Abstract
Background
Device-Associated Healthcare-Associated Infections (DA-HAI), including Ventilator-Associated Pneumonia (VAP), Central-Line-Associated Blood Stream Infection (CLABSI), and Catheter-Related Urinary Tract Infection (CAUTI), are considered as principal contributors to healthcare hazard and threat to patient safety as they can cause prolonged hospital stay, sepsis, and mortality in the ICU. The study intends to characterize DA-HAI in a tertiary care multidisciplinary ICU of a teaching hospital in eastern India.
Methods
This prospective outcome-surveillance study was conducted among 2157 ICU patients of a 760-bedded teaching hospital in Eastern India. Clinical, laboratory and environmental surveillance, and screening of HCPs were conducted using the US Centers for Disease Control and Prevention (CDC)’s National Healthcare Safety Network (NHSN) definitions and methods.
Results
With 8824 patient/bed/ICU days and 14,676 device days, pooled average device utilization ratio was 1.66, total episodes of DA-HAI were 114, and mean monthly rates of DA-HAI, VAP, CLABSI, and CAUTI were 4.75, 2, 1.4, and 1.25/1000 device days. Most common pathogens isolated from DA-HAI patients were Klebsiella pneumoniae (24.6%), Escherichia coli (21.9%), and Pseudomonas aeruginosa (20.2%). All Acinetobacter baumanii, >80% K. pneumoniae and E. coli, and >70% P. aeruginosa were susceptible only to colistin and tigecycline. One P. aeruginosa isolate was panresistant.
Conclusion
Mean rates of VAP, CLABSI, and CAUTI were 14.4, 8.1, and 4.5 per 1000 device days, which are comparable with Indian and global ICUs. Patients and HCPs form important reservoirs of infection. Resolute conviction and sustained momentum in Infection Control Initiatives are an essential step toward patient safety.
Keywords: Device-Associated Healthcare Associated Infections (DA-HAI), Ventilator-Associated Pneumonia (VAP), Central-Line –Associated Blood Stream Infections (CLABSI), Catheter-Related Urinary Tract Infection (CAUTI), Antimicrobial Resistance
Introduction
Healthcare-associated infections (HAI) are infections acquired after 48 h of admission, up to 30 days of discharge and up to one year in case of implants; which were not evident or under incubation at the time of admission.1 Ventilator-Associated Pneumonia (VAP), Central-Line-Associated Blood Stream Infections (CLABSI), and Catheter-Related Urinary Tract Infection (CAUTI) are the most commonly encountered Device-Associated Healthcare-Associated Infections (DA-HAI) and are considered as the principal contributors to healthcare hazard and threat to patient safety.
Patients in Intensive Care Unit (ICU) with multiple comorbidities are on artificial ventilation, inotropes, central venous catheterization/central-line, urinary catheterization, parenteral nutrition, and other supports, which render them susceptible to HAI.2 Multidrug-resistant (MDR) pathogens persisting in ICU environment cause opportunistic infections, more so in association with the use of devices. DA-HAI leading to bacteremia and sepsis is the leading cause of prolonged hospital stay, enhanced commitment toward barrier nursing and patient isolation, morbidity, mortality, cost escalation, and reduction in bed availability.3
The incidence of DA-HAI depends on access to ICU, frequency and duration of use of devices, infection control practices, and immune constitution of patients. The rates of HAI in high-income countries’ ICUs are approximately 5–10% vis-a-vis 2–10 times higher incidence in lower- and middle-income countries. Rates of HAI vary in different hospitals, different ICUs in the same hospital or same ICU at different periods, with higher rates in teaching hospitals.2, 4
The National Patient Safety Goals purported by WHO World Alliance on Patient Safety in 2009 advocate focused control of DA-HAI mandating a strong policy, teamwork, supervision, surveillance, and administrative patronage. Ongoing surveillance of HAI helps characterize infections, etiology, sources, DA-HAI rates, and resistograms, thus forming a guideline for targeted interventions for patients, healthcare professionals (HCP), and institutional policies. This study intends to characterize DA-HAI (VAP, CLABSI, and CAUTI) in a multidisciplinary ICU of a tertiary-care hospital in Eastern India.
Materials and methods
This prospective outcome-surveillance study was conducted among all patients admitted to multidisciplinary 14-bedded ICU of a 760-bedded tertiary-care teaching and referral hospital in Eastern India over a period of 24 months from June 2014 to May 2016, after approval from Hospital Ethics Committee. All good clinical practice and laboratory guidelines were observed. Patients staying less than 48 h, testing positive for infections within 48 h, and showing evidence of existing infections on admission were excluded.
Surveillance of DA-HAI was conducted by the Infection Control Team (ICT) under the aegis of Hospital Infection Control Committee (HICC) using the US Centers for Disease Control and Prevention (CDC)’s National Healthcare Safety Network (NHSN) definitions and methods.5 Surveillance included clinical surveillance, laboratory surveillance, environmental surveillance, and screening of HCPs in ICU.
-
1.
Clinical surveillance, conducted every morning during ICU rounds, included patient's clinicodemographic profile, diagnosis, date of admission to ICU, fever/hypothermia, abnormal leukocyte counts, use of ventilator, central-line or urinary catheter, and date of transfer/discharge/death. Daily surveillance data recorded in the HICC register was perused by a clinical microbiologist.
-
2.
Laboratory surveillance included baseline and intuitive cultures interpreted as per NHSN-CDC guidelines to arrive at the diagnosis of DA-HAI including VAP, CLABSI, and CAUTI. Baseline surveillance cultures were requested before use of devices such as endotracheal cultures immediately after tracheostomy, blood cultures on admission to ICU, and urine cultures before catheterization. Clinical intuitive cultures were requested after 48 h of use of device. Paired blood cultures after positive culture screen from BacT/ALERT® 3D blood culture system (bioMérieux, France) were aerobically incubated in O2 at 37 °C for 18–120 h. Positive blood cultures, urine obtained after clamping the catheter, and endotracheal aspirate were aerobically incubated at 37 °C for 24 h on solid media. Both standard and automated methods were used for identification. Colony characteristics, Gram staining, motility, carbon source utilization, and enzymatic activity were correlated with results from Vitek-2 compact (bioMérieux, France) automated system which was also used for determining Minimal Inhibitory Concentrations (MIC). Identification percentage >85% was taken as cutoff for final validation. Non-repeat positive cultures with antibiograms were taken into account for profiling of isolates and antimicrobial susceptibility. Quality control was performed by testing Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853.
-
3.
VAP was diagnosed by positive endotracheal aspirate cultures in a mechanically ventilated patient with at least one of CDC qualifying criteria of new onset of purulent sputum production or change in character of sputum, pathogen cultured from blood, or positive culture from endotracheal aspirate, with corroborative chest radiograph showing new, persistent or progressive infiltrate, consolidation, cavitation, or pleural effusion. Clinical correlates included fever >38 °C or hypothermia <35.5 °C, blood leukocytosis >10,103/mm3 or leucopenia <3103/mm3. Purulent secretions with >25 neutrophils and <10 squamous epithelial cells per high power field or >5% cells with intracellular bacteria in Gram Stain along with growth corresponding to >105 colony forming units per ml (CFU/ml) of sample after 1 in 1000 dilution were correlated.
