Abstract
Purpose
Whether, and if so when, a second-ear cochlear implant should be provided to older, unilaterally implanted children is an ongoing clinical question. This study evaluated rate of speech recognition progress for the second implanted ear and with bilateral cochlear implants in older sequentially implanted children and evaluated localization abilities.
Method
A prospective longitudinal study included 24 bilaterally implanted children (mean ear surgeries at 5.11 and 14.25 years). Test intervals were every 3–6 months through 24 months postbilateral. Test conditions were each ear and bilaterally for speech recognition and localization.
Results
Overall, the rate of progress for the second implanted ear was gradual. Improvements in quiet continued through the second year of bilateral use. Improvements in noise were more modest and leveled off during the second year. On all measures, results from the second ear were poorer than the first. Bilateral scores were better than either ear alone for all measures except sentences in quiet and localization.
Conclusions
Older sequentially implanted children with several years between surgeries may obtain speech understanding in the second implanted ear; however, performance may be limited and rate of progress gradual. Continued contralateral ear hearing aid use and reduced time between surgeries may enhance outcomes.
Bilateral cochlear implantation in children has become more prevalent (Peters, Wyss, & Manrique, 2010) with sequential implantation more common than simultaneous procedures. The dominance of sequential implantation has been driven by the many unilateral pediatric cochlear implant (CI) recipients who were implanted before bilateral implantation was considered and the fact that worldwide, the majority of children continue to be implanted unilaterally (Cullington, Bele, Brinton, & Lutman, 2013; Peters et al., 2010). During the decision process for individual children about second-side implantation, clinicians and families must consider the type and extent of benefit to be expected, for both the second implanted ear and bilaterally, and the rate of improvement to expect after varied years of unilateral CI experience.
Pediatric studies have focused on bilateral compared with unilateral performance in the same individual using one of three study designs: bilateral compared with the first implanted (CI1) ear, bilateral compared to the better performing ear (Galvin, Mok, & Dowell, 2007; Kim et al., 2009; Kühn-Inacker, Shehata-Dieler, Müller, & Helms, 2004; Wolfe et al., 2007), or bilateral compared with each ear individually (Galvin, Hughes, & Mok, 2010; Galvin, Mok, Dowell, & Briggs, 2008; Peters, Litovsky, Parkinson, & Lake, 2007; Steffens et al., 2008; for reviews of pediatric sequential bilateral studies, see Dowell et al., 2011; Johnston, Durieux-Smith, Angus, O'Connor, & Fitzpatrick, 2009; Lammers, Venekamp, Grolman, & van der Heijden, 2014; Sparreboom et al., 2010). Most published studies report results from a single time point with participants having varied amounts of bilateral implant experience, rather than following the same participants longitudinally. A few studies have reported longitudinal results for the second CI (CI2) ear in addition to bilateral performance as it relates to CI1 performance, with varied results for speech recognition in quiet. For example, Peters et al. (2007) found for children receiving CI1 before 5 years of age and CI2 before 8 years of age, that CI2 performance reached that of CI1 by 12 months of bilateral use. If CI2 was delayed until after 8 years of age, however, CI2 performance continued to lag that of CI1 at the 12-month test interval. Similar results for speech in quiet were found by Scherf et al. (2009), in that children receiving a second implant by 6 years of age had similar abilities to CI1 after 2 years of bilateral experience. In contrast, two separate studies reported CI2 performance significantly poorer than CI1 at the 2-year test interval for children receiving CI2 prior to (Sparreboom, Snik, & Mylanus, 2011) or near (Strom-Roum, Laurent, & Wie, 2012) 8 years of age. Bilateral performance surpassed CI1-alone performance by 2 years of bilateral use for children receiving their second implant before or after age 6 years in the Scherf et al. (2009) and Sparreboom et al. (2011) studies and by 1 year of bilateral use in the Strom-Roum, Laurent, and Wie (2012) study. Peters et al. (2007) did not identify bilateral benefit at the 12-month test interval; however, scores approached ceiling and may not have allowed identification of bilateral benefit.
Fewer results are available for performance in noise in children with sequential bilateral implants. Scherf et al. (2009) found results quite variable for CI1 and CI2 and not significantly different at any test interval. Bilateral performance was significantly better than either ear alone at the 18-month and 3-year test intervals for children receiving CI2 before 8 years of age and by the 2-year test interval for children receiving CI2 after 8 years of age. In contrast, Sparreboom et al. (2011) identified an early bilateral advantage at the 6-month test interval that was no longer present at the 12- and 24-month intervals.
With respect to localization results in this population, most studies have reported on localization improvement over time in the bilateral condition. (For reviews of pediatric bilateral localization studies see Litovsky, 2011; Litovsky & Gordon, 2016). For 11 bilaterally implanted children tested at two sessions 7–21 months apart, four children showed some improvement (>10° root-mean-square [RMS] error) and seven children had no substantial change (Grieco-Calub & Litovsky, 2010). Sparreboom et al. (2011) included a lateralization task in their longitudinal study of sequentially implanted children. After 6 months of bilateral experience, 57% of the children tested had some lateralization ability; this increased to 63% of children after 12 months. More recently, Strom-Roum, Rodvik, Osnes, Fagerland, and Wie (2012) reported 12- and 24-month postbilateral sound localization results for 63 sequentially implanted children. On average, the participants reduced their mean angular error by 3.9° at 12 months and 12.2° at 24 months using bilateral devices compared to CI1 alone. Analysis of time-based factors indicated that a shorter time between CI1 and CI2 surgeries and longer CI1 experience were related to better outcomes.
Overall, these studies report considerable variability among bilateral recipients and tend to indicate that children implanted at younger ages with less time between surgeries have superior CI2 and bilateral performance than older children with longer delays between surgeries. However, even the older groups of children obtained open-set speech understanding with CI2, and a bilateral advantage was not always evident for younger groups of children. Continued questions for sequential bilateral implantation of older children are related to rate of progress, expected outcomes, and identification of factors that may put some children at risk for poor benefit. The aims of the current study were (a) to describe the rate of progress on speech recognition measures for CI2 alone and bilaterally, (b) to compare CI2 and bilateral performance to that of CI1, (c) to evaluate the effect of patient characteristics on CI2 and bilateral CI outcomes, and (d) to evaluate localization abilities for the bilateral condition compared to CI1 or CI2 alone.
Research Design and Method
Participants
For this prospective study, all children who were to have their second ear implanted (sequential CIs) at St. Louis Children's Hospital from Summer 2006 to Summer 2009 and were able to complete the study protocol were invited to participate prior to the second ear surgery; all 30 enrolled in the study. Six children withdrew due to relocation or scheduling difficulties. Data for the remaining 24 children are reported here. All, except one participant, were implanted in the first ear prior to 2003; the make-up of the study group was reflective of children being implanted during the 1990s. Table 1 provides mean unaided and aided hearing thresholds for the group prior to receiving the second CI. On average, unaided hearing for the nonimplanted ear was in the profoundly impaired range, which greatly limited access to speech even with hearing aids (HAs). FM-tone sound-field thresholds with CI1 were 20–25 dB HL from 0.25–6 kHz. The mean age at surgery for CI1 was 5.11 years and for CI2 was 14.25 years, resulting in an average time between surgeries of 9.14 years. The majority of participants (15) had congenital or presumed congenital onset of bilateral severe-to-profound hearing loss (SPHL). Only four participants consistently wore an HA in the nonimplanted ear between the two surgeries, with two other children having some inconsistent HA use. Table 2 provides participant demographic information.
Table 1.
