92 |
| 26 | 38 | 4.92 |
| 27 | 25 | 3.23 |
| 28 | 36 | 4.66 |
| 29 | 44 | 5.69 |
| 30 | 26 | 3.36 |
| 31 | 46 | 5.95 |
| 32 | 31 | 4.01 |
| 33 | 38 | 4.92 |
| 34 | 30 | 3.88 |
| 35 | 25 | 3.23 |
| 36 | 27 | 3.49 |
| 37 | 30 | 3.88 |
| 38 | 19 | 2.46 |
| 39 | 19 | 2.46 |
| 40 | 4 | 0.52 |
| Total | 773 | 100.00 |
Table 2.
Descriptive statistics for MCA PI with respect to gestational age (weeks).
| Gestational age (weeks) | Min | Max | Average | SD | 5th percentile | Quartile 1 (25%) | Quartile 2 (50%) | Quartile 3 (75%) | 95th percentile |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 1.54 | 2.11 | 1.74 | 0.17 | 1.58 | 1.61 | 1.68 | 1.89 | 1.98 |
| 19 | 1.53 | 2.01 | 1.76 | 0.11 | 1.60 | 1.68 | 1.78 | 1.84 | 1.94 |
| 20 | 1.41 | 2.07 | 1.77 | 0.13 | 1.55 | 1.70 | 1.81 | 1.84 | 1.92 |
| 21 | 1.53 | 2.12 | 1.83 | 0.12 | 1.63 | 1.80 | 1.86 | 1.91 | 1.96 |
| 22 | 1.50 | 2.24 | 1.84 | 0.16 | 1.63 | 1.75 | 1.86 | 1.93 | 2.06 |
| 23 | 1.64 | 2.38 | 1.93 | 0.15 | 1.68 | 1.82 | 1.93 | 2.04 | 2.11 |
| 24 | 1.52 | 2.26 | 1.97 | 0.17 | 1.68 | 1.86 | 1.98 | 2.12 | 2.19 |
| 25 | 1.78 | 2.21 | 2.00 | 0.12 | 1.80 | 1.94 | 2.02 | 2.10 | 2.16 |
| 26 | 1.61 | 2.23 | 1.99 | 0.16 | 1.64 | 1.91 | 2.05 | 2.10 | 2.16 |
| 27 | 1.74 | 2.32 | 2.05 | 0.15 | 1.83 | 1.95 | 2.00 | 2.18 | 2.24 |
| 28 | 1.81 | 2.39 | 2.09 | 0.15 | 1.84 | 2.01 | 2.09 | 2.19 | 2.32 |
| 29 | 1.64 | 2.36 | 2.04 | 0.15 | 1.79 | 1.96 | 2.02 | 2.16 | 2.31 |
| 30 | 1.65 | 2.31 | 2.00 | 0.16 | 1.80 | 1.88 | 2.00 | 2.12 | 2.28 |
| 31 | 1.61 | 2.28 | 2.01 | 0.15 | 1.78 | 1.90 | 2.05 | 2.14 | 2.20 |
| 32 | 1.64 | 2.24 | 1.96 | 0.14 | 1.80 | 1.86 | 1.96 | 2.09 | 2.14 |
| 33 | 1.51 | 2.10 | 1.80 | 0.13 | 1.62 | 1.70 | 1.81 | 1.88 | 2.00 |
| 34 | 1.44 | 2.10 | 1.76 | 0.14 | 1.54 | 1.64 | 1.81 | 1.85 | 1.93 |
| 35 | 1.43 | 1.93 | 1.72 | 0.14 | 1.49 | 1.62 | 1.75 | 1.82 | 1.91 |
| 36 | 1.18 | 1.82 | 1.61 | 0.15 | 1.39 | 1.55 | 1.62 | 1.69 | 1.80 |
| 37 | 0.63 | 1.73 | 1.51 | 0.20 | 1.32 | 1.44 | 1.57 | 1.62 | 1.69 |
| 38 | 1.10 | 1.65 | 1.30 | 0.16 | 1.10 | 1.20 | 1.30 | 1.39 | 1.61 |
| 39 | 1.10 | 1.40 | 1.21 | 0.09 | 1.12 | 1.14 | 1.20 | 1.28 | 1.36 |
| 40 | 0.95 | 1.31 | 1.22 | 0.10 | 1.11 | 1.16 | 1.23 | 1.29 | 1.31 |
Table 3.
Descriptive statistics for MCA RI with respect to gestational age (weeks).
