Abstract
Purpose
The purpose of this study was to perform a comprehensive systematic review of the literature on voice-related patient-reported outcome (PRO) measures in adults and to evaluate each instrument for the presence of important measurement properties.
Method
MEDLINE, the Cumulative Index of Nursing and Allied Health Literature, and the Health and Psychosocial Instrument databases were searched using relevant vocabulary terms and key terms related to PRO measures and voice. Inclusion and exclusion criteria were developed in consultation with an expert panel. Three independent investigators assessed study methodology using criteria developed a priori. Measurement properties were examined and entered into evidence tables.
Results
A total of 3,744 studies assessing voice-related constructs were identified. This list was narrowed to 32 PRO measures on the basis of predetermined inclusion and exclusion criteria. Questionnaire measurement properties varied widely. Important thematic deficiencies were apparent: (a) lack of patient involvement in the item development process, (b) lack of robust construct validity, and (c) lack of clear interpretability and scaling.
Conclusions
PRO measures are a principal means of evaluating treatment effectiveness in voice-related conditions. Despite their prominence, available PRO measures have disparate methodological rigor. Care must be taken to understand the psychometric and measurement properties and the applicability of PRO measures before advocating for their use in clinical or research applications.
Voice disorders have an estimated point prevalence of 20 million (0.98%) in the United States (Cohen, Kim, Roy, Asche, & Courey, 2012b; Roy, Merrill, Gray, & Smith, 2005). Annual direct costs exceed $5 billion even before accounting for productivity losses due to absenteeism and presenteeism (Cohen, Kim, Roy, Asche, & Courey, 2012a; Dew, Keefe, & Small, 2005). Quality of life consequences for voice disorders have a magnitude similar to that of chronic sinusitis, sciatica, and angina pectoris (Benninger, Ahuja, Gardner, & Grywalski, 1998). A need exists to improve care, but this requires the ability to quantify a given voice disorder's effect on the patient.
Several categories of voice measurement are used in clinical practice, such as the Consensus Auditory Perceptual Evaluation of Voice (Kempster, Gerratt, Verdolini Abbott, Barkmeier-Kraemer, & Hillman, 2009); the Grade, Roughness, Breathiness, Asthenia, Strain scale (Hirano, 1981); laryngoscopy; and patient-reported outcome (PRO) measures. Of central importance are PRO measures, defined as “any report of the status of a patient's health condition that comes directly from the patient without interpretation of the patient's response by a clinician or anyone else” (Guyatt & Schunemann, 2007; Reeve et al., 2013; Snyder, Jensen, Segal, & Wu, 2013), which provide a method of systematically capturing patient perspective and experience. Including the patient experience related to treatment benefit and harm is now obligatory in the United States' preapproval regulatory setting. In fact, the U.S. Food and Drug Administration (FDA) recommends the use of PRO measures as part of the approval process for pharmacological products and devices (Ahmed et al., 2012). PRO measures have also been highlighted in the National Institutes of Health Roadmap, which identifies priority areas that have the greatest potential to drive progress in biomedical research (Patient-Centered Outcomes Research Institute, 2014).
PRO measures are increasingly used to better understand the perspectives of, and to measure concepts that matter to, the patient (Patrick et al., 2007). A National Institutes of Health/FDA working group identified three patient-centered outcome categories—feeling, function, and survival—as primary outcomes to be focused on and incorporated into all clinical trials proposing novel interventions, devices, or pharmaceuticals that aim for FDA approval (Patrick et al., 2007). Several conceptual models for PRO measure development exist, including the International Classification of Functioning, Disability and Health promoted by the World Health Organization (2001). The International Classification of Functioning, Disability and Health paradigm is considered the international standard for conceptualizing the measurement of health and disability. Its basic tenets have formed the rubric for PRO measures in a wide variety of health topics, including voice and voice disorders (Hogikyan & Sethuraman, 1999; Jacobson et al., 1997). It is important to note that although it does provide a conceptual framework, it does not provide guidance on how to develop a PRO measure.