-
4.
CLABSI was diagnosed by positive paired blood cultures revealing the same pathogen concurrently from existing central-line and peripheral vein after 48 h of catheterization, with >105 CFU growth in central-line two hours faster than growth from peripheral sample, indicating source as central-line, in the absence of an infection at another site. Clinical correlates were similar to VAP. Coagulase negative Staphylococci growing within 48 h with positive clinical correlates were considered.
-
5.
CAUTI was diagnosed from urine sample of patients having catheter in situ or catheter removed within 7 days, either with growth of not more than two pathogens corresponding to 105 CFU/ml of urine, along with clinical correlates of fever >38 °C, urgency or suprapubic tenderness, or positive dipstick analysis for leukocyte esterase or nitrate, pyuria ≥10 leukocytes/ml, pathogens seen on Gram stain, clinical diagnosis, or initiation of appropriate therapy for UTI.
-
6.The data from clinical and laboratory surveillance was independently analyzed by a clinical microbiologist under the following parameters. Any patient admitted to ICU or exposed to devices for 24 h or part thereof was calculated for full day.
-
(a)Patient/bed/ICU days, calculated by multiplying number of patients with total admission days in ICU.
-
(b)Device-days, calculated by multiplying the use of devices (ventilator, central-line or urinary catheter) by days of use in all patients.
-
(c)Device utilization ratios, calculated by dividing the total number of device-days by the total patient/bed/ICU days.
-
(d)Pooled average length of stay for all patients and after acquiring VAP/CLABSI/CAUTI.
-
(e)Rates of DA-HAI, calculated by dividing episodes of VAP/CLABSI/CAUTI by respective device-days multiplied by 1000 to be expressed in terms of rates per 1000 device-days.
-
(f)Crude mortality rate for all patients and after VAP/CLABSI/CAUTI.
-
(g)Microbiological profile, frequency, resistograms with MICs for DA-HAI.
-
(h)Comparison of rates of VAP/CLABSI/CAUTI with benchmarking parameters, Indian and global ICUs.
-
(a)
-
7.
Environmental surveillance, conducted fortnightly after cleaning and decontamination procedures, included ICU air handling unit vent air exposure cultures; surface and fomite swab cultures from bed rails, bedside trolleys, intravenous fluid frames and bottles, monitoring devices, stethoscopes, switches, telephone receivers, computer peripherals, and door-handles. Disinfectant solutions were screened by in-use test.
-
8.
Screening of HCPs for Methicillin resistant S. aureus (MRSA) was conducted fortnightly through swabs from nostrils and fingertips. HCPs carrying MRSA were shifted out of ICU followed by MRSA eradication by 2% topical intranasal mupirocin twice a day and 2% chlorhexidine wash twice a day for seven days.
-
9.
Didactic lectures and demonstrations on infection control policies and practices including sterilization and disinfection, biomedical waste, standard precautions, hand hygiene, and occupational hazards were targeted fortnightly for nursing, ancillary, and housekeeping staff.
-
10.
Data was presented quarterly in the HICC meet with Heads/Officers of departments, centers, medical stores, and logistics in attendance. Suggestions were worked and reviewed in subsequent meetings. Quarterly returns were submitted.
Results
The study included a total of 2157 patients admitted to 14-bedded multidisciplinary ICU of a tertiary-care teaching hospital in Eastern India over a period of 24 months, of which 1188 patients stayed for more than 48 h (55.1%, monthly mean 49.5 ± standard deviation 13.89 with 95% Confidence Interval/CI 48.7–50.3%). The specialty distributions of patients included 19% neurology, 15% cardiology, 12% cardiology, 10% neurosurgery, and rest other specialties. Average nurse:patient ratio in the ICU was 1:2. A two-tier Infection Control Program (ICP) and patient safety protocol included restricted entry by biometric identification system, availability of bedside alcohol-based hand rubs, and bundles of care for prevention of VAP/CLABSI/CAUTI. Average monthly ICU occupancy was 89.88 ± 14 (95% CI 59.5–122.3) patients. The total patient/bed/ICU days were 8824, monthly average 367.7 ± 40.9 (95% CI 366.8–368.5). The total device utilization days were 14,676, monthly average 611.5 ± 116.3 (95% CI 609.6–613.4). Pooled average device utilization ratio was 1.66 (Ventilator 0.4, Central-Line 0.46, and Urinary Catheter 0.80) (Table 1).
Table 1.
Device-Associated Healthcare-Associated Infections (DA-HAI) in a multidisciplinary tertiary intensive care unit of a teaching hospital in eastern India.
| S No | Month/year | Pooled data |
VAP |
CLABSI |