| Gestational age (weeks) | Min | Max | Average | SD | 5th percentile | Quartile 1 (25%) | Quartile 2 (50%) | Quartile 3 (75%) | 95th percentile |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 0.70 | 1.73 | 0.81 | 0.23 | 0.71 | 0.73 | 0.74 | 0.80 | 0.93 |
| 19 | 0.68 | 0.84 | 0.76 | 0.03 | 0.71 | 0.74 | 0.76 | 0.78 | 0.82 |
| 20 | 0.69 | 0.80 | 0.76 | 0.03 | 0.72 | 0.75 | 0.77 | 0.78 | 0.80 |
| 21 | 0.73 | 0.86 | 0.78 | 0.03 | 0.74 | 0.76 | 0.78 | 0.80 | 0.83 |
| 22 | 0.71 | 0.82 | 0.76 | 0.03 | 0.73 | 0.74 | 0.76 | 0.78 | 0.81 |
| 23 | 0.73 | 1.02 | 0.78 | 0.04 | 0.74 | 0.76 | 0.77 | 0.80 | 0.82 |
| 24 | 0.72 | 0.85 | 0.80 | 0.03 | 0.76 | 0.79 | 0.81 | 0.83 | 0.84 |
| 25 | 0.71 | 0.86 | 0.82 | 0.03 | 0.79 | 0.80 | 0.83 | 0.83 | 0.85 |
| 26 | 0.76 | 0.88 | 0.82 | 0.02 | 0.77 | 0.80 | 0.83 | 0.83 | 0.85 |
| 27 | 0.78 | 0.88 | 0.83 | 0.02 | 0.80 | 0.81 | 0.82 | 0.85 | 0.86 |
| 28 | 0.79 | 0.90 | 0.84 | 0.03 | 0.81 | 0.83 | 0.84 | 0.86 | 0.89 |
| 29 | 0.71 | 0.95 | 0.83 | 0.03 | 0.80 | 0.82 | 0.83 | 0.85 | 0.87 |
| 30 | 0.79 | 0.89 | 0.83 | 0.02 | 0.79 | 0.80 | 0.83 | 0.84 | 0.86 |
| 31 | 0.76 | 0.86 | 0.82 | 0.02 | 0.78 | 0.80 | 0.83 | 0.83 | 0.85 |
| 32 | 0.77 | 0.85 | 0.81 | 0.02 | 0.78 | 0.80 | 0.83 | 0.83 | 0.84 |
| 33 | 0.74 | 0.85 | 0.79 | 0.03 | 0.74 | 0.77 | 0.80 | 0.81 | 0.83 |
| 34 | 0.72 | 0.84 | 0.79 | 0.03 | 0.74 | 0.77 | 0.78 | 0.81 | 0.83 |
| 35 | 0.75 | 0.82 | 0.79 | 0.02 | 0.75 | 0.78 | 0.79 | 0.80 | 0.82 |
| 36 | 0.70 | 0.81 | 0.76 | 0.03 | 0.70 | 0.74 | 0.77 | 0.78 | 0.81 |
| 37 | 0.70 | 0.78 | 0.75 | 0.02 | 0.71 | 0.73 | 0.75 | 0.76 | 0.77 |
| 38 | 0.60 | 0.77 | 0.69 | 0.05 | 0.62 | 0.65 | 0.70 | 0.72 | 0.75 |
| 39 | 0.61 | 0.70 | 0.67 | 0.03 | 0.63 | 0.65 | 0.66 | 0.69 | 0.70 |
| 40 | 0.66 | 0.70 | 0.68 | 0.02 | 0.66 | 0.67 | 0.68 | 0.69 | 0.70 |
Table 4.
Descriptive statistics for UA PI with respect to gestational age (weeks).
| Gestational age (weeks) | Min | Max | Average | SD | 5th percentile | Quartile 1 (25%) | Quartile 2 (50%) | Quartile 3 (75%) | 95th percentile |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 1.03 | 1.76 | 1.38 | 0.18 | 1.09 | 1.26 | 1.32 | 1.50 | 1.62 |
| 19 | 1.04 | 1.77 | 1.38 | 0.15 | 1.16 | 1.28 | 1.36 | 1.47 | 1.66 |
| 20 | 1.03 | 1.70 | 1.34 | 0.14 | 1.06 | 1.26 | 1.34 | 1.43 | 1.55 |
| 21 | 0.92 | 1.71 | 1.27 | 0.17 | 1.06 | 1.14 | 1.24 | 1.37 | 1.53 |
| 22 | 0.97 | 1.56 | 1.21 | 0.16 | 0.98 | 1.11 | 1.19 | 1.29 | 1.54 |
| 23 | 0.87 | 1.59 | 1.14 | 0.15 | 0.94 | 1.04 | 1.10 | 1.21 | 1.41 |
| 24 | 0.81 | 1.47 | 1.12 | 0.15 | 0.88 | 1.01 | 1.12 | 1.20 | 1.42 |
| 25 | 0.94 | 1.51 | 1.14 | 0.13 | 0.97 | 1.06 | 1.12 | 1.21 | 1.31 |
| 26 | 0.77 | 1.42 | 1.05 | 0.14 | 0.83 | 0.99 | 1.05 | 1.11 | 1.24 |
| 27 | 0.73 | 1.35 | 1.03 | 0.16 | 0.82 | 0.92 | 1.00 | 1.10 | 1.32 |
| 28 | 0.77 | 1.86 | 1.03 | 0.20 | 0.86 | 0.91 | 0.98 | 1.09 | 1.33 |
| 29 | 0.72 | 1.42 | 0.96 | 0.15 | 0.74 | 0.86 | 0.95 | 1.05 | 1.25 |
| 30 | 0.68 | 1.38 | 0.93 | 0.14 | 0.74 | 0.84 | 0.93 | 1.00 | 1.08 |
| 31 | 0.63 | 1.28 | 0.95 | 0.13 | 0.74 | 0.89 | 0.95 | 1.06 | 1.12 |
| 32 | 0.68 | 1.21 | 0.91 | 0.13 | 0.71 | 0.81 | 0.91 | 1.00 | 1.10 |
| 33 | 0.66 | 1.19 | 0.90 | 0.15 | 0.69 | 0.81 | 0.87 | 0.97 | 1.15 |
| 34 | 0.60 | 1.28 | 0.87 | 0.16 | 0.67 | 0.79 | 0.84 | 0.91 | 1.20 |
| 35 | 0.60 | 1.18 | 0.81 | 0.14 | 0.60 | 0.73 | 0.80 | 0.88 | 1.05 |
| 36 | 0.54 | 1.24 | 0.84 | 0.15 | 0.65 | 0.77 | 0.82 | 0.93 | 1.05 |
| 37 | 0.59 | 1.08 | 0.83 | 0.12 | 0.65 | 0.74 | 0.84 | 0.93 | 1.00 |
| 38 | 0.55 | 1.09 | 0.79 | 0.17 | 0.58 | 0.66 | 0.78 | 0.87 | 1.08 |
| 39 | 0.55 | 1.02 | 0.74 | 0.14 | 0.58 | 0.61 | 0.75 | 0.80 | 0.95 |
| 40 | 0.64 | 0.82 | 0.75 | 0.08 | 0.66 | 0.72 | 0.77 | 0.80 | 0.82 |
Table 5.