Group mean hearing thresholds prior to second ear cochlear implantation.
| 0.25 Hz | 0.5 Hz | 1 kHz | 2 kHz | 4 kHz | 6 kHz | 8 kHz | |
|---|---|---|---|---|---|---|---|
| Prebilateral nonimplant ear | |||||||
| Unaided thresholds | 92.7 (15.3) | 102.5 (11.6) | 110.9 (8.5) | 113.1 (8.8) | 111.5 (12.9) | 104.2 (9.7) | 92.5 (9.0) |
| Aided SF thresholds | 46.1 (17.6) | 51.1 (18.5) | 55.4 (17.9) | 67.0 (19.6) | 83.6 (12.3) | 77.6 (18.4) | 83.2 (13.4) |
| CI SF thresholds (CI1) | 20.6 (4.4) | 23.2 (5.6) | 21.2 (6.0) | 20.3 (4.6) | 25.3 (6.8) | 24.1 (8.7) |
Note. SF = sound field; CI = cochlear implant; CI1 = first cochlear implant. Standard deviations are indicated in parentheses.
Table 2.
Participant demographic and hearing history information.
| Participant | Etiology CI1 and CI2 | Ear CI1 | Age at surgery |
Age SPHL onset |
LOD |
HA use CI2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CI1 | CI2 | TBS | CI1 | CI2 | CI1 | CI2 | ||||
| P1 | Unknown | LE | 5.92 | 20.08 | 14.16 | 0 | 0 | 5.92 | 20.08 | No |
| P2 | Unknown | RE | 5.50 | 13.83 | 8.33 | 0 | 0 | 5.50 | 13.83 | Yes |
| P3 | Ushers | RE | 2.75 | 17.25 | 14.50 | 0 | 0 | 2.75 | 17.25 | No |
| P4 | CMV | RE | 6.58 | 14.67 | 8.09 | 0 | 0 | 6.58 | 14.67 | No |
| P5 | Waardenburg | RE | 3.50 | 10.42 | 6.92 | 0 | 0 | 3.50 | 10.42 | No |
| P6 | Unknown | LE | 2.17 | 12.33 | 10.16 | 0 | 0 | 2.17 | 12.33 | No |
| P7 | Unknown | LE | 4.92 | 13.25 | 8.33 | 0 | 0 | 4.92 | 13.25 | No |
| P8 | Meningitis | RE | 10.50 | 16.50 | 6.00 | 2.67 | 4.25 | 7.83 | 12.25 | Yes |
| P9 | Unknown | RE | 7.17 | 17.17 | 10.00 | 2.33 | 2.33 | 4.84 | 14.84 | No |
| P10 | Unknown | RE | 4.17 | 14.92 | 10.75 | 0 | 0 | 4.17 | 14.92 | Yes |
| P11 | Unknown | RE | 9.42 | 14.83 | 5.41 | 9.00 | 0.50 | 0.42 | 14.33 | No |
| P12 | Unknown | RE | 2.92 | 12.83 | 9.91 | 0 | 0 | 2.92 | 12.83 | No |
| P13 | Familial | RE | 5.92 | 13.83 | 7.91 | 0 | 0 | 5.92 | 13.83 | No |
| P14 | Unknown | RE | 3.50 | 8.08 | 4.58 | 0 | 0 | 3.50 | 8.08 | Yes |
| P15 | Familial | LE | 4.42 | 11.33 | 6.91 | 2.33 | 2.33 | 2.09 | 9.00 | Yes |
| P16 | Unknown | RE | 3.17 | 12.42 | 9.25 | 0 | 0 | 3.17 | 12.42 | No |
| P17 | Unknown | RE | 4.17 | 11.75 | 7.58 | 0 | 0 | 4.17 | 11.75 | No |
| P18 | Unknown | RE | 6.25 | 15.92 | 9.67 | 2.00 | 2.00 | 4.25 | 13.92 | No |
| P19 | Cx26 | RE | 5.83 | 15.58 | 9.75 | 4.00 | 4.00 | 1.83 | 11.58 | Yes |
| P20 | EVA | RE | 6.92 | 19.83 | 12.91 | 2.67 | 6.08 | 4.25 | 13.75 | No |
| P21 | Unknown | LE | 2.17 | 14.42 | 12.25 | 0 | 0 | 2.17 | 14.42 | No |
| P22 | Unknown | LE | 6.17 | 12.00 | 5.83 | 5.67 | 0 | 0.50 | 12.00 | No |
| P23 | Unknown | RE | 3.00 | 18.00 | 15.00 | 0 | 0 | 3.00 | 18.00 | No |
| P24 | Meningitis | LE | 5.58 | 10.83 | 5.25 | 5.42 | 5.42 | 0.16 | 5.41 | No |
| Group | ||||||||||
| M | 5.11 | 14.25 | 9.14 | 1.50 | 1.12 | 3.61 | 13.13 | |||
| SD | 2.14 | 2.96 | 2.98 | 2.41 | 1.93 | 1.96 | 3.09 | |||
| Minimum | 2.17 | 8.08 | 4.58 | 0 | 0 | 0.42 | 5.41 | |||
| Maximum | 10.50 | 20.08 | 15.00 | 5.67 | 6.08 | 7.83 | 20.08 | |||
Note. CI1 = first cochlear implant; CI2 = second cochlear implant; TBS = time between surgery; SPHL = severe-to-profound hearing loss; LOD = length of deafness; HA = hearing aid; CMV = cytomegalovirus; LE = left ear; RE = right ear; EVA = enlarged vestibular aqueduct; Cx26 = Connexin 26. All numeric information is in years.
Nine participants had older technology (Nucleus 22 or Clarion 1.2) at the first ear and another 15 had the Nucleus 24/24RE or the Advanced Bionics CII/90K device. Two participants (P6 and P18) had newer technology in the first ear due to a device failure and replacement prior to enrollment in this study. In the second implanted ear, 17 participants used a Nucleus 24RE, one had the Nucleus 512, and six used an Advanced Bionics 90k. All participants used a device from the same manufacturer in each ear.
Procedures
Test Schedule
The initial test session was prior to implantation of the second ear (prebilateral), and testing was conducted with each ear individually (CI1 alone, HA alone) and with both together (bimodal). Postimplant test sessions occurred after 1, 3, 6, 9, 12, 15, 18, and 24 months of bilateral CI experience (CI1 alone, CI2 alone, and bilateral conditions). Word and sentence recognition measures were administered at each test interval with the exception of the Hearing In Noise Test in R-Space and localization that were conducted only at the 12- and 24-month postbilateral test intervals.
Sound-field Detection Threshold and Speech Recognition Measures
FM-tone sound-field thresholds with the CI were obtained at each postimplant test interval for each ear individually using a standard Hughson–Westlake procedure (Carhart & Jerger, 1959) from 0.25–6 kHz to confirm good audibility prior to testing. Speech recognition measures included words and sentences at various presentation levels with multiple noise conditions, including quiet, to mimic everyday listening while avoiding ceiling and floor effects as much as possible. Unless indicated otherwise, stimuli were presented from 0° azimuth with the participant seated approximately 1 m from the source loudspeaker. Stimuli were calibrated to ensure accuracy and consistency over time. All testing was conducted in a double-walled audio booth using recorded test materials. For each measure, participants were evaluated with CI1, CI2, and the bilateral condition. Test and condition order were pseudorandomized across test intervals and participants.