There is also a misconception that these instruments are designed only to measure health-related quality of life. In reality, they can be designed as symptom indices (Belafsky, Postma, & Koufman, 2002; Garrow et al., 2015); measure general (McHorney, Ware, & Raczek, 1993; Ware & Sherbourne, 1992) or condition-specific (Andrae, Patrick, Drossman, & Covington, 2013; Hutchings et al., 2015) health-related quality of life, utility (Feeny, Furlong, Boyle, & Torrance, 1995; Torrance, Furlong, Feeny, & Boyle, 1995), well-being (Monk, 1981; Pouwer, Snoek, van der Ploeg, Ader, & Heine, 2000), or social health (Sherbourne & Stewart, 1991); or can focus on latent structures such as self-efficacy (Riehm et al., 2016) and willingness to change (Pleil et al., 2005; Wegener et al., 2014). Increased focus on patient-centered outcomes research has resulted in a proliferation of PRO measures with variable psychometric rigor (Johnston et al., 2015).
Methodological experts in measurement theory and survey design have disseminated several consensus statements to guide appropriate development and implementation of these measures (Aaronson et al., 2002; Feeny, Eckstrom, Whitlock, & Perdue, 2013; Mokkink et al., 2010; Patrick et al., 2007; Reeve et al., 2013; Terwee et al., 2007). Use of a poorly developed PRO measures or those designed for a purpose that differs from its use can have significant implications and lead to distorted, inaccurate, or equivocal findings (Mokkink et al., 2009, 2010; Regnault, Hamel, & Patrick, 2015). Measures should be chosen on the basis of relevance and their track record in the context of the proposed study. Therefore, it is incumbent upon researchers and other end users to carefully consider a measure's properties and weigh its strengths and potential weaknesses before implementing it in practice, clinical trials, quality improvement initiatives, or population-level studies.
To date, no criterion objective test supersedes the importance of the patient's perspective in the evaluation of voice disorders. In fact, objective metrics used in clinical practice have correlated poorly with subjective patient-reported improvement and current PRO measures (Cheng & Woo, 2010; Hsiung, Pai, & Wang, 2002; Wheeler, Collins, & Sapienza, 2006; Woisard, Bodin, Yardeni, & Puech, 2007). Although a complete voice evaluation requires some level of objective assessment, results should be contextualized within each patient's perspective and expectations (Roy et al., 2013). For instance, some people have known only a rough voice. This is their “normal” despite acoustic, cepstral, audio-perceptual, and visual-perceptual assessments indicating a disordered voice.
Practitioners recognize that if patients are satisfied with their voices and no negative health consequence exists (e.g., malignancy), then further intervention (e.g., surgery, voice therapy) is difficult to justify even if the practitioner rates the voice as disordered. As such, PRO measures are arguably the primary tools for systematically assessing both the individual's perspective and population-level burden of voice-related disease. The Cochrane Collaboration routinely uses PRO measures as primary outcome assessments in its systematic reviews of associated topics (Hopkins, Yousaf, & Pedersen, 2006; Ruotsalainen, Sellman, Lehto, Jauhiainen, & Verbeek, 2007a, 2007b).
Recognition of the importance of the patient-centered approach has led to the proliferation of PRO measures for a variety of voice-related constructs. Methodological inconsistency exists because of the complexity of proper PRO measure development. The use of questionnaires that do not adhere to meticulous measure-instrument construction principles—for example, psychometrics (Anastasi, 1988) or clinimetrics (Feinstein, 1983)—can yield spurious data and incorrect conclusions (Penson, Litwin, & Aaronson, 2003). To date, three systematic reviews of voice-related PRO measures have been published. One was performed as part of a larger 2002 Agency for Healthcare Research and Quality systematic review, at which time (1996–2000) few voice-related PRO measures existed (Biddle, Watson, Hooper, Lohr, & Sutton, 2002). A subsequent review found that all five voice quality of life instruments that it identified were incomplete in their psychometric development (Franic, Bramlett, & Bothe, 2005). Another found content validity inadequacies in all nine of the questionnaires it identified (Branski et al., 2010). These reviews had different objectives and assessed few measures currently available in the literature. Differences in number of identified studies likely relates to earlier publication dates, a specific focus on quality of life instruments (perhaps resulting in exclusion of PRO measures with different constructs; e.g., symptom severity, self-efficacy), and variable literature search strategies.