CAUTI |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients admitted | Patients staying >48 h (%) | Patient/bed days | Device days | Device utilization ratio | Episodes of DA-HAI | Overall rate of DA-HAI | Ventilator days | Ventilator utilization ratio | Episodes of VAP (%) | Rate of VAP | Central line days | Central line utilization ratio | Episodes of CLABSI (%) | Rate of CLABSI | Urinary catheter days | Urinary catheter utilization ratio | Episodes of CAUTI (%) | Rate of CAUTI | ||
| 1 | June 14 | 64 | 44 (68.7) | 336 | 452 | 1.35 | 4 | 8.8 | 119 | 0.35 | 2 (50) | 16.8 | 114 | 0.34 | 0 | 0 | 219 | 0.65 | 2 (50) | 9.1 |
| 2 | July 14 | 87 | 54 (62.1) | 385 | 625 | 1.62 | 3 | 4.8 | 166 | 0.43 | 2 (66.7) | 12 | 115 | 0.30 | 1 (33.3) | 8.7 | 344 | 0.89 | 0 | 0 |
| 3 | August 14 | 85 | 57 (67.1) | 402 | 729 | 1.81 | 7 | 9.6 | 175 | 0.44 | 3 (42.8) | 17.1 | 203 | 0.50 | 2 (28.6) | 9.8 | 351 | 0.87 | 2 (28.6) | 5.7 |
| 4 | September 14 | 87 | 48 (55.2) | 374 | 637 | 1.70 | 5 | 7.8 | 127 | 0.34 | 2 (40) | 15.7 | 329 | 0.88 | 3 (60) | 9.1 | 181 | 0.48 | 0 | 0 |
| 5 | October 14 | 83 | 61 (73.5) | 407 | 727 | 1.79 | 3 | 4.1 | 106 | 0.26 | 2 (66.7) | 18.9 | 202 | 0.50 | 1 (33.3) | 4.9 | 419 | 1.03 | 0 | 0 |
| 6 | November 14 | 104 | 62 (59.6) | 430 | 707 | 1.64 | 4 | 5.7 | 144 | 0.33 | 3 (75) | 20.8 | 183 | 0.43 | 1 (25) | 5.4 | 380 | 0.88 | 0 | 0 |
| 7 | December 14 | 93 | 60 (64.5) | 426 | 686 | 1.61 | 3 | 4.4 | 150 | 0.35 | 2 (66.7) | 13.3 | 161 | 0.38 | 1 (33.3) | 6.2 | 375 | 0.88 | 0 | 0 |
| 8 | January 15 | 85 | 53 (62.4) | 423 | 753 | 1.78 | 5 | 6.6 | 181 | 0.43 | 3 (60) | 16.5 | 187 | 0.44 | 1 (20) | 5.3 | 385 | 0.91 | 1 (20) | 2.6 |
| 9 | February 15 | 84 | 37 (44) | 351 | 603 | 1.72 | 4 | 6.6 | 152 | 0.43 | 0 | 0 | 144 | 0.41 | 2 (50) | 13.9 | 307 | 0.87 | 2 (50) | 6.5 |
| 10 | March 15 | 104 | 34 (35.8) | 392 | 443 | 1.13 | 1 | 2.3 | 118 | 0.30 | 0 | 0 | 79 | 0.20 | 0 | 0 | 246 | 0.63 | 1 (100) | 4 |
| 11 | April 15 | 74 | 54 (73) | 374 | 587 | 1.57 | 4 | 6.8 | 103 | 0.28 | 2 (50) | 19.4 | 169 | 0.45 | 1 (25) | 5.9 | 315 | 0.84 | 1 (25) | 3.2 |
| 12 | May 15 | 75 | 38 (55.9) | 333 | 659 | 1.98 | 5 | 7.6 | 189 | 0.57 | 3 (60) | 15.8 | 168 | 0.50 | 1 (20) | 5.9 | 302 | 0.91 | 1 (20) | 3.3 |
| 13 | June 15 | 84 | 31 (36.9) | 300 | 471 | 1.57 | 6 | 12.7 | 101 | 0.34 | 3 (50) | 29.7 | 224 | 0.75 | 3 (50) | 13.4 | 146 | 0.49 | 0 | 0 |
| 14 | July 15 | 116 | 23 (19.8) | 359 | 551 | 1.53 | 5 | 9.1 | 142 | 0.40 | 3 (60) | 21.1 | 123 | 0.34 | 0 | 0 | 286 | 0.80 | 2 (40) | 7 |
| 15 | August 15 | 106 | 86 (81.1) | 415 | 705 | 1.70 | 7 | 9.9 | 151 | 0.36 | 3 (42.8) | 19.8 | 203 | 0.49 | 2 (28.5) | 9.8 | 351 | 0.85 | 2 (28.6) | 5.7 |
| 16 | September 15 | 88 | 60 (68.2) | 289 | 398 | 1.38 | 6 | 15.1 | 45 | 0.16 | 0 | 0 | 145 | 0.50 | 1 (16.7) | 6.9 | 208 | 0.72 | 5 (83.3) | 24 |
| 17 | October 15 | 105 | 56 (53.3) | 352 | 529 | 1.50 | 5 | 9.5 | 122 | 0.35 | 3 (60) | 24.6 | 142 | 0.40 | 1 (20) | 7 | 265 | 0.75 | 1 (20) | 3.8 |
| 18 | November 15 | 88 | 31 (35.2) | 285 | 430 | 1.51 | 2 | 4.7 | 93 | 0.33 | 0 | 0 | 109 | 0.38 | 1 (50) | 9.2 | 228 | 0.80 | 1 (50) | 4.4 |
| 19 | December 15 | 100 | 49 (49) | 356 | 662 | 1.86 | 5 | 7.6 | 163 | 0.46 | 2 (40) | 12.3 | 183 | 0.51 | 1 (20) | 5.5 | 316 | 0.89 | 2 (40) | 6.3 |
| 20 | January 16 | 108 | 65 (60.2) | 401 | 556 | 1.39 | 8 | 14.4 | 153 | 0.38 | 3 (37.5) | 19.6 | 139 | 0.35 | 3 (37.5) | 21.6 | 264 | 0.66 | 2 (25) | 7.6 |
| 21 | February 16 | 110 | 56 (50.9) | 361 | 642 | 1.78 | 8 | 12.5 | 152 | 0.42 | 3 (37.5) | 19.7 | 182 | 0.50 | 2 (25) | 11 | 308 | 0.85 | 3 (37.5) | 9.7 |
| 22 | March 16 | 82 | 42 (51.2) | 361 | 868 | 2.40 | 5 | 5.7 | 321 | 0.89 | 2 (40) | 6.2 | 218 | 0.60 | 2 (40) | 9.2 | 329 | 0.91 | 1 (20) | 3 |
| 23 | April 16 | 80 | 37 (46.2) | 348 | 654 | 1.88 | 5 | 7.6 | 179 | 0.51 | 2 (40) | 11.2 | 166 | 0.48 | 2 (40) | 12 | 309 | 0.89 | 1 (20) | 3.2 |
| 24 | May 16 | 65 | 50 (76.9) | 364 | 602 | 1.65 | 4 | 6.6 | 149 | 0.41 | 2 (50) | 13.4 | 143 | 0.39 | 2 (50) | 14 | 310 | 0.85 | 0 | 0 |
| Total | 2157 | 1188 | 8824 | 14,676 | – | 114 | – | 3501 | – | 50 | – | 4031 | – | 34 | – | 7144 | – | 29 | – | |
| Average | 89.88 | 49.50 | 367.67 | 611.50 | 1.66 | 4.75 | 7.94 | 145.88 | 0.40 | 2.08 | 14.35 | 167.96 | 0.46 | 1.42 | 8.13 | 297.67 | 0.80 | 1.32 | 4.55 | |
| SD | 14.03 | 13.89 | 40.88 | 116.31 | 0.25 | 1.73 | 3.26 | 49.98 | 0.13 | 1.06 | 8.06 | 50.49 | 0.14 | 0.88 | 4.91 | 67.81 | 0.14 | 1.21 | 5.15 | |
| 95% CI | 59.47 | 48.71 | 366.81 | 609.62 | 1.58 | 4.43 | 7.48 | 144.22 | 0.31 | 1.79 | 13.50 | 166.40 | 0.38 | 1.12 | 7.44 | 296.09 | 0.87 | 0.88 | 3.59 | |
| 120.28 | 50.29 | 368.52 | 613.38 | 1.74 | 5.07 | 8.40 | 147.53 | 0.48 | 2.38 | 15.20 | 169.52 | 0.54 | 1.71 | 8.81 | 299.24 | 0.74 | 1.76 | 5.52 | ||
VAP – Ventilator-Associated Pneumonia, CLABSI – Central-Line-Associated Blood Stream Infections, CAUTI – Catheter Related Urinary Tract Infection.
