Abstract
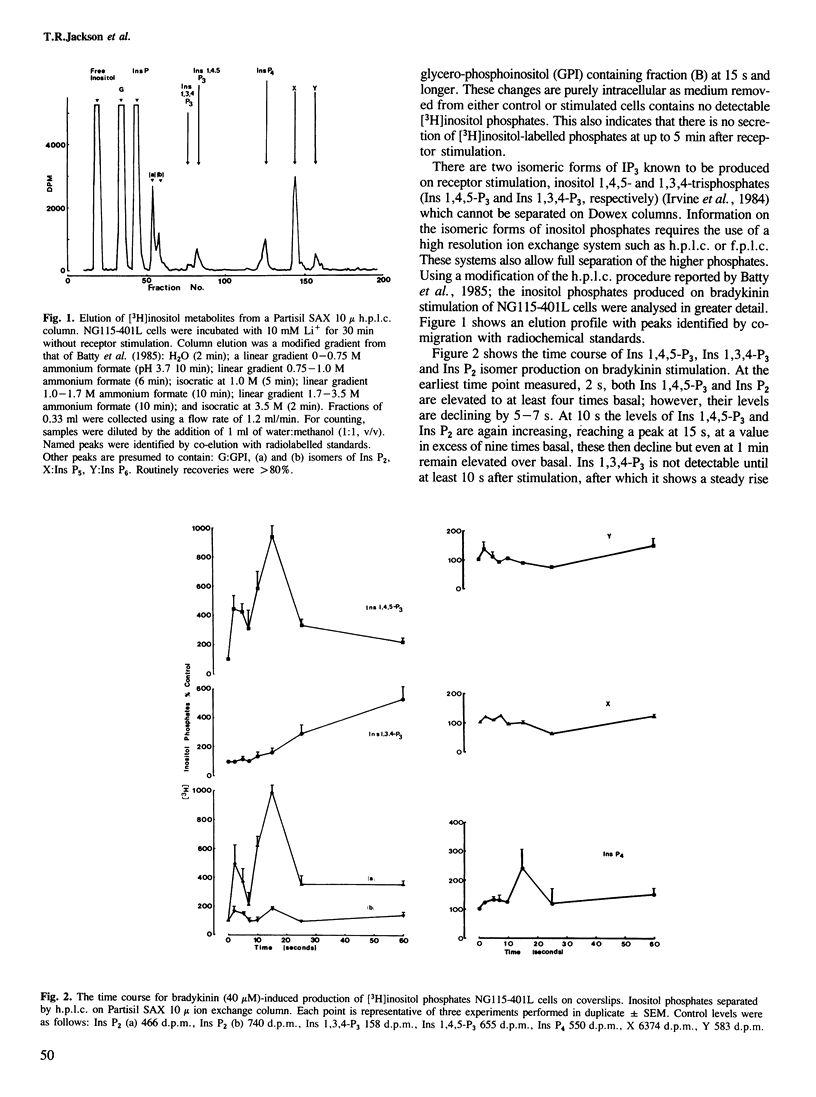
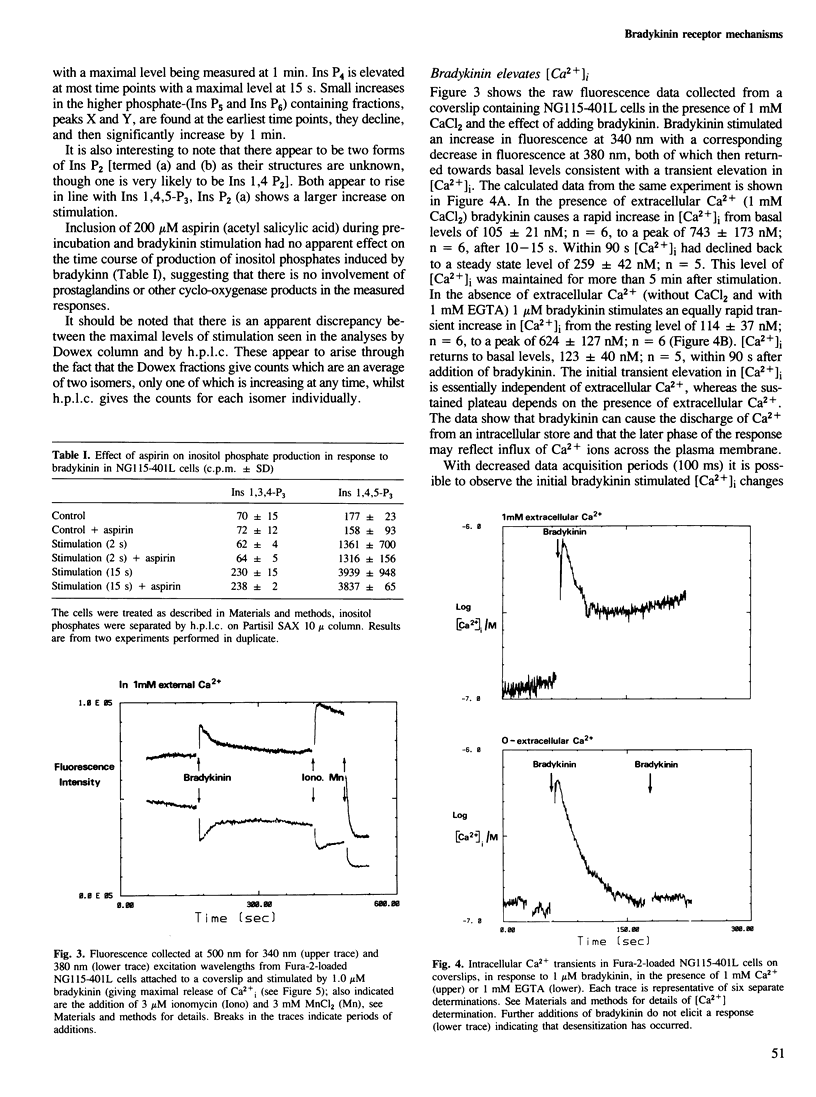
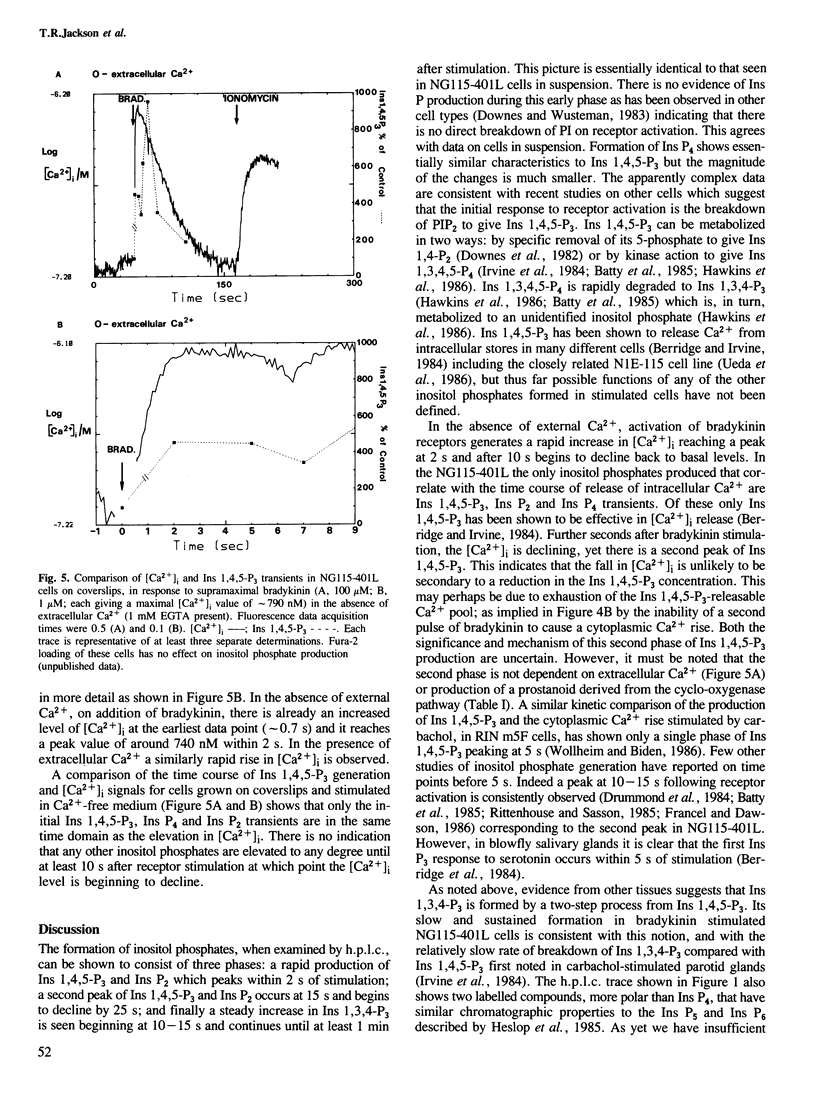
The addition of bradykinin to NG115-401L cells grown on coverslips results in the generation of rapid transient increases in intracellular [Ca2+] and inositol phosphates. Changes in intracellular Ca2+, measured using the fluorescent indicator dye Fura-2, show two components; an initial rapid peak in [Ca2+]i which is essentially independent of extracellular Ca2+, and a sustained plateau dependent on the presence of extracellular Ca2+. Analysis of bradykinin stimulated production of [3H]inositol phosphates, by h.p.l.c., shows a rapid biphasic production of inositol 1,4,5-trisphosphate, inositol tetrakisphosphate and inositol bisphosphates, followed by a sustained rise in inositol 1,3,4-trisphosphate production. Quantitative measurements have indicated the presence of other, more polar, [3H]inositol-labelled metabolites which do not show major changes on bradykinin stimulation. The initial phase of inositol phosphate production parallels the rapid transient increase in intracellular [Ca2+], however, the second phase of inositol phosphate production occurs when intracellular [Ca2+] is declining and implies a complex series of regulatory events following receptor stimulation. Similar time courses of inositol 1,4,5-trisphosphate and Ca2+ signals provides supporting evidence that inositol 1,4,5-trisphosphate is the second messenger coupling bradykinin receptor stimulation to release of Ca2+ from intracellular stores.