Monosyllabic words were presented at an average conversational level of 60 dB SPL in quiet as well as in noise at a fixed +8 dB signal-to-noise ratio (SNR). Lists were scored as percent correct. The study began using half lists of phonetically balanced kindergarten (PBK) words (Haskins, 1949). However, this was changed to consonant–nucleus–consonant words (CNC; Peterson & Lehiste, 1962) because more lists were available. Subsequent analysis indicated no significant effect for measure between the PBK and CNC (p < .05). The increased number of lists enabled a full list of 50 words for each condition. The noise was four-talker babble for CNC words and multitalker babble for PBK words. For testing in noise, the speech and noise were both presented from the front loudspeaker.
A single list of 20 Bamford–Kowal–Bench sentences (BKB; Bench, Kowal, & Bamford, 1979) was presented at 50 dB SPL in quiet and scored as a percent of key words correct. A softer level was used for this measure than for the monosyllabic words to help avoid ceiling effects and determine abilities for soft conversational speech. Two additional sentence tests were administered in noise using varied SNRs. The BKB Sentence-in-Noise (BKB-SIN; Etymotic Research, 2005) test was administered with sentences at 65 dB SPL and four-talker babble at SNRs that progressed from +21 dB to 0 dB in 3 dB steps across a set of eight sentences, twice for each condition. The lists were scored for key words correct, and an SNR for 50% accuracy was determined following the BKB-SIN manual scoring instructions. The poorest SNR possible was 23.5 dB. The Hearing In Noise Test (Nilsson, Soli, & Sullivan, 1994) was presented in restaurant noise (Compton-Conley, Neuman, Killion, & Levitt, 2004) at 60 dB SPL using the R-Space (Revit, Schulein, & Julstrom, 2002). The R-Space is a system with eight loudspeakers positioned 360° around the participant, each 24 in. from the head. Single lists of 20 sentences were presented from the front. The first sentence was presented at a favorable SNR of +12 dB. If the sentence wasn't repeated correctly in its entirety, the sentence was re-presented at progressively greater SNR values until it was repeated correctly. Once the first sentence was repeated correctly, the following sentences were presented at more difficult (smaller) SNR levels if correct and at easier (larger) SNRs if incorrect. The step size was ±4 dB for the first four sentences and ±2 dB for the remaining 16 sentences. This resulted in a presentation SNR for each of the 20 sentences, plus the SNR that would be used for a 21st sentence. The score was an average of the final 17 SNRs and represented 50% accuracy.
Localization
Localization testing was conducted following procedures described elsewhere (Firszt, Holden, Reeder, Cowdrey, & King, 2012; Potts, Skinner, Litovsky, Strube, & Kuk, 2009) and is summarized here. The participants faced 15 loudspeakers equally spaced along a 140° horizontal plane arc. One hundred monosyllabic words (60 dB SPL, roved ±3 dB) were presented pseudorandomly from 10 active loudspeakers (±70°, ±50°, ±30°, ±20°, and ±10°), 10 words from each active loudspeaker. The participant was unaware that some loudspeakers were inactive and identified the source loudspeaker by number from 1 (−70°) to 15 (+70°) to indicate the perceived location of the target sound for each presentation.
Device Verification
Either the participant's or a clinic-owned HA was used for prebilateral testing. Participants who did not use a HA consistently and had the potential to receive any benefit from a HA underwent a several-week HA trial. The length of the trial varied by participant and ranged from 4 to 16 weeks; a number of participants had tolerance issues at the initial HA fitting on the nonimplanted ear and required a several-month trial prior to testing. Participants who were unable to detect conversational speech with the HA were scored at 0% or a maximum SNR for the HA-only condition and bimodal testing was not conducted. Following Saint Louis Children's Hospital's standard clinical practice, HA-fitting verification was completed with an Audio Scan Verifit (Audioscan, Division of Etymotic Design Inc.; Dorchester, Ontario, Canada) for 50, 65, and 80 dB inputs. An attempt was made to reach desired sensation level targets; however, given the severity of the HL for most participants, these targets could not be reached, particularly in the high frequencies. Participants had several years of CI1 device use with stable speech processor programs. The CI2 was fit using standard clinic procedures to provide detection of soft sounds and comfort for loud sounds across the frequency range. Adjustments to programming parameters were made as needed to ensure comfort and balance between ears when both devices were worn together.
Data Analysis
Initial analysis was completed using standard analyses of variance (ANOVA; Keppel, 1991; Maxwell & Delaney, 1990) and the latest test interval, typically 24 months, for each participant under each CI condition (CI1, CI2, bilateral). Although occasionally the latest test interval was not at 24 months, this approach allowed an adequate sample size for repeated-measure ANOVAs (CI condition as the repeated measure). Bonferroni-adjusted post hoc comparisons were used when the overall F test was significant.
Hierarchical linear modeling (HLM; Heck & Thomas, 2009; Hox, 2010; Raudenbush & Bryk, 2002; Snijders & Bosker, 2012) was the primary approach to analyze longitudinal effects (e.g., rate of improvement) due to the hierarchical structure of the data (the repeated measures over time nested within CI conditions, which are nested within participants). Outcomes were examined with a three-level growth curve model. Linear growth (i.e., growth rate; tangent to the growth curve at specific intervals) and quadratic growth (i.e., change in linear growth over time or departure from an overall linear or straight line pattern) were included in the model. Because standard errors may be reduced when HLM is used with smaller samples, p values ≤ .005 were used to determine significance. If significant variation was found by an overall chi-square test in the first hierarchical model among CI conditions for the intercept, the linear component, and the quadratic component, the nature of the variation was examined using CI condition pairwise comparisons (see Reeder, Firszt, Holden, & Strube, 2014, for a detailed description of the HLM analysis).
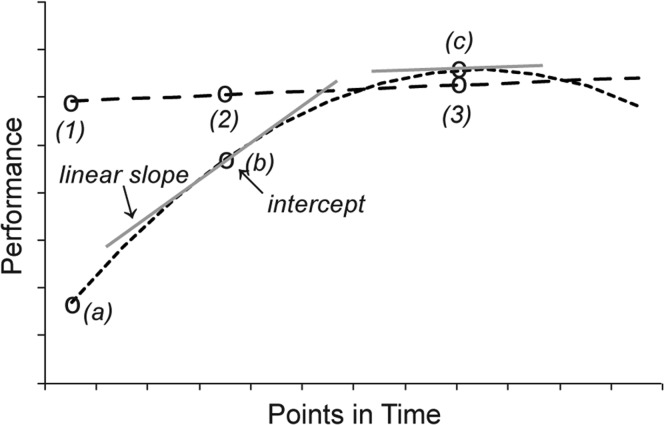
The intercept for data centered at a particular point in time is the expected performance at that point in time, and the linear component is the growth rate (the tangent to the growth curve) at that point in time. Curvilinearity over time (i.e., how the HLM curve differs from a straight line) indicates the quadratic component. Figure 1 (taken from Reeder et al., 2014) provides a theoretical example of two HLM curves and the analyzed components. Analysis of these three HLM components (intercept – expected performance at a particular point in time; growth rate – the tangent to the growth curve at the same point in time; and curvilinearity over time – how the HLM curve differs from a straight line) identified significant differences between the three conditions (CI1, CI2, bilateral) and at various time points. The HLM analysis was completed for the prebilateral and 3-, 6-, 12-, and 24-month test intervals.
Figure 1.