Descriptive statistics for UA RI with respect to gestational age (weeks).
| Gestational age (weeks) | Min | Max | Average | SD | 5th percentile | Quartile 1 (25%) | Quartile 2 (50%) | Quartile 2 (75%) | 95th percentile |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 0.65 | 0.92 | 0.77 | 0.07 | 0.67 | 0.71 | 0.76 | 0.80 | 0.90 |
| 19 | 0.64 | 1.02 | 0.75 | 0.06 | 0.66 | 0.71 | 0.75 | 0.79 | 0.86 |
| 20 | 0.65 | 0.89 | 0.75 | 0.05 | 0.67 | 0.73 | 0.76 | 0.78 | 0.82 |
| 21 | 0.62 | 0.98 | 0.75 | 0.07 | 0.69 | 0.71 | 0.74 | 0.77 | 0.84 |
| 22 | 0.65 | 0.85 | 0.73 | 0.05 | 0.65 | 0.69 | 0.71 | 0.77 | 0.83 |
| 23 | 0.59 | 0.86 | 0.71 | 0.05 | 0.64 | 0.67 | 0.70 | 0.74 | 0.81 |
| 24 | 0.60 | 0.82 | 0.70 | 0.06 | 0.60 | 0.66 | 0.71 | 0.73 | 0.79 |
| 25 | 0.61 | 0.84 | 0.71 | 0.05 | 0.65 | 0.67 | 0.70 | 0.75 | 0.77 |
| 26 | 0.55 | 0.80 | 0.68 | 0.05 | 0.58 | 0.66 | 0.69 | 0.70 | 0.75 |
| 27 | 0.54 | 0.79 | 0.66 | 0.07 | 0.56 | 0.62 | 0.65 | 0.70 | 0.78 |
| 28 | 0.56 | 0.80 | 0.66 | 0.05 | 0.60 | 0.62 | 0.65 | 0.68 | 0.76 |
| 29 | 0.53 | 0.82 | 0.65 | 0.06 | 0.56 | 0.62 | 0.64 | 0.67 | 0.76 |
| 30 | 0.53 | 0.79 | 0.63 | 0.06 | 0.53 | 0.59 | 0.63 | 0.66 | 0.70 |
| 31 | 0.48 | 0.76 | 0.64 | 0.06 | 0.52 | 0.61 | 0.64 | 0.69 | 0.71 |
| 32 | 0.49 | 0.74 | 0.61 | 0.06 | 0.53 | 0.57 | 0.60 | 0.65 | 0.73 |
| 33 | 0.49 | 0.75 | 0.61 | 0.07 | 0.52 | 0.57 | 0.60 | 0.66 | 0.73 |
| 34 | 0.46 | 0.77 | 0.60 | 0.07 | 0.51 | 0.55 | 0.58 | 0.62 | 0.74 |
| 35 | 0.46 | 0.72 | 0.57 | 0.06 | 0.46 | 0.54 | 0.56 | 0.61 | 0.66 |
| 36 | 0.42 | 0.74 | 0.57 | 0.07 | 0.46 | 0.54 | 0.57 | 0.61 | 0.66 |
| 37 | 0.42 | 0.68 | 0.57 | 0.06 | 0.47 | 0.54 | 0.58 | 0.62 | 0.65 |
| 38 | 0.44 | 0.68 | 0.55 | 0.08 | 0.44 | 0.50 | 0.54 | 0.58 | 0.68 |
| 39 | 0.42 | 0.63 | 0.53 | 0.07 | 0.45 | 0.47 | 0.53 | 0.58 | 0.62 |
| 40 | 0.47 | 0.58 | 0.53 | 0.05 | 0.48 | 0.51 | 0.54 | 0.56 | 0.58 |
Table 6.