Total ventilator days, mean monthly ventilator days, and ventilator utilization ratio were 3501, 145.9 ± 49.9 (95% CI 144.2–147.5), and 0.4 ± 0.1 (95% CI 0.31–0.48). Total central-line days, mean monthly central-line days, and central-line utilization ratio were 4031, 167.9 ± 50.5 (95% CI 166.5–169.5), and 0.46 ± 0.14 (95% CI 0.38–0.54). Right subclavian was used for most patients, internal jugular was avoided in neurosurgery patients, and femoral catheterization was utilized for rapid access. Total urinary-catheter days, mean monthly urinary-catheter days, and urinary-catheter utilization ratio were 7144, 297.7 ± 67.8 (95% CI 296.1–299.2), and 0.80 ± 0.14 (95% CI 0.74–0.87).
The total episodes of DA-HAI, mean monthly episodes, and rate were 114, 4.7 ± 1.7 (95% CI 4.4–5.1), and 7.94 ± 3.3 (95% CI 7.5–8.4). Total episodes of VAP, mean monthly episodes, and rate were 50 (43.8% of DA-HAI), 2.1 ± 1.1 (95% CI 1.8–2.4), and 14.35 ± 8.1/1000 ventilator days (95% CI 13.5–15.2). Total episodes of CLABSI, mean monthly episodes, and rate were 34 (29.8% of DA-HAI), 1.4 ± 0.9 (95% CI 1.1–1.7), and 8.1 ± 4.9/1000 central-line days (95% CI 7.4–8.8). Total episodes of CAUTI, mean monthly episodes, and rate were 29 (25.4% of DA-HAI), 1.32 ± 1.2 (95% CI 0.88–1.76), and 4.55 ± 5.2/1000 urinary catheter days (95% CI 3.6–5.5) (Table 1).
Pooled unadjusted average length of stay (LOS) of all patients was 4.09 ± 2.9 (95% CI 3.1–6.2) days. Pooled average LOS after acquiring DA-HAI, VAP, CLABSI, and CAUTI were 6.12, 6.95, 5.68, and 5.73 days. Crude unadjusted mortality in ICU was 33.5%. Crude unadjusted mortality in patients with DA-HAI, VAP, CLABSI, and CAUTI was 4.9%, 30%, 1.6%, and 0.3% of total deaths in ICU.
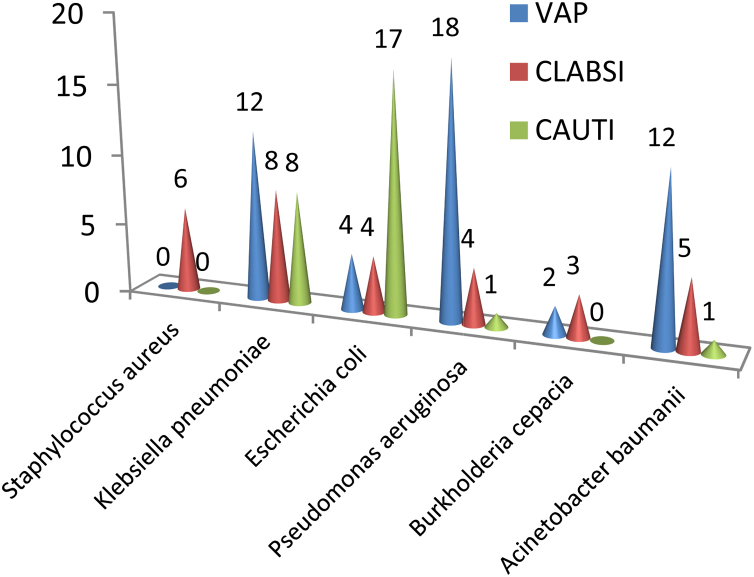
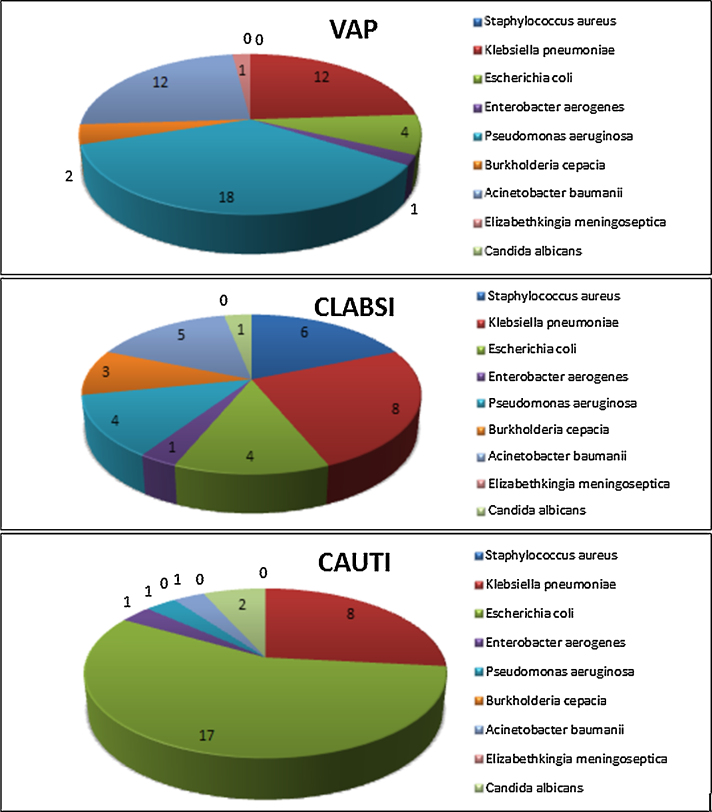
Most common pathogens isolated from DA-HAI patients were Klebsiella pneumoniae (24.6%), Escherichia coli (21.9%) and Pseudomonas aeruginosa (20.2%). Acinetobacter baumanii comprised 15.8% of all infections (Fig. 1, Fig. 2). All Acinetobacter baumanii, > 80% Klebsiella pneumoniae and Escherichia coli, and >70% Pseudomonas aeruginosa were susceptible only to colistin and tigecycline. Higher MIC values for tigecycline were seen in some Klebsiella pneumoniae isolates. Enterobacter aerogenes was susceptible to ciprofloxacin, colistin and tigecycline. One Pseudomonas aeruginosa isolate was panresistant. Burkholderia cepacia and Elizabethkingia meningoseptica exhibited susceptibility only to cotrimoxazole. All Staphylococcus aureus isolates were MRSA, susceptible only to vancomycin, linezolid and rifampicin. Candida albicans were resistant only to fluconazole (Table 2).
Fig. 1.
Organism profile of DA-HAI (VAP, CLABSI, and CAUTI) from ICU of a Teaching Hospital in Eastern India.
Fig. 2.
Organism distribution of DA-HAI (VAP, CLABSI, and CAUTI) from ICU of a Teaching Hospital in Eastern India.
Table 2.