Examples of two theoretical HLM curves are shown (dotted and dashed lines) with the components indicated. Intercept is the expected performance at a point in time (the centering point) on the basis of the HLM analysis of the group data. Three example intercepts are indicated with open circles along each curve (1, 2, and 3 along the dashed line and a, b, and c along the dotted line). Expected performance at the earliest point is much higher along the dashed line (1) than the dotted line (a). Linear slope is the rate of change at a specific point in time (the centering point)—that is, the slope of the tangent (gray lines) to the curve at the given time point. The linear slope at the intercept (b) is considerably steeper than the linear slope at the intercept (c). The rate of change is greater at the earlier time point. Curvilinearity is change in the linear component over time. The two example HLM curves differ in curvilinearity. The change over time is minimal for the dashed line and substantial for the dotted line. Each component and each condition has its own significance test that indicates if the parameter estimate is different from 0. For example, it is likely that the linear slope at (b) in Figure 1 would differ from 0, but the linear slope at (c) would not differ from 0. Comparisons of the components between CI conditions were conducted using chi-square tests.
Figure from Reeder, Firszt, Holden, and Strube (2014, p. 1113). Reprinted with permission.
One advantage of HLM analysis was that it allowed for missing data across time that is common with longitudinal pediatric studies. Data were most complete for monosyllabic words in quiet and in noise (ns = 16–24 participants). Sentence measures, particularly for the prebilateral testing were obtained for fewer participants, primarily due to age, attention, hearing, and clinical needs that did not allow for administration of these measures for some children at some time points. Postbilateral data were available for 14–19 participants; prebilateral data were available for 8–10 participants. Localization and R-Space testing occurred for 13 participants at the 12-month test interval and 12 participants at the 24-month test interval.
Results
FM-tone sound-field thresholds were obtained from 0.25 to 6 kHz for CI1 and CI2 at each interval to ensure good audibility prior to testing. Thresholds were relatively similar between ears and stable over time. The average thresholds across frequencies and participants ranged from 10.3 to 31.7 dB HL for CI1 and from 12.7 to 38.3 for CI2. The average difference between CI1 and CI2 at each frequency was 5.9 dB.
Speech Recognition
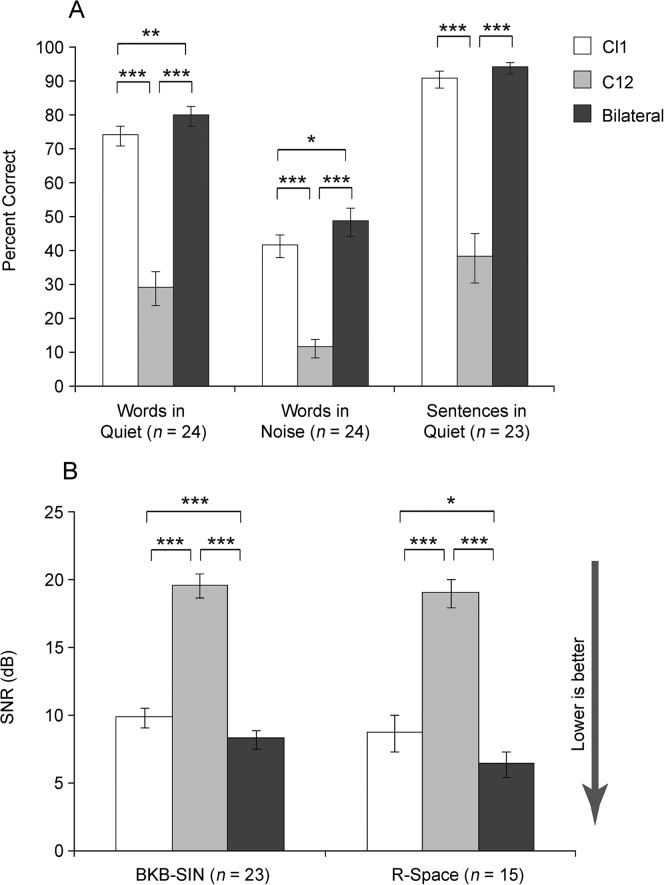
Figure 2 shows mean latest test interval scores for monosyllabic words at 60 dB SPL in quiet and in noise, BKB sentences in quiet at 50 dB SPL, the BKB-SIN, and the R-Space for each CI condition. Note that for the BKB-SIN and R-Space (Panel B), lower scores (SNR) indicate better performance. For all five measures, there was a significant CI condition effect, words in quiet F(1.1, 25.9) = 105.04, p < .001; words in noise F(2, 46) = 72.95, p < .001; BKB sentences in quiet F(1.1, 23.1) = 56.04, p < .001; BKB-SIN F(1.2, 26.8) = 119.01, p <.001; and R-Space F(1.3, 18.0) = 57.45, p < .001. Post hoc analysis indicated significantly better bilateral scores than either ear alone (p < .05) for all measures except BKB sentences in quiet, for which bilateral performance was significantly better than CI2 (p < .001) but not CI1. On all measures, CI1 scores were significantly better than CI2.
Figure 2.
Group mean speech recognition scores for words in quiet, words in noise, and sentences in quiet are shown as percent correct in Panel A and for sentences in noise as dB SNR in Panel B. Scores represent the latest test interval for the three CI conditions: CI1 (white bars), CI2 (gray bars), and bilateral CIs (black bars). Error bars represent 1 SE. Asterisk(s) indicate significant differences: *p < .05, **p < .01, ***p < .001.
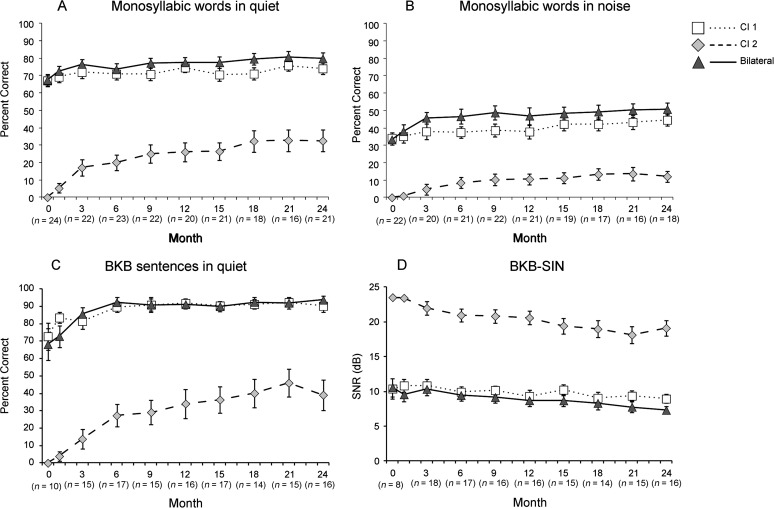
Figure 3 Panels A–D shows group means over time for measures that were administered at most test intervals: words in quiet (A), words in noise (B), BKB sentences in quiet (C), and BKB-SIN (D). Test intervals are indicated along the x-axis from 0, which refers to prebilateral through 24 months of CI2/bilateral experience. Recall that at the prebilateral test interval testing with the CI2 ear was with the HA alone and bilateral testing was conducted in the bimodal condition, CI + HA. For clarity, actual means are provided in the figure rather than the generated HLM curves and components that were analyzed. Table 3 provides the HLM analysis results summary for these four speech recognition measures.
Figure 3.
Group mean speech recognition results over time are shown for words and sentences in quiet and noise for the three CI conditions by test measure. The squares, diamonds, and triangles represent group means at each test interval for the CI1, CI2, and the bilateral conditions, respectively. Error bars represent 1 SE. Significant differences are indicated in Table 3.
Table 3.