Descriptive statistics for CP ratio with respect to gestational age (weeks).
| Gestational age (weeks) | Min | Max | Average | SD | 5th percentile | Quartile 1 (25%) | Quartile 2 (50%) | Quartile 2 (75%) | 95th percentile |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 1.00 | 2.05 | 1.29 | 0.25 | 1.03 | 1.10 | 1.27 | 1.37 | 1.62 |
| 19 | 1.00 | 1.68 | 1.29 | 0.12 | 1.12 | 1.21 | 1.28 | 1.38 | 1.47 |
| 20 | 1.13 | 1.85 | 1.33 | 0.13 | 1.16 | 1.26 | 1.34 | 1.39 | 1.48 |
| 21 | 1.12 | 1.78 | 1.46 | 0.16 | 1.18 | 1.37 | 1.47 | 1.55 | 1.70 |
| 22 | 1.16 | 1.92 | 1.54 | 0.14 | 1.34 | 1.49 | 1.54 | 1.60 | 1.68 |
| 23 | 1.31 | 2.15 | 1.72 | 0.18 | 1.45 | 1.60 | 1.72 | 1.86 | 1.98 |
| 24 | 1.44 | 2.23 | 1.77 | 0.18 | 1.50 | 1.65 | 1.76 | 1.89 | 2.06 |
| 25 | 1.46 | 2.12 | 1.78 | 0.17 | 1.52 | 1.66 | 1.75 | 1.86 | 2.09 |
| 26 | 1.54 | 2.34 | 1.91 | 0.17 | 1.65 | 1.80 | 1.90 | 2.03 | 2.14 |
| 27 | 1.63 | 2.38 | 2.02 | 0.24 | 1.64 | 1.78 | 2.03 | 2.21 | 2.34 |
| 28 | 1.14 | 2.69 | 2.07 | 0.28 | 1.70 | 1.95 | 2.11 | 2.25 | 2.46 |
| 29 | 1.65 | 2.73 | 2.15 | 0.24 | 1.82 | 1.99 | 2.12 | 2.28 | 2.57 |
| 30 | 1.67 | 2.81 | 2.17 | 0.23 | 1.82 | 2.06 | 2.17 | 2.30 | 2.47 |
| 31 | 1.78 | 2.57 | 2.15 | 0.21 | 1.84 | 2.00 | 2.16 | 2.29 | 2.52 |
| 32 | 1.65 | 2.62 | 2.19 | 0.25 | 1.84 | 2.03 | 2.13 | 2.38 | 2.61 |
| 33 | 1.52 | 2.78 | 2.04 | 0.29 | 1.60 | 1.84 | 2.01 | 2.19 | 2.48 |
| 34 | 1.58 | 2.40 | 2.07 | 0.24 | 1.62 | 1.90 | 2.12 | 2.24 | 2.37 |
| 35 | 1.64 | 2.64 | 2.15 | 0.26 | 1.81 | 1.98 | 2.12 | 2.28 | 2.55 |
| 36 | 1.47 | 2.42 | 1.95 | 0.25 | 1.65 | 1.73 | 1.91 | 2.11 | 2.37 |
| 37 | 0.84 | 2.47 | 1.85 | 0.32 | 1.45 | 1.67 | 1.85 | 1.99 | 2.36 |
| 38 | 1.27 | 2.06 | 1.69 | 0.25 | 1.28 | 1.55 | 1.71 | 1.90 | 2.01 |
| 39 | 1.18 | 2.30 | 1.69 | 0.28 | 1.25 | 1.53 | 1.70 | 1.86 | 2.06 |
| 40 | 1.27 | 1.72 | 1.55 | 0.20 | 1.32 | 1.52 | 1.61 | 1.64 | 1.70 |
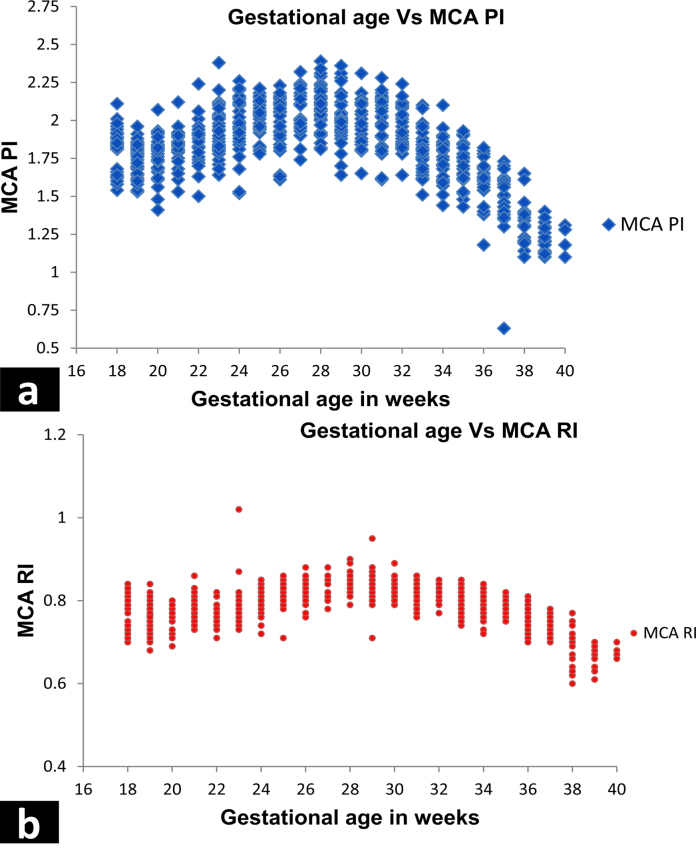
The MCA PI and RI values for various gestational ages are plotted in a scatter chart as shown in Fig. 2. The scatter plot shows a parabolic curve with plateau between 28 and 30 weeks and thereafter a turn at 30 weeks onwards of gestation. From 18 to 30 weeks, the MCA PI values show a positive correlation with gestational age. From 30 weeks till 40 weeks, MCA PI values demonstrate a negative correlation with gestational age. The scatter plot of MCA RI shows a parabolic curve with plateau between 28 and 30 weeks and thereafter a turn at 30 weeks onwards of gestation just like the MCA PI curve. From 18 to 30 weeks, the MCA RI values show a positive correlation with gestational age. From 30 weeks till 40 weeks, MCA RI values demonstrate a negative correlation with gestational age. Correlation was done between MCA PI and RI for each gestational week (Table 7) which has shown a strong positive correlation between the two for each gestation week.