The intent of the present systematic review is to assess the measurement characteristics of all currently available adult voice-related PRO measures in order to identify their strengths, weaknesses, and applicability. It aims to evaluate each instrument's measurement properties, including conceptual model, content validity, reliability, construct validity, responsiveness to change, scoring and interpretation, and respondent burden and presentation. These important parameters have significant ramifications for the applicability of PRO measures. The ultimate goal of the present study is to fill these voids and to provide guidance in terms of how to evaluate a PRO measure and to aid in selection of an appropriate instrument for a specific application.
Methods
Search Strategy
MEDLINE via the PubMed interface, the Cumulative Index of Nursing and Allied Health Literature, and the Health and Psychosocial Instrument database were searched using relevant vocabulary terms and key terms related to PRO measures and voice (see Appendixes A–C). No restrictions on publication date were used. The initial literature search was conducted in November 2014 and was updated in April 2015. Reference lists of the included articles and recent reviews related to measurement of voice were hand searched to identify additional relevant articles.
Study Selection
Inclusion and exclusion criteria were developed in consultation with an expert panel that included a statistician with expertise in measurement theory (the seventh author), systematic review methodologists (the fourth and eighth authors), and researchers and clinicians who treat and study voice and voice disorders (the first, second, and fifth authors). Abstracts for all studies identified in the literature search were independently reviewed by three investigators (the first, second, and third authors), and those meeting predetermined abstract screening criteria (see Table 1) were advanced to full-text review. Measures focused on singing voice and pediatric voice were excluded. Articles lacking adequate information in their title or abstract to determine eligibility were also included in the full-text review phase. Three independent reviewers performed full-text review of articles to determine eligibility for data extraction. Disagreements were resolved through discussion or adjudication by a senior investigator (the seventh author). When necessary, article authors were personally contacted for further information.
Table 1.
Screening criteria for abstract review.
| Original research (includes systematic reviews and meta-analysis but not narrative reviews)? |
|---|
| Research is on human subjects? |
| Study addresses voice problems or hoarseness? |
| Study addresses a patient-reported outcome, instrument, questionnaire, or survey? |
| Study addresses development, validity testing, and/or reliability testing of a patient-reported outcome measure, instrument, survey, or questionnaire? |
| Study performed in adult population (≥18 years of age)? |
Data Extraction
One reviewer extracted all relevant data from studies meeting criteria at the full-text review phase. A second reviewer independently verified data accuracy. Components of PRO measure development were critically examined and entered into evidence tables. These included PRO measure name and acronym, authors, year published, objective and intended construct, setting of development (e.g., tertiary care, community) and country, population targeted and involved in instrument development, type of scale used (e.g., Likert, visual analog scale), number of items or questions, and, when present, what subscales or domains they were designed to specifically measure.
PRO Measure Assessment
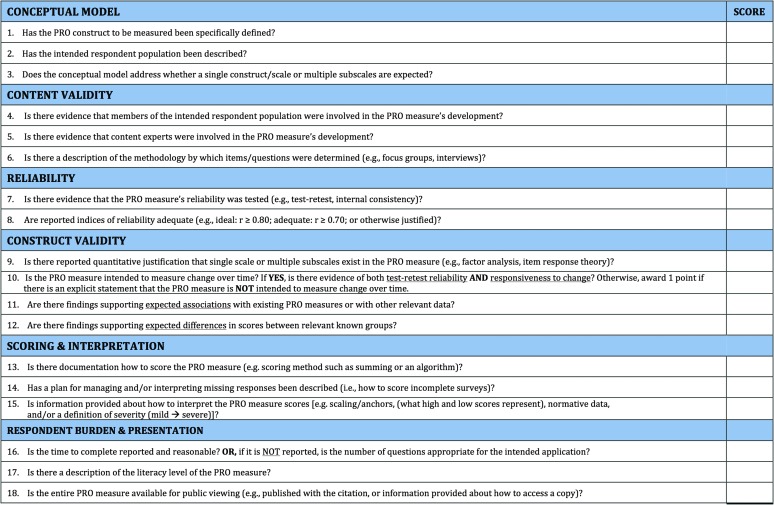
Three investigators independently assessed each study's methodology using a criteria checklist developed a priori (see Figure 1; Francis, McPheeters, Noud, Penson, & Feurer, 2016). In brief, the checklist used was designed as a tool to help systematic reviewers identify components considered important in the development of questionnaires. The checklist helps users evaluate a prospective PRO measure's conceptual model, content validity, reliability, construct validity, responsiveness to change, scoring and interpretation, and respondent burden and presentation. A glossary of important measurement properties is shown in Table 2. This tool is not meant to yield a total score, as that implies equal weighting of included items. Instead, it is intended as a guide to identify whether important measurement properties are present in current PRO measures. Each item is scored in a dichotomous manner (i.e., presence or absence of a component) and does not attempt to grade the quality of particular parameters.