Hierarchical linear modeling summary of significant differences
| Test | Component | CI1 | CI2 | Bilateral | CI1 vs. CI2 | CI1 vs. Bil | CI2 vs. Bil |
|---|---|---|---|---|---|---|---|
| Words Q | Curvilinearity | * | * | * | |||
| Linear slope | *12 | *P, 3, 6, 12 | *P, 3, 6, 12 | *P, 3, 6, 12 | *P, 3, 6, 12 | ||
| Intercept | *P, 3, 6, 12, 24 | *3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | ||
| Words N | Curvilinearity | * | |||||
| Linear slope | *12 | *P, 3, 6, 12 | *P, 3, 6 | ||||
| Intercept | *P, 3, 6, 12, 24 | *6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12 | ||
| Sentences Q | Curvilinearity | * | * | * | |||
| Linear slope | *P, 3, 6 | *P, 3, 6, 12 | *6 | *P, 3, 6, 12 | *P, 3, 6, 12 | ||
| Intercept | *P, 3, 6, 12, 24 | *3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | ||
| Sentences N | Curvilinearity | ||||||
| Linear slope | *12 | *P, 3, 6, 12 | *12 | *3, 6, 12 | |||
| Intercept | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 | *P, 3, 6, 12, 24 |
Note. CI1 = first cochlear implant; CI2 = second cochlear implant; Bil = bilateral; Q = quiet; N = noise; P = prebilateral interval; 1–24 indicate postbilateral intervals. Significant differences (from zero or between CI conditions) are indicated with an asterisk or an asterisk followed by the test intervals for which significant differences occurred. For example, on Words Q (first row), the first three significant findings columns indicate the quadratic component of the CI2 curve (but not CI1 or bilateral) was significantly different from zero (straight). The three far-right columns indicate that the CI2 quadratic curve component was significantly different from that of CI1 and bilateral, which were not significantly different from each other. In the second row, CI1, CI2, and bilateral linear slopes (rate of change) were significantly different from zero at 12; at P, 3, 6, and 12; and at P, 3, 6, and 12, respectively. CI2 linear slope differed significantly from CI1 and bilateral at P, 1, 3, 6, and 12. CI1 linear slope did not differ significantly from bilateral at any interval. Significant differences for intercept are indicated in the same manner as for linear slope. Only significant post hoc results following significant overall tests are indicated.
p ≤ .005
Although values varied by measure and condition, the results followed a similar pattern across measures. As can be seen in Figure 3, on average, this group of older sequentially implanted children did obtain open-set speech understanding in the second implanted ear. After 24 months of bilateral experience, the average for words in quiet was 32%, for words in noise, 12%, and for sentences in quiet, 39%, and the average SNR for sentences in noise was 19 dB. The CI2 condition exhibited gradual but significant growth in performance over time for measures in quiet. The small changes that occurred for measures in noise were during the first 12 months and then leveled off. Sentences in quiet for CI1 and both word measures for the bilateral condition demonstrated some minimal early growth. The only measure with significant change over time was words in noise in the bilateral condition.
Variation among CI conditions was analyzed with chi-square tests and follow-up pairwise comparisons (see results in Table 3). Results indicated that for measures in quiet, the change over time (curvilinearity) and growth rate (linear components) at each test interval through 12 months were significantly different between CI1 and CI2 (ps ≤ .005). The intercepts for CI1 and CI2 were significantly different at all test intervals (ps ≤ .005). The change over time and growth rate at each test interval for words in noise did not differ between CI1 and CI2 (p > .005). For sentences in noise, the change over time did not differ between CI1 and CI2 (p > .005); however, the growth rate was significantly greater for CI2 than CI1 from the 3–12-month test intervals. As with the measures in quiet, the intercepts for CI1 and CI2 were significantly different at all test intervals (ps ≤ .005). In other words, CI2 performance did not reach that of CI1 performance for any measure at any test interval. Although in general bilateral scores at each interval were equal to or better than CI1 scores, this difference was not statistically different. Bilateral performance was comparable to that of CI1 for all four measures.
Demographic Factors
Inclusion of demographic variables in the ANOVAs identified a significant main effect of CI2 length of SPHL for words in quiet (p < .05) and the BKB-SIN (p < .001) and a significant main effect of time between CI surgeries for words in quiet (p < .05), words in noise (p < .05), and the BKB-SIN (p < .01). Analysis indicated that for every additional year of time between surgery (which is also longer CI2 length of SPHL), the bilateral CI word recognition score declined by approximately 2% for both words in quiet (p < .05) and in noise (p < .001). Likewise, every additional year in the time between surgeries resulted in decreased sentence understanding in noise by approximately ½ dB SNR (p < .05). There was not a significant main effect of age at CI1, age at CI2, or age at CI2 SPHL.
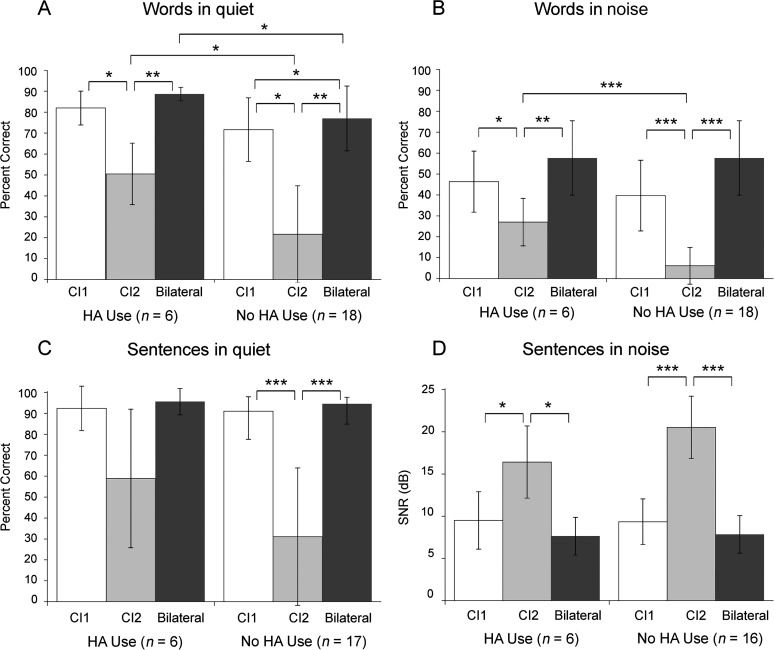
In addition to these time-based factors, we were particularly interested in the relation between HA use in the nonimplanted ear during the time between surgeries and speech recognition outcomes. Given the relatively common practice of discontinuing HA use at the time of CI1 when these children received their first CI, it was not surprising that only six of the study participants had worn an HA at the nonimplanted ear after the CI1 surgery, and consequently results should be viewed as preliminary. It is of note that these participants also had somewhat better hearing thresholds (in the nonimplanted ear prior to CI2 surgery) than those who had discontinued HA use. The mean pure-tone thresholds at 0.5, 1, and 2 kHz (pure-tone average [PTA]) were 104.27 dB HL (SD = 9.3 dB) for the no-HA use group and 90.55 dB HL (SD = 5.7 dB) for the HA use group, t(22) = 3.37, p < .01. Figure 4 shows the mean latest test interval scores in each of the three conditions for the HA use group on the left and for the no-HA use group on the right in each panel. Results of a one-way ANOVA for each measure indicated a significant condition effect for each group (F values ranged from 50.3 to 123.3 for the no-HA use group and 11.0 to 31.2 for the HA use group, all ps < .001). Post hoc Bonferroni corrected pairwise comparisons indicated that the primary difference between the two HA use groups was in the CI2 condition for words. The HA use group had significantly higher CI2 word scores in quiet and in noise than the no-HA use group (ps < .05). The HA use group's CI2 mean score for sentences in quiet was lower than the CI1 and bilateral mean scores, yet not significantly different (p > .05); this was not the case for the no-HA use group. For sentences in noise, the two groups' mean SNRs for the CI1 and bilateral conditions were very similar, and although the CI2 SNR for the no-HA use group was poorer than for the HA use group, the difference was not statistically significant. Comparison of the bilateral scores between groups shows only words in quiet to be significantly better for the HA use group.