Fig. 2.
Scatter plot diagram of MCA PI (a) and MCA RI (b).
Table 7.
Correlation between MCA PI and RI, UA PI and RI, CP ratio and MCA PI, and CP ratio and UA PI for gestational period 18–40 weeks.
| Gestational age in weeks (N) | Correlation of MCA PI and RI (P value) |
Correlation of UA PI and RI (P value) |
Correlation of CP ratio and MCA PI (P value) |
Correlation of CP ratio and UA PI (P value) |
|---|---|---|---|---|
| 18 (34) | 0.077 (0.665) | 0.431 (0.011) | 0.635 (0.000) | −0.806 (0.000) |
| 19 (66) | 0.838 (0.000) | 0.688 (0.000) | −0.175 (0.160) | −0.859 (0.000) |
| 20 (38) | 0.376 (0.020) | 0.863 (0.000) | 0.394 (0.014) | −0.517 (0.000) |
| 21 (31) | 0.722 (0.000) | 0.808 (0.000) | 0.137 (0.462) | −0.614 (0.000) |
| 22 (23) | 0.446 (0.033) | 0.935 (0.000) | −0.057 (0.797) | −0.724 (0.000) |
| 23 (56) | 0.691 (0.000) | 0.942 (0.000) | 0.041 (0.763) | −0.746 (0.000) |
| 24 (46) | 0.796 (0.000) | 0.851 (0.000) | −0.028 (0.854) | −0.592 (0.000) |
| 25 (39) | 0.543 (0.000) | 0.932 (0.000) | 0.151 (0.358) | −0.775 (0.000) |
| 26 (39) | 0.829 (0.000) | 0.933 (0.000) | 0.131 (0.476) | −0.729 (0.000) |
| 27 (25) | 0.818 (0.000) | 0.961 (0.000) | 0.027 (0.897) | −0.772 (0.000) |
| 28 (37) | 0.695 (0.000) | 0.774 (0.000) | 0.061 (0.721) | −0.869 (0.000) |
| 29 (43) | 0.803 (0.000) | 0.951 (0.000) | −0.193 (0.215) | −0.840 (0.000) |
| 30 (27) | 0.471 (0.013) | 0.911 (0.000) | −0.028 (0.888) | −0.803 (0.000) |
| 31 (46) | 0.809 (0.000) | 0.959 (0.000) | −0.264 (0.076) | −0.863 (0.000) |
| 32 (31) | 0.634 (0.000) | 0.932 (0.000) | −0.169 (0.363) | −0.879 (0.000) |
| 33 (38) | 0.804 (0.000) | 0.935 (0.000) | 0.036 (0.829) | −0.862 (0.000) |
| 34 (29) | 0.884 (0.000) | 0.968 (0.000) | −0.436 (0.018) | −0.898 (0.000) |
| 35 (25) | 0.728 (0.000) | 0.905 (0.000) | −0.366 (0.072) | −0.857 (0.000) |
| 36 (27) | 0.739 (0.000) | 0.907 (0.000) | 0.039 (0.849) | −0.822 (0.000) |
| 37 (29) | 0.549 (0.002) | 0.943 (0.000) | 0.555 (0.002) | −0.594 (0.001) |
| 38 (18) | 0.849 (0.000) | 0.984 (0.000) | −0.035 (0.890) | −0.833 (0.000) |
| 39 (17) | 0.781 (0.000) | 0.971 (0.000) | 0.107 (0.683) | −0.899 (0.000) |
| 40 (3) | 0.317 (0.795) | 0.994 (0.071) | −0.999 (0.028) | −1.000 (0.013) |
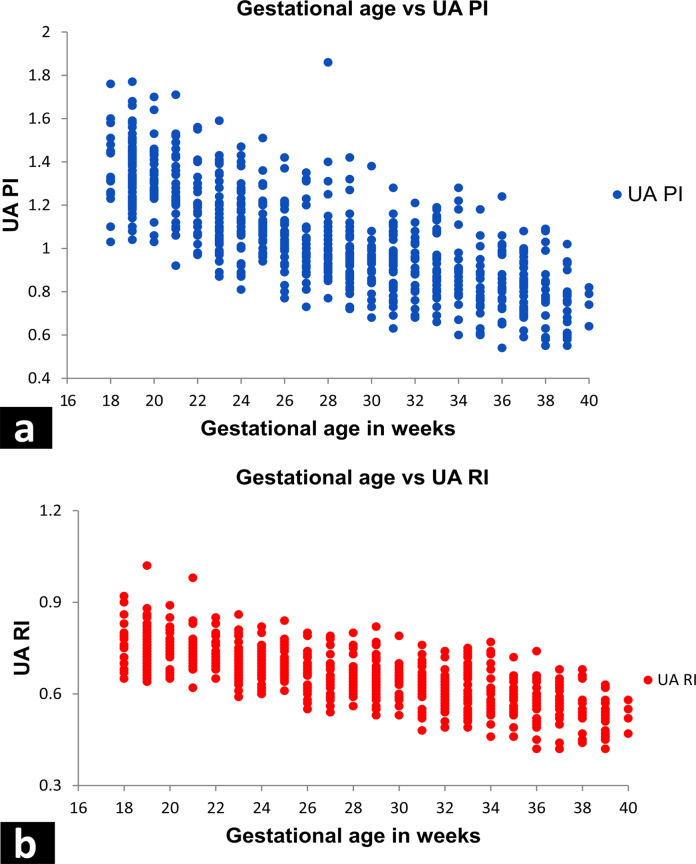
The UA PI and RI value for various gestational ages is plotted in a scatter chart as shown in Fig. 3. The scatter plot shows a linear graph with a strong negative correlation between gestational age and UA PI throughout the gestation from 18 weeks till 40 weeks. The scatter plot of UA RI shows a linear graph with a strong negative correlation between gestational age and UA RI throughout the gestation from 18 weeks till 40 weeks. Correlation was done between UA PI and RI for each gestational week (Table 7) which has shown a strong positive correlation between the two for each gestation week.