Figure 1.
Checklist of key characteristics to consider when evaluating a patient-reported outcome (PRO) measure. Indicate in the score column whether the information provided in the citation/source document meets each of the criteria (0 = criterion not met, 1 = criterion met).
Table 2.
Glossary of measurement properties of patient-reported outcome (PRO) measures.
| Domain | Explanation |
|---|---|
| Conceptual model | A conceptual model provides a rationale for and description of the concepts and target population that a measure is intended to assess. |
| Content validity | Content validity refers to evidence that a PRO measure's domain(s) are appropriate for its intended use. Items and conceptual domains should be relevant to the target population's concerns. The PRO measure's development should include direct input from patients and from content experts. There should be a clear description of the process by which included questions were derived. |
| Reliability | Reliability is the degree to which scores are free from random (measurement) error. |
| Internal consistency reliability, the degree to which segments of a test (e.g., individual items) are associated with one another, reflects precision at a single time point. | |
| Test–retest reliability refers to the reproducibility of scores over two administrations, typically in close temporal proximity, among respondents who are assumed not to have changed on the relevant domains. | |
| Cited minimum levels for reliability coefficients traditionally are .70 for group-level comparisons and .90 to .95 for individual comparisons. Reliability estimates lower than these conventions should be justified in the context of the proposed PRO measure's intended application. | |
| Construct validity | Construct validity refers to whether a test measures intended theoretic constructs or traits and directly affects the appropriateness of the measurement-based inferences. Several different forms exist and are outlined below. |
| Empirical demonstration of dimensionality (e.g., factor analysis) provides evidence of whether a single scale or multiple subscales exist in the PRO measure. | |
| Responsiveness to change (longitudinal validity) is the extent to which a PRO measure detects meaningful change over time when it is known to have occurred. It is predicated on demonstration of both test–retest-reliability (stability when no change is expected) and clinically meaningful change when it is expected. | |
| Convergent validity is the degree to which a PRO measure's scores correlate with other instruments that measure the same construct or with related clinical indicators (e.g., diagnostic test). A priori hypotheses about expected associations between a PRO measure and similar or dissimilar measures should be documented. | |
| Known-groups validity is the degree to which a PRO measure is able to differentiate between groups that empiric evidence has shown to be different (e.g., cases and controls). | |
| Interpretability and scoring | Interpretability is the degree to which the meaning of the scores can be easily understood. Scoring refers to the “rules” for computing total scores or scales, if relevant. A description of how to score the measure (e.g., summation, algorithm) should be provided. |
| Missing responses are a common occurrence in both clinical and research settings and can affect an end user's ability to interpret results. A prespecified plan for managing missing responses can mitigate the risk of bias resulting from the necessity to exclude cases with missing data. | |
| Scaling is the process of distributing the full range of respondents' possible scores with respect to the measured attribute. A relative score then represents a subject's location in relation to others on a common scale. It allows cross-sectional and longitudinal quantification of the magnitude of the attribute that is reported and its change over time. Both cross-sectional and longitudinal changes in scores need to be contextualized to allow interpretation of their meaning. Ideally, scaling should be based on an understanding of what represents a clinically important or patient-important change in the construct being measured. | |
| Burden and presentation | Burden refers to the time, effort, or other demands placed on respondents or those administering the instrument. This includes number and complexity of items. The literacy level needed to understand and complete the measure is another important aspect of burden. Although most experts recommend literacy be at the sixth-grade reading level or lower, this criterion should be contextualized to the intended target population. |
| Presentation refers to a questionnaire's appearance in light of its intended mode of administration. It is important that prospective users be able to preview a measure in its entirety (e.g., items and response options) to ensure its appropriateness for the intended application. |
For this study, each reviewer was trained and calibrated on appropriate application of the checklist using a methodology described separately (Francis et al., 2016). Each reviewer reviewed six voice and swallowing PRO measures with variable psychometric construction approaches and different measurement properties without instruction. Their scoring was compared to that of individuals with extensive experience in instrument development and psychometrics. If agreement was insufficient on the first pass, they received directed education and repeated the scoring process. Reviewers for this study needed one round of instruction, after which they demonstrated near-perfect agreement with experts. Once determined to be competent, they were then independently tasked with evaluating all identified voice-related PRO measures. Upon completion, reviewers met to discuss and come to consensus on scoring discrepancies. Initial agreement was greater than 75% among reviewers for all but three parameters: justification of subscales (72%), longitudinal validity (75%), and description of item development (75%; see Table 3). All discrepancies were discussed, and articles in question were reviewed together until consensus was achieved. A senior psychometrician (the seventh author) adjudicated the few remaining discrepancies.