Figure 4.
Group mean speech recognition results at the latest test interval for the three CI conditions (CI1, CI2, and bilateral) are plotted separately for participants with and without HA use. Panels A and B display group mean results for words in quiet and noise; Panels C and D display results for sentences in quiet and noise. Error bars represent 1 SD. Asterisk(s) indicate significant differences: *p < .05, **p < .01, ***p < .001.
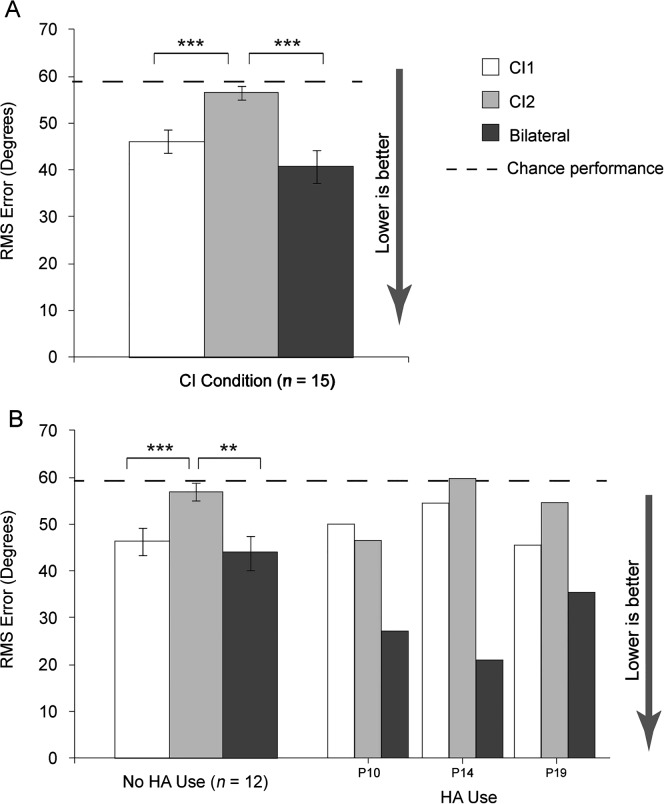
Localization
Figure 5A shows group mean latest test interval RMS error scores by CI condition. There was a significant CI condition effect, F(1.4, 19.3) = 18.4, p < .001, and post hoc analysis indicated CI1 and bilateral localization was significantly better than CI2 localization (p < .001), but the bilateral condition was not significantly better than CI1 (p > .05). Localization testing was completed by three of the HA use participants. Figure 5B has mean RMS scores for the no-HA use group and individual results for the three HA use participants. Whereas, for the no-HA use group, CI1 and bilateral performance was similar (CI1 mean 47.9°; bilateral mean 44.6°), for the three HA use participants this was not true; the bilateral condition was approximately 10°–30° better than CI1.
Figure 5.
Panel A shows group mean localization results for RMS error in degrees at the latest test interval for CI1, CI2, and the bilateral condition. Panel B shows group mean localization RMS error in degrees at the latest test interval for participants with no HA use and three individual participants with HA use. Scores are displayed as white bars for CI1, gray bars for CI2, and black bars for bilateral CIs. Chance performance is indicated by the dashed line at 59°. Error bars represent 1 SE. Asterisk(s) indicate significant differences: **p < .01; ***p < .001.
Discussion
This study reports on results of older children (n = 24) who obtained their first CI after at least 2 years of age (2–10 years) and then obtained a second CI after several years CI1 experience (5–15 years). In particular, these longitudinal results provide insights into CI2 and bilateral speech recognition rates of progress, CI2 and bilateral performance compared to CI1 performance over time, and the effect of patient characteristics on CI2 outcomes for this population.
Speech Recognition
Rate of CI2 Progress Compared to CI1 Performance
In general, CI2 progress was gradual. Improvements in word and sentence understanding in quiet continued on through the second year of bilateral device use. Improvements in noise were more modest and leveled off during the second year of device use. Although mean CI2 performance continued to be significantly poorer than CI1 performance, there was evidence of open-set word and sentence understanding with CI2 alone on all measures. This is consistent with the findings of Peters et al. (2007) for their oldest group (8–13 years at the time of CI2 surgery) whose mean 12-month word and sentence scores in quiet were similar to scores in the current study. The discrepancy in performance between ears for the current study is in contrast to the results of Scherf et al. (2009), who did not find a statistical difference between CI1 and CI2 word scores at any postbilateral test interval (through 36 months). The difference in findings might be attributed to differences in test materials and conditions (lists of 12 consonant–vowel–consonant words scored for phonemes vs. lists of 25–50 consonant–vowel–consonant words scored for words; SNR for noise testing of +10 dB with speech weighted noise vs. +8 dB with four-talker babble). Results from Strom-Roum, Laurent, and Wie (2012) suggested CI2 improvement during the first year that leveled off during the second year for words in quiet, whereas in the current study, CI2 word recognition in quiet improved during the second year. Participants in the Strom-Roum study were younger than those in the current study at CI1 (mean 3 vs. 5 years) and at CI2 (mean 8 vs. 14 years). In addition, mean 12- and 24-month word scores were higher for participants in Strom-Roum than the current study. It may be that later CI2 implantation requires more time for recipients to reach maximal performance.
Bilateral Performance Compared to Each Individual Ear
Group mean bilateral performance was somewhat better than, but not statistically different from, CI1 at all postimplant intervals for words in quiet (differences ranged by 2.5–8.6 percentage points), words in noise (differences ranged by 2.8–10.2 percentage points), and BKB-SIN (differences ranged by 0.5–1.6 dB) for the analysis over time. In the analysis of the latest test interval results, the mean bilateral performance was significantly better than CI1 for these measures as well as the R-Space, although the differences were modest (5.6 percentage points for words in quiet, 7.5 percentage points for words in noise, 1.6 dB for BKB-SIN, and 2.1 dB for R-Space). Strom-Roum, Laurent, and Wie (2012) also found a small but statistically significant improvement for the bilateral condition over unilateral CI improvement in sequentially implanted children for words in quiet. Asp et al. (2015), in a group of younger children, most of whom were implanted sequentially (mean age at CI1 of 1.1 years and CI2 of 4.8 years), found a small but statistically significant word recognition improvement in quiet at the first study visit but not at the two subsequent annual visits; however, a ceiling effect was present for this measure. In that study, group mean word recognition ability in noise was significantly better in the bilateral condition compared to best ear condition by 8–13 percentage points across visits. For the older participants in the current study, the significant bilateral versus CI1 performance differences are considered clinically significant for the measures in noise. Each dB improvement in SNR translates to approximately 10.6% improvement in sentence intelligibility (Soli & Wong, 2008), which for this study would be an improvement of 14–19 percentage points for sentence understanding in noise.