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Buchan P. B., Heslop J. P. Relationship of polyphosphoinositide metabolism to the hormonal activation of the inset salivary gland by 5-hydroxytryptamine. Mol Cell Endocrinol. 1984 Jun;36(1-2):37–42. doi: 10.1016/0303-7207(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Francel P. C., Dawson G. Bradykinin induces a rapid release of inositol trisphosphate from a neuroblastoma hybrid cell line NCB-20 that is not antagonized by enkephalin. Biochem Biophys Res Commun. 1986 Mar 13;135(2):507–514. doi: 10.1016/0006-291x(86)90023-9. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Sanchez A., Rink T. J. Stimulus-response coupling in human platelets. Changes evoked by platelet-activating factor in cytoplasmic free calcium monitored with the fluorescent calcium indicator quin2. Biochem J. 1984 Mar 15;218(3):819–827. doi: 10.1042/bj2180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart M., Landis D. M., Reese T. S. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol. 1976 Aug;70(2 Pt 1):338–347. doi: 10.1083/jcb.70.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttinger D., Hernandez D. E., Nemeroff C. B., Prange A. J., Jr Peptides and nociception. Int Rev Neurobiol. 1984;25:185–241. doi: 10.1016/s0074-7742(08)60680-7. [DOI] [PubMed] [Google Scholar]
- Ogura A., Amano T. Transmitter responsiveness in two newly isolated clones of neuroblastoma X glioma hybrid. Brain Res. 1983 Jan 10;258(2):243–249. doi: 10.1016/0006-8993(83)91147-2. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Di Virgilio F., Vicentini L. M., Meldolesi J. Activation of muscarinic receptors in PC12 cells. Stimulation of Ca2+ influx and redistribution. Biochem J. 1986 Mar 15;234(3):547–553. doi: 10.1042/bj2340547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Dubyak G. R. Agonist-induced calcium transients in cultured smooth muscle cells: measurements with fura-2 loaded monolayers. Biochem Biophys Res Commun. 1986 May 14;136(3):927–934. doi: 10.1016/0006-291x(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Snider R. M., Richelson E. Bradykinin receptor-mediated cyclic GMP formation in a nerve cell population (murine neuroblastoma clone N1E-115). J Neurochem. 1984 Dec;43(6):1749–1754. doi: 10.1111/j.1471-4159.1984.tb06104.x. [DOI] [PubMed] [Google Scholar]
- Snider R. M., Richelson E. Bradykinin receptor-mediated cyclic GMP formation in a nerve cell population (murine neuroblastoma clone N1E-115). J Neurochem. 1984 Dec;43(6):1749–1754. doi: 10.1111/j.1471-4159.1984.tb06104.x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Ueda T., Chueh S. H., Noel M. W., Gill D. L. Influence of inositol 1,4,5-trisphosphate and guanine nucleotides on intracellular calcium release within the N1E-115 neuronal cell line. J Biol Chem. 1986 Mar 5;261(7):3184–3192. [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Yano K., Higashida H., Hattori H., Nozawa Y. Bradykinin-induced transient accumulation of inositol trisphosphate in neuron-like cell line NG108-15 cells. FEBS Lett. 1985 Feb 25;181(2):403–406. doi: 10.1016/0014-5793(85)80301-x. [DOI] [PubMed] [Google Scholar]
- Yano K., Higashida H., Inoue R., Nozawa Y. Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem. 1984 Aug 25;259(16):10201–10207. [PubMed] [Google Scholar]