Microorganism profile and resistogram from Device-Associated Healthcare-Associated Infections (DA-HAI) from ICU of a teaching hospital in eastern India.
| Pathogens (n = 114)/DA-HAI/antimicrobials |
Klebsiella pneumoniae (n = 28) (24.6%) |
Escherichia coli (n = 25) (21.9%) |
Enterobacter aerogenes (n = 3) (2.6%) |
Pseudomonas aeruginosa (n = 23) (20.2%) |
Acinetobacter baumanii (n = 18) (15.8%) |
Burkholderia cepacia (n = 5) (4.4%) |
Elizabethkingia meningoseptica (n = 1) (0.9%) |
Staphylococcus aureus (n = 6) (5.3%) |
Candida albicans (n = 3) (2.6%) |
|---|---|---|---|---|---|---|---|---|---|
| VAP | 12 | 4 | 1 | 18 | 12 | 2 | 1 | 0 | 0 |
| CLABSI | 8 | 4 | 1 | 4 | 5 | 3 | 0 | 6 | 1 |
| CAUTI | 8 | 17 | 1 | 1 | 1 | 0 | 0 | 0 | 2 |
| Resistance | R (%) | MIC | R (%) | MIC | R (%) | MIC | R (%) | MIC | R (%) | MIC | R (%) | MIC | R (%) | MIC | R (%) | MIC | R (%) | MIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coamoxiclav | 82.1 | ≥32 | 80 | ≥32 | 100 | ≥32 | 82.6 | ≥32 | 100 | ≥32 | 100 | ≥32 | 100 | ≥32 | – | – | – | – |
| Ciprofloxacin | 92.8 | ≥4 | 96 | ≥4 | 0 | ≥4 | 86.9 | ≥4 | 100 | ≥4 | 100 | ≥4 | 100 | ≥4 | – | – | – | – |
| Ceftriaxone/oxacillin | 82.1 | ≥64 | 80 | ≥64 | 100 | ≥64 | 82.6 | ≥64 | 100 | ≥64 | 100 | ≥64 | 100 | ≥64 | 100 | ≥4 | – | – |
| Cefoperazone/subactam | 82.1 | ≥64 | 80 | ≥64 | 100 | ≥64 | 82.6 | ≥64 | 100 | ≥64 | 100 | ≥64 | 100 | ≥64 | – | – | – | – |
| Amikacin | 82.1 | ≥64 | 60 | ≥64 | 100 | ≥64 | 69.5 | ≥64 | 100 | ≥64 | 100 | ≥64 | 100 | ≥64 | – | – | – | – |
| Imipenem | 82.1 | ≥16 | 52 | ≥16 | 100 | 4 | 69.5 | 2 | 100 | ≥16 | 100 | ≥16 | 100 | ≥16 | – | – | – | – |
| Meropenem | 82.1 | ≥16 | 52 | ≥16 | 100 | 8 | 69.5 | 8 | 100 | ≥16 | 100 | 4 | 100 | ≥16 | – | – | – | – |
| Piperacillin-tazobactam | 82.1 | ≥128 | 68 | ≥128 | 100 | ≥128 | 69.5 | ≥128 | 100 | ≥128 | 100 | ≥128 | 100 | ≥128 | – | – | – | – |
| Cotrimoxazole | 92.8 | ≥320 | 100 | ≥320 | 100 | ≥320 | 100 | ≥320 | 100 | ≥320 | 0 | ≤20 | 0 | 40 | 100 | ≥320 | – | – |
| Colistin | 0 | ≤0.5 | 0 | ≤0.5 | 0 | ≤0.5 | 4.3 | ≤0.5–≥8 | 0 | ≤0.5 | 100 | ≤0.5 | 100 | ≥16 | – | – | – | – |
| Tigecycline | 0 | 0.5–2 | 0 | ≤0.5 | 0 | ≤0.5 | 4.3 | 0.5–2 | 0 | 2 | 100 | ≤0.5 | 0 | ≥8 | – | – | – | – |
| Vancomycin | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 1 | – | – |
| Linezolid | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 2 | – | – |
| Rifampicin | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | ≤0.03 | – | – |
| Fluconazole | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 66.7 | ≥2 |
| Voriconazole | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | ≤0.12 |
| Amphotericin B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 0.5 |
| Flucytosine | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | ≤1 |
| Caspofungin | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | ≤0.25 |
| Micafungin | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | ≤0.06 |
R – resistance, MIC – Mean Minimal Inhibitory Concentration in μg/ml for resistant isolates, n represents cumulative in the respective category.
Environmental surveillance revealed coagulase negative staphylococci, aerobic spore bearers, and K. pneumoniae susceptible to all antimicrobials. Average prevalence of MRSA was 31.67% among detected Staphylococci from HCPs. MRSA eradication was confirmed through subsequent repeat cultures. The surveillance parameters and rates of DA-HAI have been compared with benchmarking data, ICUs in India, and other countries (Table 3).
Table 3.
Comparison of rates of DA-HAI (VAP, CLABSI, and CAUTI) from ICU of a teaching hospital in eastern India, with India and the World.
| S. No. | Country/region | Year(s) of surveillance | No of hospitals/ICUs | No of patients | No of patient/bed/ICU days | VAP | CLABSI | CAUTI |
|---|---|---|---|---|---|---|---|---|
| Benchmarking parameters | ||||||||
| 1 | Present study | 2014–16 | 1/1 | 2157 | 8824 | 14.4 | 8.1 | 4.5 |
| 2 | INICC Rates6 | 2004–09 | 36/422 | 313,008 | 2,194,897 | 15.8 | 6.8 | 6.3 |
| 3 | INICC Rates7 | 2007–12 | 43/503 | 605,310 | 3,338,396 | 16.8 | 4.9 | 5.5 |
| 4 | US CDC-NHSN Rates8 | 2013 | 4567 | – | 18,910,558 | 0.6 | 0.5 | 0.7 |
| 5 | US CDC-NHSN Rates9 | 2012 | 4444 | – | 19,177,288 | 1.6 | 1.3 | 2.6 |
| Comparison within India | ||||||||
| 6 | AIIMS, Delhi10 | – | 1 | – | – | 31.4 | 3.4 | 11.3 |
| 7 | Chandigarh11 | 2010–11 | 1/2 | 679 | – | 6.0 | 13.9 | 9.1 |
| 8 | Pune2 | 2009–10 | 1/1 | 293 | – | 32 | 16 | 9 |
| 9 | 20 Indian cities12 | 2004–13 | 40/40 | 236,700 | 970,713 | 9.4 | 5.1 | 2.1 |
| Comparison with lower middle income countries excluding India | ||||||||
| 10 | Eight countries4 | 2002–05 | 46/55 | 21,069 | 137,740 | 24.1 | 12.5 | 8.9 |
| 11 | Turkey13 | 2003–12 | 29/63 | 94,498 | 647,316 | 21.4 | 11.1 | 7.5 |
| 12 | China14 | 2004–09 | 70/398 | 391,527 | 3,245,244 | 20.8 | 3.1 | 6.4 |
| 13 | Mongolia15 | 2013–15 | 3/3 | 467 | 2133 | 43.7 | 19.7 | 15.7 |
| 14 | Brazil16 | 2003–06 | 3/5 | 1031 | 10,293 | 20.9 | 9.1 | 9.6 |
| 15 | Philippines17 | 2005–09 | 9/9 | 4952 | 40,733 | 16.7 | 4.6 | 4.2 |
| Comparison with upper middle income countries | ||||||||
| 16 | Iran18 | 2014 | 1/1 | 2584 | 16,796 | 7.9 | 5.8 | 9.0 |
| 17 | Mexico19 | 2005 | 4/5 | 1055 | – | 21.8 | 23.1 | 13.4 |
| Comparison with high income countries | ||||||||
| 18 | Argentina20 | 2003 | 6/6 | – | – | 46.3 | 30.3 | 18.5 |
| 19 | Poland21 | 2007–10 | 1/1 | 847 | 9386 | 18.2 | 4.0 | 4.8 |
| 20 | Saudi Arabia22 | 2004–11 | 1/1 | – | – | 4.5 | 10 | 8.2 |
| 21 | Kuwait23 | 2013–15 | 7/7 | 3732 | 21,611 | 4.0 | 3.5 | 3.3 |
| 22 | Korea24 | 2012 | 162/162 | – | – | 1.6 | 2.6 | 1.6 |
Discussion
The occurrence of HAI among patients and HCPs in ICU is a healthcare hazard as it may lead to cross-infections and further transmission to lower dependency units, other healthcare facilities, and the community through patient transfers and discharge. The source of HAI can be patients, visitors, HCP, environmental reservoirs, and biomedical waste, which can be traced by ongoing surveillance programs and transmission reduced or stopped by appropriate intervention.