Effect of Time-Based Factors and Hearing Aid Use
There was no main effect of age at CI1 or CI2 surgery or age at CI2 SPHL on outcomes at the latest test interval (generally 24 months). Age at CI is a well-established factor related to speech recognition outcomes. However, the age at CI1 for the current study participants was relatively old, with a mean of 5.11 years and no children implanted prior to 2 years of age. This may account for the lack of age at CI1 influence on study outcomes. The age at SPHL onset was presumed to be congenital for several participants; however, this often was not documented because it was prior to the implementation of newborn hearing screening and may have affected results related to onset of SPHL. There was a significant main effect of time between surgeries and CI2 length of SPHL (two highly correlated variables) on speech recognition in quiet and noise. Children with longer time between surgeries and longer CI2 length of SPHL had poorer outcomes. These findings were somewhat inconsistent with those of previous studies. Participants in the study reported by Asp et al. (2015) had varied amounts of bilateral CI experience at the time of enrollment. As in the current study, they did not find a significant relationship between age at CI2 and word recognition in noise. Results from Strom-Roum, Rodvik, et al. (2012) differed from the current study. They found that a shorter time between surgeries correlated with the amount of bilateral benefit after 12 months of bilateral experience, but this correlation was not present at the 24-month test interval.
Although most study participants received CI1 at a time when only the most profoundly impaired children were implanted and use of an HA on the nonimplanted ear was often discontinued, six participants had worn an HA on the nonimplanted ear for some time between CI1 and CI2 surgeries. On average, these children also had PTAs that were 13.7 dB better than the other participants' PTAs, which may explain the reason that HA use was not completely discontinued. Preliminary results suggest greater CI2 word understanding for these children than the participants without any HA use between surgeries. Latest test interval means for the HA use group were 50% for words in quiet and 27% for words in noise, which were significantly higher than means for the no-HA use group—22% for words in quiet and 6% for words in noise. Note that the PTA of the children in the HA use group was still in the profound hearing loss range (mean PTA of 90.55 dB HL). Although the HA use group was small, these results support the growing clinical trend to encourage continued amplification, even when losses are in the severe-to-profound range in the contralateral ear of children using unilateral implants—children who may become bilateral recipients in the future. It may be that some acoustic stimulation, albeit not ideal, helps to maintain that ear and pathway for later electrical stimulation via CIs. Additional research regarding the effect of continued HA use on the nonimplanted ear of unilaterally implanted children is needed before drawing any definitive conclusions.
Localization
For the group as a whole and the subgroup without HA use, there was no significant difference in sound localization bilaterally compared to CI1 alone. Three of the participants who had HA use prior to CI2 surgery had 10°–30° better localization bilaterally compared to either ear alone. Studies of children receiving CI2 at a younger age have reported bilateral lateralization or localization benefit. Sparreboom et al. (2011) reported significant bilateral improvement at the 24-month postbilateral interval. Sound localization improved significantly for 74%–83% of participants across visits in Asp et al. (2015) and sound localization was significantly related to age at CI2. Grieco-Calub and Litovsky (2010) found a significant effect of age at CI2 on localization ability among a group of bilaterally implanted children, most sequentially implanted and all receiving CI2 under 3 years of age. In the current study, there was no correlation between bilateral localization ability and age at CI2, potentially because the children were older at CI2 and had longer periods of unilateral CI experience than the participants in Asp et al. (2015) or Grieco-Calub and Litovsky (2010). Findings by Jiwani, Papsin, and Gordon (2016) suggest long periods of unilateral CI experience, as seen in the current study population, can result in ongoing disruption in the auditory system and consequentially limit abilities that rely on the deprived pathways. Killan, Royle, Totten, Raine, and Lovett (2015) found no difference in localization ability between a group of children implanted bilaterally (82% simultaneously) by 3.5 years of age (mean age at CI2 = 1 year 11 months) and a group of children implanted in the first ear by 3.5 years of age (mean age at CI1 = 2 years 4 months) and in the second ear after 3.5 years (mean age at CI2 = 6 years 9 months). The authors postulate that the lack of early acoustic experience was detrimental for both groups of children. A third group of bilaterally implanted children who heard normally until at least 3.5 years of age and acquired a SPHL after that time (mean age at SPHL = 8 years 2 months) localized significantly better than the children without early acoustic experience. Early acoustic experience (i.e., age at SPHL onset) was not correlated with outcomes for the current study, although SPHL onset was presumed congenital for the majority of participants.
The current study is parallel to an adult longitudinal sequential bilateral study reported by Reeder et al. (2014) using the same or similar measures. Compared to the postlingually implanted adults in that study, the children in this study had a much slower rate of CI2 progress and poorer outcomes at the latest test interval despite having had 24 months of experience compared to the 12-month experience of the adults. The children's speech recognition results were more similar to those adults with longer term CI2 deafness (>30 years). Localization results for the children differed from those of the adults (both those with longer and shorter term deafness). The children had no bilateral benefit compared to CI1 and were significantly poorer localizers with CI2 alone compared to the other conditions. The comparison of results between children and adults with bilateral SPHL suggests an interaction between the length, or perhaps percentage of a person's life, of unilateral CI experience, and the timing of auditory deprivation onset.
Summary
Results from this study in sequentially implanted older children have implications for clinical practice and are summarized below.
These sequentially implanted children were able to obtain bilateral benefit for speech recognition in quiet and in noise.
A shorter time between surgeries (resulting in shorter length of CI2 SPHL) resulted in better bilateral speech understanding, both in quiet and in noise.
Although some children with several years between surgeries obtained CI2 open-set speech understanding, performance was more limited and rate of progress more gradual as the time between surgeries increased.
Bilateral localization was not improved over CI1 localization for these older, sequentially implanted children.
Preliminary findings suggest that continued HA use on the nonimplanted ear during the time between surgeries may enhance CI2 and bilateral outcomes, even when hearing thresholds are in the severe-to-profound range. The impact of acoustic hearing and the quality of that acoustic hearing for the nonimplanted ear needs further investigation.
Acknowledgments
This work was supported by R01DC009010 from the National Institute on Deafness and Other Communication Disorders. We acknowledge and thank the following: The St. Louis Children's Hospital Cochlear Implant Team, particularly Janet Vance, Jerrica Kettle, Heather Strader, and Rose Wright for assistance with data collection; Beverly Fears formerly at St. Joseph Institute for the Deaf for assistance with data collection; Tim Holden for test equipment and stimuli calibration; and our patients for their time and participation in this study.
Funding Statement
This work was supported by R01DC009010 from the National Institute on Deafness and Other Communication Disorders.