Fig. 3.
Scatter plot diagram of UA PI (a) and UA RI (b).
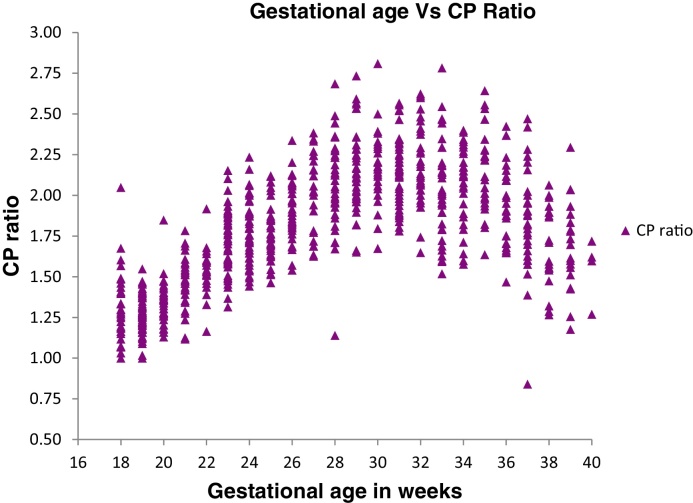
The CP ratio value for various gestational ages is plotted in a scatter chart as shown in Fig. 4. The scatter plot shows a parabolic curve with plateau between 29 and 31 weeks and thereafter a turn at 31 weeks onwards of gestation. From 18 to 30 weeks, the CP ratio values show positive correlation with gestational age. From 30 weeks till 40 weeks, MCA RI values demonstrate a negative correlation with gestational age. Correlation was done between CP ratio with MCA PI and UA PI for each gestational week (Table 7) which has shown a negative correlation between CP ratio and UA PI for each gestation week.
Fig. 4.
Scatter plot diagram of CP ratio.
Discussion
Doppler velocimetry of uteroplacental, umbilical and fetal vessels have become an established method for fetal monitoring in day-to-day obstetric practice.11 Circulatory changes, reflected in fetal Doppler waveforms, can reliably predict adverse perinatal outcome. Several investigators have highlighted the utility of DU of umbilical and fetal vessels for monitoring fetal well-being, IUGR, fetal anemia, and perinatal outcomes.12
MCA Doppler indices
The MCA Doppler studies define the fetal response to abnormal placental function. There is cerebral vasodilation with redistribution of the blood flow from the fetal periphery to the brain known as “brain-sparing effect”, an adaptive phenomenon which occurs initially for some time. The MCA PI and RI values change throughout normal pregnancy. Previous studies done on MCA PI and RI by Ebbing et al.,7 Mari et al.,10 Tarzamni et al.,13 and Komwilaisak et al.14 have shown a parabolic curve for MCA PI and RI with a plateau between 28 and 30 weeks likely due to increased requirement of brain during early and late pregnancy.
Our study has also shown a similar parabolic curve with a peak at 30 weeks and then a gradual fall comparable with the previously described studies.7, 10, 13, 14 Our study has shown a moderate positive correlation of MCA PI and RI with gestational age till 30 weeks of gestation followed by a strong negative correlation with the gestation age after 30 weeks which is again similar to the previous studies.7, 10, 13, 14 The MCA PI and RI value ranges in our study in different gestation are similar with what was found by Tarzamni et al.,13 but were lower as compared to what were recorded by Ebbing et al.,7 Mari et al.,10 and Komwilaisak et al.14 The exact cause for this deviation is not clear. However, this may be due to a different subset of population, socioeconomic status, demographic indices, and statistical methods. A strong positive correlation was noted between MCA PI and RI in our study as was also seen in the study done by Tarzamni et al.13
UA Doppler indices
UC plays a crucial role in fetal health and development. Several complications like IUGR, cord accidents, and stillbirths are attributed to an abnormal fetoplacental circulation. Various studies have shown definite associations of abnormal values of UA PI, RI, and S/D ratio in IUGR, hypertensive disease of pregnancies, and intra-ventricular haemorrhage.15, 16, 17 Bhide et al.18 and many other researchers have described normal reference ranges for UA Doppler indices. However, as these reference ranges were prepared in a specific subset of populations, these reference values may not be reproducible in all populations. Acharya et al.,9 Chanprapaph et al.,19 Harneet et al.,20 and Kurmanavicius et al.21 described that UA PI and RI show a gradual fall over gestation period. Our study has also followed a similar linear graph of UA PI and RI with gradual fall over gestation period with a strong negative correlation between UA PI and RI with gestation period.