Table 3.
Initial rater agreement for patient-reported outcome measure assessment domain and criterion.
| Domain | Criterion | Initial agreement (%) |
|---|---|---|
| Conceptual model | Construct defined | 97 |
| Target population defined | 100 | |
| Expected subscales defined | 81 | |
| Content validity | Patients devised items | 78 |
| Content experts involved | 100 | |
| Description of item development | 75 | |
| Reliability | Reliability tested | 94 |
| Coefficients adequate | 78 | |
| Construct validity | Justification of subscales | 72 |
| Convergent validity | 81 | |
| Known-group validity | 84 | |
| Responsiveness | Longitudinal validity | 75 |
| Interpretation and scoring | Plan for scoring measure | 84 |
| Plan for missing data | 84 | |
| Scaling described | 78 | |
| Burden and presentation | Length reasonable | 94 |
| Literacy level | 97 | |
| Items viewable | 91 |
Data Synthesis
Data from unique PRO measures demonstrated wide heterogeneity in constructs, methodology, and intended purpose. Thus, data were not appropriate for aggregation or meta-analysis. Instead, individual PRO characteristics were summarized independently with respect to instrument construction and psychometric rigor.
Results
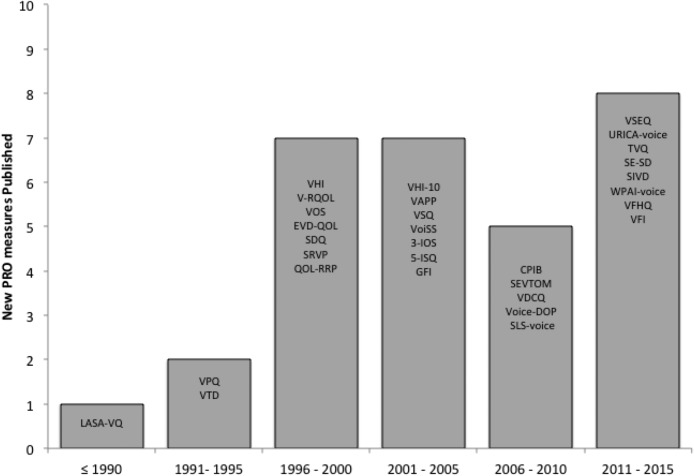
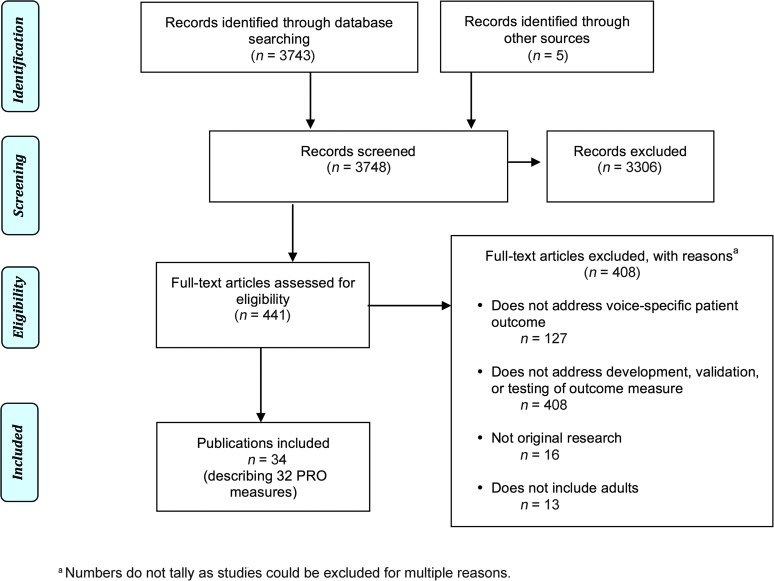
Figure 2 is the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) diagram describing the study flow and inclusions. The most common reasons for excluding articles were that they lacked relevance, did not describe de novo development or validation of an existing PRO measure, or involved a primarily pediatric population. A total of 34 studies were identified that provided initial development process data on 32 voice-related PRO measures (see Tables 4 and 5).