Surveillance programs on DA-HAI started with the US CDC-NHSN collaboration in 1988, now comprising 4567 hospitals inclusive of 34 US military hospitals.5 The results of the surveillance were utilized for well-coordinated ICPs focused on DA-HAI, which brought forth reduction in incidence of DA-HAI by 30%, reduction in proportionate morbidity, mortality, and healthcare costs. The International Nosocomial Infection Control Consortium (INICC), started in 2008 in Argentina, coordinates a network of 250 ICUs from 38 countries in South America, Asia, Africa, and Europe, for surveillance of DA-HAI.6, 7 While INICC includes middle-income country ICUs, most of the work on DA-HAI has been carried out in high-income countries.6, 7
Diagnosis of DA-HAI requires standardized protocols corroborating clinical and laboratory parameters with baseline and intuitive clinical correlates. Muted clinical response in critically ill patients can delay requisition of cultures from ICU. The recently approved CDC definitions on tiered ventilator-associated events comprising ventilator-associated condition, infection-related ventilator-associated condition, and possible and probable VAP, are likely to objectify surveillance of VAP through extensive monitoring of FiO2 and PEEP; however, it is not intended for clinical management of patients. Standardization of definitions and criteria from US CDC-NHSN have streamlined diagnosis of DA-HAI along with benchmarking of ICU data on DA-HAI from both CDC-NHSN and INICC, facilitating comparison between hospitals and countries.
The current analysis has been classified as per latest World Bank classification of countries into low-, lower middle (India), upper middle and high-income countries. The rates of VAP/CLABSI/CAUTI are comparable to INICC benchmarks although much higher than US CDC-NHSN rates.6, 7, 8, 9 There is a great variation in the rates reported in India and the world. The range of VAP, CLABSI, and CAUTI rates in India has been 6–32, 3.4–16, and 2.1–11.3 per 1000 device-days respectively.2, 10, 11, 12 Among the lower middle income countries, the range of VAP, CLABSI, and CAUTI rates was 8.1–43.7, 3.1–19.7, and 4.1–20.3.4, 13, 14, 15, 16, 17 Even upper middle and high income countries have reported very high rates of 46.3, 30.3, and 18.5 for VAP, CLABSI, and CAUTI respectively.18, 19, 20, 21, 22, 23, 24 However, countries such as Saudi Arabia and Kuwait, which are developing high-income countries, have low rates of DA-HAI, and the rates in Korea are closer to CDC-NHSN rates.22, 23, 24 The LOS after DA-HAI and associated mortality vary due to patient transfer and discharge. While mortality associated with VAP, CLABSI, and CAUTI varies from 15 to 50%, 12 to 25%, and 7 to 35%, crude mortality in ICU is attributable to (a) Admissions of patients having undergone extensive traumatic or pathophysiological compromise who get referred through various echelons of care; (b) Geriatric clientele with multiple comorbidities in decompensated state; (c) Deteriorating patients of other wards being transferred to ICU for critical care, who could not be revived from cardiac arrest prior to demise.4, 6, 25
Most common pathogens causing HAI have been abbreviated as ESKAPE pathogens which include Enterococcus faecium, S. aureus, K. pneumoniae, A. baumanii, P. aeruginosa, Enterobacter species, and E. coli. Serratia, Proteus, Citrobacter, Enterobacter, Morganella, Hemophilus influenza, coagulase negative Staphylococci, Providencia stuartii, and Elizabethkingia meningoseptica are also implicated in HAI, along with Candida, and Trichosporon which are common in patients on prolonged antibacterial therapy.3, 26, 27 The magnitude of MDR including panresistance, as reported by this study as well as various studies, has reached alarming proportions and acts as a caveat to the conduct of ICP.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Multiple coexistent resistance phenotypes such as Modification of PBP (mecA) and Macrolide/Lincosamide/Streptogramin B resistance (MLSB) among Gram-positive bacteria as well as Extended Spectrum Beta Lactamases (ESBL), Metallo Beta Lactamases (MBL), AmpC, and Carbapenemase-resistant Enterobacteriaceae (CRE) in Gram-negative bacteria reduce treatment options to reserve antimicrobials which are expensive and further the development of antimicrobial resistance due to exposure and increased selection pressure.3, 27 Environmental surveillance revealing K. pneumoniae susceptible to all antimicrobials could not be correlated with any clinical isolates; however, such ICU flora have the potential to develop multidrug resistance.
The control of HAI mandates a strict infection control policy, ongoing surveillance of infections, monitoring of preventive practices, and overall administrative control, all of which are executed under the ambit of Hospital Infection Control Committee (HICC) in our hospital. The Head of the hospital leads a team steered by a clinical microbiologist keeping the hospital management, clinicians, ICU administrators, hospital epidemiologist, and nursing staff in a coherent team effectively committed to ICP through sterilization and disinfection procedures, two-tier approach, barrier nursing, hand hygiene, proper disposal of biomedical waste, and interactive teaching sessions. However, there is a scope of improving adherence to guidelines and bundles of care for devices in a multidisciplinary ICU. The study is limited by lack of assessment of risk factors, severity of illness, and complications such as sepsis. There are likely variations in efficiency of surveillance temporally due to (a) Infrastructural and logistic limitations such as increasing patient commitments in ICU, change of staff, rapid discharge/deaths/transfers; (b) Patient limitations and morbidity restricting early discharge from ICU, weaning-off from devices, deficiencies in patient, and attendant education; (c) Research limitations such as interpersonal variation in requesting intuitive cultures from ICU; (d) Microbiological limitations of timing of sample collection, detection of small colony variants, biofilms, single colony on plate, interpretation of semiquantitative cultures in patients exposed to antimicrobials before admission to ICU, sensitivity of culture-based methods, and heteroresistance.