In our study, mean value obtained for UA PI and RI for different gestation period is almost similar to the value obtained by Acharya et al.9 However, the reference values obtained in our study are found to be higher as compared to a similar study done by Harneet et al.,20 which may be due to a different subset of population and a different sample size.
Cerebro-placental ratio
CP ratio is a combined parameter of MCA PI and UA PI. CP ratio is a better indicator of fetoplacental circulation when compared to UA PI and MCA PI alone in the evaluation of various antenatal and perinatal complications.22, 23 CP ratio reflects the status of redistribution of the cardiac output to the cerebral circulation, which improves accuracy in predicating adverse outcome compared to MCA and UA Doppler alone.24 The CP ratio is also considered to be more physiological in the measurement of centralization of fetal blood flow. Shahinaj et al. have studied the correlation of CP ratio and IUGR and found a strong positive correlation of IUGR with reduced CP ratio.23 CP ratio is proven to be an important adjunct parameter to help in monitoring perinatal and antenatal complications in high-risk pregnancies.25 In our study, we have evaluated and formed a reference curve for CP ratio from 18 to 40 weeks of gestation which has shown a parabolic curve with turning point at around 31–32 weeks of gestation. CP ratio showed a strong positive correlation with gestation age till 30 weeks of gestation followed by strong negative correlation thereafter till 40 weeks of gestation. This is probably due to different amount of blood volume required by brain in different gestation. There is paucity of information in the literature on this account. There are few studies26 which have tried to formulate reference ranges for CP ratio over the gestation period in normal pregnancies.
The mean value of CP ratio obtained in our study is similar to the values described by Baschat et al.26 To the best of our knowledge, only Baschat et al.26 have suggested a reference range for CP ratio. No study to the best of our knowledge has correlated CP ratio with MCA PI and UA PI.
Strength of the study
-
1.
This study is a longitudinal observation study. Previous studies done by Chanprapaph et al.,19 Tarzamni et al.,13 Harneet et al.,20 and Kurmanavicius et al.21 are cross-sectional studies with no serial Doppler correlation. Although Ebbing et al.7 and Acharya et al.9 did longitudinal studies, they had smaller sample size (131 and 161 cases) as compared to ours (200 cases). As mentioned earlier, a total of 773 Doppler examination findings were analyzed in our study.
-
2.
Our study population is of varied culture and socioeconomic status; hence, it could represent the general population.
-
3.
In our study, we have proposed a reference curve for CP ratio, which has been described by very few studies.26
-
4.
To the best of our knowledge, no such study about reference values of UA, and MCA Doppler velocimetry in normal gestation has been conducted for the Indian population.
Limitations of the study
-
1.
This study is limited by the fact that total numbers of cases of 38–40 weeks gestation were relatively less to enable meaningful derivation of the Doppler indices for these gestation periods.
-
2.
There are outliers in a few variables which may have influenced the study results.
-
3.
The study was done in a single center which could have also affected the results.
-
4.
The study also suffers from inherent pitfalls of a single investigator observational study.
Conclusion
The study shows that fetal MCA and UA Doppler indices follow a definite pattern depending on the gestational age. The MCA PI and RI has shown a parabolic curve which is possibly due to the relative increase in the requirement of blood supply by fetal brain in the initial and later part of pregnancy. The UA PI and RI has shown a gradual decline throughout the gestation likely due to decrease in placental resistance as the pregnancy progresses. CP ratio, which is derived by using both MCA and UA indices, is considered to be a better physiological parameter than MCA PI and UA PI alone. The CP ratio has shown a parabolic curve during normal gestation. CP ratio has also shown a strong negative correlation with UA PI. Hence, it may be inferred that CP ratio can also be used as an additional parameter along with the UA Doppler indices in cases of IUGR. Our study results can form a baseline for the Obstetric Doppler indices which in turn will help in picking up abnormal Doppler values, especially in high-risk cases, especially in the Indian context. However, further studies with a larger sample size, preferably a multicenter study may be required before the results of the present study can be extrapolated to the general population.
Conflicts of interest
The authors have none to declare.