References
- Asp F., Mäki-Torkko E., Karltorp E., Harder H., Hergils L., Eskilsson G., & Stenfelt S. (2015). A longitudinal study of the bilateral benefit in children with bilateral cochlear implants. International Journal of Audiology, 54, 77–88. [DOI] [PubMed] [Google Scholar]
- Bench J., Kowal A., & Bamford J. (1979). The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. British Journal of Audiology, 13, 108–112. [DOI] [PubMed] [Google Scholar]
- Carhart R., & Jerger J. (1959). Preferred method for clinical determination of pure-tone thresholds. Journal of Speech and Hearing Disorders, 24, 330–345. [Google Scholar]
- Compton-Conley C. L., Neuman A. C., Killion M. C., & Levitt H. (2004). Performance of directional microphones for hearing aids: Real-world versus simulation. Journal of the American Academy of Audiology, 15, 440–455. [DOI] [PubMed] [Google Scholar]
- Cullington H., Bele D., Brinton J., & Lutman M. (2013). United Kingdom national paediatric bilateral cochlear implant audit: Preliminary results. Cochlear Implants International, 14(Suppl. 4), S22–S26. [DOI] [PubMed] [Google Scholar]
- Dowell R. C., Galvin K. L., Dettman S. J., Leigh J. R., Hughes K. C., & van Hoesel R. (2011). Bilateral cochlear implants in children. Seminars in Hearing, 32, 53–72. [Google Scholar]
- Etymotic Research. (2005). BKB-SIN Speech-in-Noise Test. Version 1.03. Elk Grove Village, IL: Author. [Google Scholar]
- Firszt J. B., Holden L. K., Reeder R. M., Cowdrey L., & King S. (2012). Cochlear implantation in adults with asymmetric hearing loss. Ear and Hearing, 33, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin K. L., Hughes K. C., & Mok M. (2010). Can adolescents and young adults with prelingual hearing loss benefit from a second, sequential cochlear implant? International Journal of Audiology, 49, 368–377. [DOI] [PubMed] [Google Scholar]
- Galvin K. L., Mok M., & Dowell R. C. (2007). Perceptual benefit and functional outcomes for children using sequential bilateral cochlear implants. Ear and Hearing, 28, 470–482. [DOI] [PubMed] [Google Scholar]
- Galvin K. L., Mok M., Dowell R. C., & Briggs R. J. (2008). Speech detection and localization results and clinical outcomes for children receiving sequential bilateral cochlear implants before four years of age. International Journal of Audiology, 47, 636–646. [DOI] [PubMed] [Google Scholar]
- Grieco-Calub T. M., & Litovsky R. Y. (2010). Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear and Hearing, 31, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins H. L. (1949). A phonetically balanced test of speech discrimination for children (Unpublished master's thesis). Northwestern University, Evanston, IL. [Google Scholar]
- Heck R. H., & Thomas S. L. (2009). An introduction to multilevel modeling techniques. New York, NY: Routledge. [Google Scholar]
- Hox J. J. (2010). Multilevel analysis: Techniques and applications. Mahwah, NJ: Erlbaum. [Google Scholar]
- Jiwani S., Papsin B. C., & Gordon K. A. (2016). Early unilateral cochlear implantation promotes mature cortical asymmetries in adolescents who are deaf. Human Brain Mapping, 37, 135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. C., Durieux-Smith A., Angus D., O'Connor A., & Fitzpatrick E. (2009). Bilateral paediatric cochlear implants: A critical review. International Journal of Audiology, 48, 601–617. [DOI] [PubMed] [Google Scholar]
- Keppel G. (1991). Design and analysis: A researcher's handbook (3rd ed.). Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Killan C. F., Royle N., Totten C. L., Raine C. H., & Lovett R. E. S. (2015). The effect of early auditory experience on spatial listening skills of children with bilateral cochlear implants. International Journal of Pediatric Otorhinolaryngology, 79, 2159–2165. [DOI] [PubMed] [Google Scholar]
- Kim L.-S., Jang Y. S., Choi A.-H., Ahn S.-Y., Park J.-S., Lee Y.-M., & Jeong S.-W. (2009). Bilateral cochlear implants in children. Cochlear Implants International, 10(Suppl. 1), 74–77. [DOI] [PubMed] [Google Scholar]
- Kühn-Inacker H., Shehata-Dieler W., Müller J., & Helms J. (2004). Bilateral cochlear implants: A way to optimize auditory perception abilities in deaf children? International Journal of Pediatric Otorhinolaryngology, 68, 1257–1266. [DOI] [PubMed] [Google Scholar]
- Lammers M. J., Venekamp R. P., Grolman W., & van der Heijden G. J. (2014). Bilateral cochlear implantation in children and the impact of the inter-implant interval. Laryngoscope, 124, 993–999. [DOI] [PubMed] [Google Scholar]
- Litovsky R. Y. (2011). Review of recent work on spatial hearing skills in children with bilateral cochlear implants. Cochlear Implants International, 12(Suppl. 1), S30–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky R. Y., & Gordon K. (2016). Bilateral cochlear implants in children: Effects of auditory experience and deprivation on auditory perception. Hearing Research, 338, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S. E., & Delaney H. D. (1990). Designing experiments and analyzing data: A model comparison perspective. Belmont, CA: Wadsworth. [Google Scholar]
- Nilsson M., Soli S. D., & Sullivan J. A. (1994). Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. The Journal of the Acoustical Society of America, 95, 1085–1099. [DOI] [PubMed] [Google Scholar]
- Peters B. R., Litovsky R., Parkinson A., & Lake J. (2007). Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otology & Neurotology, 28, 649–657. [DOI] [PubMed] [Google Scholar]
- Peters B. R., Wyss J., & Manrique M. (2010). Worldwide trends in bilateral cochlear implantation. The Laryngoscope, 120, S17–S44. [DOI] [PubMed] [Google Scholar]
- Peterson G. E., & Lehiste I. (1962). Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders, 27, 62–70. [DOI] [PubMed] [Google Scholar]
- Potts L. G., Skinner M. W., Litovsky R. A., Strube M. J., & Kuk F. (2009). Recognition and localization of speech by adult cochlear implant recipients wearing a digital hearing aid in the nonimplanted ear (bimodal hearing). Journal of the American Acadamy of Audiology, 20, 353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W., & Bryk A. S. (2002). Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage. [Google Scholar]
- Reeder R. M., Firszt J. B., Holden L. K., & Strube M. J. (2014). A longitudinal study in adults with sequential bilateral cochlear implants: Time course for individual ear and bilateral performance. Journal of Speech, Language, and Hearing Research, 57, 1108–1126. Retrieved from http://jslhr.pubs.asha.org/article.aspx?articleid=1832501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revit L. J., Schulein R. B., & Julstrom S. D. (2002). Toward accurate assessment of real-world hearing aid benefit. Hearing Review, 9, 34–38, 51. [Google Scholar]
- Scherf F., Van Deun L., van Wieringen A., Wouters J., Desloovere C., Dhooge I., … Van de Heyning P. (2009). Three-year postimplantation auditory outcomes in children with sequential bilateral cochlear implantation. The Annals of Otology, Rhinology, and Laryngology, 118, 336–344. [DOI] [PubMed] [Google Scholar]
- Snijders T., & Bosker R. (2012). Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage. [Google Scholar]
- Soli S. D., & Wong L. L. (2008). Assessment of speech intelligibility in noise with the Hearing in Noise Test. International Journal of Audiology, 47, 356–361. [DOI] [PubMed] [Google Scholar]
- Sparreboom M., Snik A. F., & Mylanus E. A. (2011). Sequential bilateral cochlear implantation in children: Development of the primary auditory abilities of bilateral stimulation. Audiology & Neurotology, 16, 203–213. [DOI] [PubMed] [Google Scholar]
- Sparreboom M., van Schoonhoven J., van Zanten B. G., Scholten R. J., Mylanus E. A., Grolman W., & Maat B. (2010). The effectiveness of bilateral cochlear implants for severe-to-profound deafness in children: A systematic review. Otology & Neurotology, 31, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Steffens T., Lesinski-Schiedat A., Strutz J., Aschendorff A., Klenzner T., Rühl S., … Lenarz T. (2008). The benefits of sequential bilateral cochlear implantation for hearing-impaired children. Acta Oto-Laryngologica, 128, 164–176. [DOI] [PubMed] [Google Scholar]
- Strom-Roum H., Laurent C., & Wie O. B. (2012). Comparison of bilateral and unilateral cochlear implants in children with sequential surgery. International Journal of Pediatric Otorhinolaryngology, 76, 95–99. [DOI] [PubMed] [Google Scholar]
- Strom-Roum H., Rodvik A. K., Osnes T. A., Fagerland M. W., & Wie O. B. (2012). Sound localising ability in children with bilateral sequential cochlear implants. International Journal of Pediatric Otorhinolaryngology 76, 1245–1248. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Baker S., Caraway T., Kasulis H., Mears A., Smith J., … Wood M. (2007). 1-year postactivation results for sequentially implanted bilateral cochlear implant users. Otology & Neurotology, 28, 589–596. [DOI] [PubMed] [Google Scholar]