The concept of HAI has wider ramifications. DA-HAI is pertinent not only in ICU settings, but also other managed care facilities including home-based hospital care (HBHC), ambulatory care, critical-access hospitals, disease-specific care, long-term care, office-based surgery, and behavioral healthcare. While DA-HAI in ICU focuses on VAP, CLABSI, and CAUTI, implantable cardiac, orthopedic, intrauterine devices, and external devices such as endoscopes and humidifiers also cause HAI. Non-device-associated HAI are also increasingly being encountered.28
Surveillance methodology for DA-HAI is evolving to standardized active real-time electronic surveillance through cross-platform infection control software for validation and quality control of hospital epidemiology database including emerging pathogens and resistograms. Automated camera-aided process surveillance of hand hygiene opportunities, which forms the most effective strategies to control HAI as well as emerging antimicrobial resistance, is mandated. While resistograms form a guideline for formulating guided prescription policies curbing empiricism and facilitating antimicrobial stewardship efforts, molecular epidemiology studies help trace clonal expansion, and antimicrobial impregnated catheters and antimicrobial lock techniques improve patient safety.3
The National Patient Safety Goals purported by WHO World Alliance on Patient Safety in 2009 emphasize role of HAI in all unanticipated death or major permanent loss of function. There is also an emphasis on implementing evidence-based practices to prevent HAI due to MDR pathogens in acute care hospitals along with patients’ active involvement in their own care as a patient safety strategy. HAI have been categorized as ‘never events’ by the National Quality Forum, with farfetched effects toward mandatory reporting of HAI, creation of performance benchmarks, notification to patients and patient safety organizations, and treatment cost waivers coupled with implementing disincentives in insurance payments to hospitals. However, there is a Policy-Practice Gap (PPG) possibly due to Knowledge, Attitude, Practices, and Behavior (KAPB) gap between policy makers and healthcare providers as infections are inevitable despite the best of practices owing to unpredictable infection dynamics, and HAI can never be classified as ‘never events’.29
While Indian ICUs have been networked under INICC, the Armed Forces Medical Services are traversing through an opportunistic model which can be replicated by standardizing surveillance parameters in networked Armed Forces hospitals to enhance patient safety parameters.
The future of infection control beckons point of care diagnostic technologies, zero-error prevention strategies, and surveillance with modern mathematical and statistical modeling including logistic regression, receiver operating curves, and Artificial Neural Networks.30 Nevertheless, the sociotechnical context and macroergonomics of patient-centered care remain important elements in infection control and patient safety.
Conclusion
The rates of VAP, CLABSI, and CAUTI of our center are comparable to average unadjusted rates in India, lower middle, upper middle and high-income countries. Rates are, however, much higher than US CDC-NHSN and Korea. Patients and HCPs form important reservoirs of infection. One-third HCPs may harbor MRSA which can be eradicated by topical mupirocin.
Resolute conviction and sustained momentum in Infection Control Initiatives targeted at ongoing surveillance and suitable interventions is an essential step toward patient safety. Reiteration of policy interventions to focus on patient education, device use, and antimicrobial stewardship; human resource interventions to focus on behavioral modification for hand hygiene and biomedical waste disposal; and procedural interventions regarding bundles of care, barrier nursing, and two-tier approach in infection control need to be emphasized time and again.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The authors thank the Hospital Infection Control Team for their efforts toward Infection Control.
References
- 1.Rosenthal V.D., Maki D.G., Graves N. The International Nosocomial Infection Control Consortium (INICC): goals and objectives, description of surveillance methods, and operational activities. Am J Infect Control. 2008;36(9):e1–e12. doi: 10.1016/j.ajic.2008.06.003. http://www.ncbi.nlm.nih.gov/pubmed/18992646 [DOI] [PubMed] [Google Scholar]
- 2.Singh S., Chaturvedi R., Garg S.M., Datta R., Kumar A. Incidence of healthcare associated infection in the surgical intensive care unit of a tertiary service hospital. Med J Armed Forces India. 2013;69(2):124–129. doi: 10.1016/j.mjafi.2012.08.028. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3862707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan I.D., Sahni A.K., Bharadwaj R., Lall M., Jindal A.K., Sashindran V.K. Emerging organisms in a tertiary healthcare set up. Med J Armed Forces India. 2014;70(2):120–128. doi: 10.1016/j.mjafi.2013.09.005. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC24843199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal V.D., Maki D.G., Salomao R. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:582–591. doi: 10.7326/0003-4819-145-8-200610170-00007. http://www.ncbi.nlm.nih.gov/pubmed/26607300 [DOI] [PubMed] [Google Scholar]
- 5.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. http://www.ncbi.nlm.nih.gov/pubmed/18538699 [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal V.D., Bijie H., Maki D.G. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control. 2012;40:396–407. doi: 10.1016/j.ajic.2011.05.020. http://www.ncbi.nlm.nih.gov/pubmed/21908073 [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal V.D., Maki D.G., Mehta Y. International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module. Am J Infect Control. 2014;42:942–956. doi: 10.1016/j.ajic.2014.05.029. http://www.ncbi.nlm.nih.gov/pubmed/25179325 [DOI] [PubMed] [Google Scholar]
- 8.Dudeck M.A., Edwards J.R., Allen-Bridson K. National Healthcare Safety Network (NHSN) Report, data summary for 2013, device-associated module. Am J Infect Control. 2015;43(3):206–221. doi: 10.1016/j.ajic.2014.11.014. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4653815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudeck M.A., Weiner L.M., Allen-Bridson K. National Healthcare Safety Network (NHSN) Report, data summary for 2012, device-associated module. Am J Infect Control. 2013;41(12):1148–1166. doi: 10.1016/j.ajic.2013.09.002. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4629786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habibi S., Wig N., Agarwal S. Epidemiology of nosocomial infections in medicine intensive care unit at a tertiary care hospital in northern India. Trop Doct. 2008;38:233–235. doi: 10.1258/td.2008.070395. http://www.ncbi.nlm.nih.gov/pubmed/18820195 [DOI] [PubMed] [Google Scholar]