References
- 1.Tarzamni M.K., Nezami N., Gatreh-Samani F., Vahedinia S., Tarzamni M. Doppler waveform indices of fetal middle cerebral artery in normal 20 to 40 weeks pregnancies. Arch Iran Med. 2009;12(1):29–34. [PubMed] [Google Scholar]
- 2.Mari G. Doppler ultrasonography in obstetrics: from the diagnosis of fetal anemia to the treatment of intrauterine growth-restricted fetuses. Am J Obstet Gynecol. 2009;200(6) doi: 10.1016/j.ajog.2008.10.054. 613.e1–e9. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald D., Drumm J. Non-invasive measurement of human fetal circulation using ultrasound: a new method. Br Med J. 1977;2(6100):1450. doi: 10.1136/bmj.2.6100.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maulik D., Mundy D., Heitmann E., Maulik D. Umbilical artery Doppler in the assessment of fetal growth restriction. Clin Perinatol. 2011;38(1):65–82. doi: 10.1016/j.clp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Mari G. Middle cerebral artery peak systolic velocity for the diagnosis of fetal anemia: the untold story. Ultrasound Obstet Gynecol. 2005;25(4):323–330. doi: 10.1002/uog.1882. [DOI] [PubMed] [Google Scholar]
- 6.Carol M.R., Stephanie R.W., Charboneau J.W., Deborah L., editors. Diagnostic Ultrasound. 4th ed. Elsevier Mosby; Philadelphia: 2011. p. 1474. [Google Scholar]
- 7.Ebbing C., Rasmussen S., Kiserud T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol. 2007;30(3):287–296. doi: 10.1002/uog.4088. [DOI] [PubMed] [Google Scholar]
- 8.Hershkovitz R., Kingdom J.C., Geary M., Rodeck C.H. Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2000;15(3):209–212. doi: 10.1046/j.1469-0705.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- 9.Acharya G., Wilsgaard T., Berntsen G.K., Maltau J.M., Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192(3):937–944. doi: 10.1016/j.ajog.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Mari G., Deter R.L. Middle cerebral artery flow velocity waveforms in normal and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1992;166(4):1262–1270. doi: 10.1016/s0002-9378(11)90620-6. [DOI] [PubMed] [Google Scholar]
- 11.Dubiel M., Breborowicz G.H., Marsal K., Gudmundsson S. Fetal adrenal and middle cerebral artery Doppler velocimetry in high-risk pregnancy. Ultrasound Obstet Gynecol. 2000;16(5):414–418. doi: 10.1046/j.1469-0705.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- 12.Signore C., Freeman R.K., Spong C.Y. Antenatal testing-a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701. doi: 10.1097/AOG.0b013e318197bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarzamni M.K., Nezami N., Sobhani N., Eshraghi N., Tarzamni M., Talebi Y. Nomograms of Iranian fetal middle cerebral artery Doppler waveforms and uniformity of their pattern with other populations’ nomograms. BMC Pregnancy Childbirth. 2008;8:50. doi: 10.1186/1471-2393-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komwilaisak R., Saksiriwuttho P., Ratanasiri T., Kleebkaow P., Seejorn K. Pulsatility index of the middle cerebral artery in normal fetuses. J Med Assoc Thai. 2004;87(suppl 3):S34–S37. [PubMed] [Google Scholar]
- 15.Shah N.S., Nandita M., Verma R.N., Desai V.A. Umbilical and cerebral arterial flow velocity waveforms and neonatal outcome in high risk pregnancy. J Obstet Gynecol India. 2007;57(3):216–220. [Google Scholar]
- 16.Baschat A.A., Gembruch U., Viscardi R.M., Gortner L., Harman C.R. Antenatal prediction of intraventricular hemorrhage in fetal growth restriction: what is the role of Doppler? Ultrasound Obstet Gynecol. 2002;19(4):334–339. doi: 10.1046/j.1469-0705.2002.00661.x. [DOI] [PubMed] [Google Scholar]
- 17.Ali A., Ara I., Sultana R., Akram F., Zaib M.J. Comparison of perinatal outcome of growth restricted fetuses with normal and abnormal umbilical artery Doppler waveforms. J Ayub Med Coll Abbottabad. 2014;26(3):344–348. [PubMed] [Google Scholar]
- 18.Bhide A., Acharya G., Bilardo C.M. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):233–239. doi: 10.1002/uog.12371. [DOI] [PubMed] [Google Scholar]
- 19.Chanprapaph P., Wanapitak C., Tongsong T. Umbilical artery Doppler waveform indices in normal pregnancies. Thai J Obstet Gynaecol. 2000;12:103–107. [Google Scholar]
- 20.Harneet N., Kapila A., Kaur M.M. Cerebral and umbilical arterial blood flow velocity in normal and growth retarded pregnancy. J Obstet Gynecol India. 2009;59(1):47–52. [Google Scholar]
- 21.Kurmanavicius J., Florio I., Wisser J. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound Obstet Gynecol. 1997;10(2):112–120. doi: 10.1046/j.1469-0705.1997.10020112.x. [DOI] [PubMed] [Google Scholar]
- 22.Bano S., Chaudhary V., Pande S., Mehta V., Sharma A. Color Doppler evaluation of cerebral-umbilical pulsatility ratio and its usefulness in the diagnosis of intrauterine growth retardation and prediction of adverse perinatal outcome. Indian J Radiol Imaging. 2010;20(1):20–25. doi: 10.4103/0971-3026.59747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahinaj R., Manoku N., Kroi E., Tasha I. The value of the middle cerebral to umbilical artery Doppler ratio in the prediction of neonatal outcome in patient with preeclampsia and gestational hypertension. J Prenat Med. 2010;4(2):17–21. [PMC free article] [PubMed] [Google Scholar]
- 24.Vergani P., Roncaglia N., Locatelli A. Antenatal predictors of neonatal outcome in fetal growth restriction with absent end-diastolic flow in the umbilical artery. Am J Obstet Gynecol. 2005;193(3 Pt 2):1213–1218. doi: 10.1016/j.ajog.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Yalti S., Oral O., Gurbuz B., Ozden S., Atar F. Ratio of middle cerebral to umbilical artery blood velocity in pre-eclamptic & hypertensive women in the prediction of poor perinatal outcome. Indian J Med Res. 2004;120(1):44–50. [PubMed] [Google Scholar]
- 26.Baschat A.A., Gembruch U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol. 2003;21(2):124–127. doi: 10.1002/uog.20. [DOI] [PubMed] [Google Scholar]