Figure 2.
Number and acronyms of new voice-related patient-reported outcome measures over time.
Table 4.
Measurement aims, target populations, and item characteristics of voice-related patient-reported outcome measures.
| Patient-reported outcome measure | Year | Measurement aim | Target population | Language a | Number of items/subscales | Response options | Subscales |
|---|---|---|---|---|---|---|---|
| Linear Analog Scale of AssessmentVoice Quality (LASA-VQ) | 1984 | To determine whether patients' self-assessment of voice gives reliable data and whether this method of assessment would be sensitive to clinical change occurring during the course of radiation therapy | Individuals with laryngeal cancer | English | 16 items in 2 subscales | Visual analog scales | Vocal symptoms Functional abilities |
| Vocal Performance Questionnaire (VPQ) | 1992 | To investigate the effectiveness of speech therapy in the treatment of nonorganic dysphonia | Individuals with nonorganic dysphonia | English | 12 items | 5-point Likert scale (a–e) | |
| Vocal Tract Discomfort (VTD) | 1993 | (a) To determine incidence of vocal tract discomfort in a group of patients with hyperfunctional dysphonia; (b) to assess qualitative differences in the discomfort experienced; (c) to correlate between discomfort and vocal fold mucosal damage; (d) to assess discomfort resolution time during treatment | Individuals with hyperfunctional voice disorders | English | 8 items | Dichotomous (yes/no) | |
| Evaluating Voice Disability–Quality of Life Questionnaire (EVD-QOL) | 1996 | To assess the general range of functional problems experienced with people diagnosed with voice disorders rather than to differentiate patterns of outcomes by diagnostic category | Individuals seeking evaluation for voice disorders | English | 28 items in 5 subscales | 5-point Likert scale (1–5) | Work Social Psychological Physical Communicative |
| Speech Disability Questionnaire (SDQ) | 1997 | To examine the effect of Botox injection on voice quality of individuals with spasmodic dysphonia and specifically on the disability arising from their voice problem | Individuals with spasmodic dysphonia | English | 28 items in 5 subscales | 5-point Likert scale (1–5) | Social isolation Negative communication Public avoidance Limited understanding Communication difficulty |
| Voice Handicap Index (VHI) | 1997 | To develop a psychometrically robust voice disability/handicap inventory that could be used with patients exhibiting a variety of voice disorders | Individuals with voice disorders | English | 30 items in 3 subscales | 5-point Likert scale (0–4) | Functional Physical Emotional |
| Voice Outcome Survey (VOS) | 1999 | To develop and validate a patient-relevant health-related quality of life instrument to evaluate vocal status of patients with uncompensated unilateral vocal fold paralysis | Individuals with uncompensated unilateral vocal fold paralysis | English | 5 items | 5-point Likert scale (a–e) (4 items); 3-point Likert scale (a–c) (1 item) | |
| Voice-Related Quality of Life (V-RQOL) | 1999 | To develop and validate a clinically useful instrument for measuring voice-related quality of life | Individuals with voice disorders | English | 10 items in 2 subscales | 5-point Likert scale (1–5) | Social-emotional physical functioning |
| Self-Ratings of Vocal Performance (SRVP) | 1999 | To assess vocal performance related to voice quality and vocal function of patients diagnosed with early glottic cancer, 6 months to 10 years after radiotherapy, compared to control speakers and to investigate consequences of voice impairment in daily life | Individuals with T1N0M0 glottic cancer | Dutch | 9 items | 7-point Likert scale | |
| Quality of Life in Recurrent Respiratory Papillomatosis (QOL-RRP) | 2000 | To develop a questionnaire that could be used to monitor the burden of disease on the recurrent respiratory papilloma patient in the ear, nose, and throat clinic and the speech therapy department | Individuals with recurrent respiratory papillomatosis | English | 23 items | Dichotomous (yes/no) | |