- 11.Datta P., Rani H., Chauhan R. Health-care-associated infections: risk factors and epidemiology from an intensive care unit in Northern India. Indian J Anaesth. 2014;58(January–February (1)):30–35. doi: 10.4103/0019-5049.126785. http://www.ncbi.nlm.nih.gov/pubmed/3968648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta Y., Jaggi N., Rosenthal V.D. Device-associated infection rates in 20 cities of India. Data summary for 2004–2013: findings of the International Nosocomial Infection Control Consortium. Infect Control Hosp Epidemiol. 2016;37(February (2)):172–181. doi: 10.1017/ice.2015.276. http://www.ncbi.nlm.nih.gov/pubmed/26607300 [DOI] [PubMed] [Google Scholar]
- 13.Leblebicioglu H., Rosenthal V.D., Arikan O.A. Device-associated hospital-acquired infection rates in Turkish intensive care units. Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;65(March (3)):251–257. doi: 10.1016/j.jhin.2006.10.012. http://www.ncbi.nlm.nih.gov/pubmed/17257710 [DOI] [PubMed] [Google Scholar]
- 14.Tao L., Hu B., Rosenthal V.D., Gao X., He L. Device-associated infection rates in 398 intensive care units in Shanghai, China: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2011;15(November (11)):e774–e780. doi: 10.1016/j.ijid.2011.06.009. http://www.ncbi.nlm.nih.gov/pubmed/21846591 [DOI] [PubMed] [Google Scholar]
- 15.Ider B.E., Baatar O., Rosenthal V.D. Multicenter study of device-associated infection rates in hospitals of Mongolia: findings of the International Nosocomial Infection Control Consortium (INICC) Am J Infect Control. 2015 doi: 10.1016/j.ajic.2015.10.010. http://www.ncbi.nlm.nih.gov/pubmed/26684368 [DOI] [PubMed] [Google Scholar]
- 16.Salomao R., Rosenthal V.D., Grimberg G. Device-associated infection rates in intensive care units of Brazilian hospitals: findings of the International Nosocomial Infection Control Consortium. Rev Panam Salud Publica. 2008;24(September (3)):195–202. doi: 10.1590/s1020-49892008000900006. http://www.ncbi.nlm.nih.gov/pubmed/19115547 [DOI] [PubMed] [Google Scholar]
- 17.Navoa-Ng J.A., Berba R., Galapia Y.A. Device-associated infections rates in adult, pediatric, and neonatal intensive care units of hospitals in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. Am J Infect Control. 2011;39(September (7)):548–554. doi: 10.1016/j.ajic.2010.10.018. http://www.ncbi.nlm.nih.gov/pubmed/21616564 [DOI] [PubMed] [Google Scholar]
- 18.Jahani-Sherafat S., Rasaghi M., Rosenthal V.D. Device-associated infection rates and bacterial resistance in six academic teaching hospitals of Iran: findings from the International Nocosomial Infection Control Consortium (INICC) J Infect Public Health. 2015;8(November–December (6)):553–561. doi: 10.1016/j.jiph.2015.04.028. http://www.ncbi.nlm.nih.gov/pubmed/26027477 [DOI] [PubMed] [Google Scholar]
- 19.Ramirez Barba E.J., Rosenthal V.D., Higuera F. Device-associated nosocomial infection rates in intensive care units in four Mexican public hospitals. Am J Infect Control. 2006;34(May (4)):244–247. doi: 10.1016/j.ajic.2005.05.024. http://www.ncbi.nlm.nih.gov/pubmed/16679185 [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal V.D., Guzmán S., Crnich C. Device-associated nosocomial infection rates in intensive care units of Argentina. Infect Control Hosp Epidemiol. 2004;25(March (3)):251–255. doi: 10.1086/502386. http://www.ncbi.nlm.nih.gov/pubmed/15061418 [DOI] [PubMed] [Google Scholar]
- 21.Kubler A., Duszynska W., Rosenthal V.D. Device-associated infection rates and extra length of stay in an intensive care unit of a university hospital in Wroclaw, Poland: International Nosocomial Infection Control Consortium's (INICC) findings. J Crit Care. 2012;27(February (1)) doi: 10.1016/j.jcrc.2011.05.018. 105.e5–10. http://www.ncbi.nlm.nih.gov/pubmed/21737244. [DOI] [PubMed] [Google Scholar]
- 22.Al-Tawfiq J.A., Amalraj A., Memish Z.A. Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004–2011. Int J Infect Dis. 2013;17(December (12)):e1207–e1211. doi: 10.1016/j.ijid.2013.06.015. http://www.ncbi.nlm.nih.gov/pubmed/23932872 [DOI] [PubMed] [Google Scholar]
- 23.Al-Mousa H.H., Omar A.A., Rosenthal V.D. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: International Nosocomial Infection Consortium findings. Am J Infect Control. 2016 doi: 10.1016/j.ajic.2015.10.031. http://www.ncbi.nlm.nih.gov/pubmed/26775929 pii:S0196-6553(15)01117-7. [DOI] [PubMed] [Google Scholar]
- 24.Choi J.Y., Kwak Y.G., Yoo H. Trends in the incidence rate of device-associated infections in intensive care units after the establishment of the Korean Nosocomial Infections Surveillance System (KONIS) J Hosp Infect. 2015;91(September (1)):28–34. doi: 10.1016/j.jhin.2015.06.002. http://www.ncbi.nlm.nih.gov/pubmed/26149593 [DOI] [PubMed] [Google Scholar]
- 25.Khan I.D., Basu A., Trivedi S. Battlefield, bullets and bugs: the vicious circle in gunshots. J Basic Clin Med. 2016;5(1):11–12. www.sspublications.org/index.php/JBCM/article/view/63 [Google Scholar]
- 26.Khan I.D., Lall M., Sen S., Ninawe S.M., Chandola P. Multiresistant Elizabethkingia meningoseptica infections in tertiary care. Med J Armed Forces India. 2014;71(3):66–67. doi: 10.1016/j.mjafi.2014.02.002. http://www.ncbi.nlm.nih.gov/pubmed/26288498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan I.D., Sahni A.K., Basu A., Haleem S. Trichosporon asahii UTI in immunocompetent patients. Med J Armed Forces India. 2014;71(4):373–376. doi: 10.1016/j.mjafi.2014.08.013. http://www.ncbi.nlm.nih.gov/pubmed/26663967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiBiase L.M., Weber D.J., Sickbert-Bennett E.E., Anderson D.J., Rutala W.A. The growing importance of non-device-associated healthcare-associated infections: a relative proportion and incidence study at an Academic Medical Center, 2008–2012. Infect Control Hosp Epidemiol. 2014;35(2):200–202. doi: 10.1086/674847. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3977707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Leapfrog Group. Leapfrog Group Position Statement on Never Events. http://www.leapfroggroup.org/Hospitals/SurveyInfo/never_events. Accessed 15.03.16.
- 30.Chang Y.-J., Yeh M.-L., Li Y.-C. Predicting hospital-acquired infections by scoring system with simple parameters. PLoS ONE. 2011;6(8):e23137. doi: 10.1371/journal.pone.0023137. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3160843 [DOI] [PMC free article] [PubMed] [Google Scholar]