| Voice Activity and Participation Profile (VAPP) | 2001 | To develop a reliable and valid tool that could be used to assess voice activity limitation and participation restriction separately | Individuals with dysphonia | Cantonese | 28 items in 5 subscales | Visual analog scales (first item = normal to severe; remainder = never to always) | Self-perceived severity Job Daily communication Social communication Emotion |
| Voice Symptom Questionnaire (VSQ) | 2003 | To find out (a) how often telephone workers experience vocal symptoms, (b) how a short vocal training course affects subjective vocal symptoms, (c) relationship between change in voice symptoms and subjective effect of vocal training, and (d) how vocal training is experienced in general | Telephone customer advisors | Finnish | 11 items | 4-point Likert scale (1–4) | |
| Voice Symptom Scale (VoiSS) | 2003 | To devise and validate a patient-derived inventory of voice symptoms for use as a sensitive assessment tool of (a) baseline pathology and (b) response to change in adult dysphonia clinics | Individuals with dysphonia | English | 43 items in 5 subscales | 5-point Likert scale (1–5) | Communication problems Throat infection Psychosocial distress Voice sound and variability Phlegm |
| 3-Item Outcome Scale (3-IOS) | 2004 | (a) To determine the effects of voice therapy practiced by speech therapists for patients with a chronic voice disorder and (b) to compare two self-assessment instruments in order to determine their specific utility | Individuals with chronic dysphonia | Dutch | 3 items | Visual analog scales (normal to extreme impairment) | |
| Voice Handicap Index-10 (VHI-10) | 2004 | To explore and possibly develop a shortened Voice Handicap Index as a vocal function assessment tool both for initial evaluation and for longitudinal assessment of patients with voice disorders | Individuals with dysphonia | English | 10 items | 5-point Likert scale (0–4) | |
| 5-Item Screening Questionnaire (5-ISQ) | 2005 | To assess psychometric properties of a screening questionnaire designed for detection of voice impairment in clinical practice | Individuals treated for early laryngeal cancer | Dutch | 5 items | 10-point Likert scale (1–10) | |
| Glottal Function Index (GFI) | 2005 | To evaluate the validity and reliability of the Glottal Function Index and to assess its utility in evaluating patients presenting with a variety of clinical entities and following treatment thereof | Individuals with vocal fold paralysis, paresis, presbylaryngis, and others | English | 4 items | 6-point Likert scale (0–5) | |
| Voice Disability Coping Questionnaire (VDCQ) | 2007 | To develop a disease-specific measure that would allow clinicians to focus on known symptoms and selection of items, which would contain relevant problem-focused strategies for coping with dysphonia, in a shorter form | Individuals with adductor spasmodic dysphonia and muscle tension dysphonia | English | 15 items in 4 subscales | 6-point Likert scale (0-5) | Social support Passive coping Avoidance Information seeking |
| Voice Disorder Outcome Profile (Voice-DOP) | 2008 | To develop a culture-specific quality of life assessment tool for individuals with voice disorders in India | Individuals with current dysphonia | Kannada | 32 items in 3 subscales | Visual analog scales (never to always) | Physical Emotional Functional |
| Communicative Participation Item Bank (CPIB) | 2009 | To create an item bank to measure communicative participation across different communication disorder populations | Individuals with communication disorders | English | 46 items (long); 10 items (short) | 5-point Likert scale (not at all to extremely) | |
| Self-Evaluation of Voice as Treatment Outcome Measure (SEVTOM) | 2009 | To test two simple, easy-to-use questionnaires in (a) disclosing the effects of vocal loading and (b) assessing outcome of various voice hygiene interventions | Female primary school teachers | Finnish | 6 items | Visual analog scale (variable) | |
| Scleroderma Logopedic Scale (SLS-Voice) | 2010 | To develop a valid and reliable tool for the assessment of oropharyngolaryngeal manifestations of scleroderma in order to obtain a quantifiable and repeatable measure | Individuals with scleroderma | Italian English |
39 items in 5 subscales; 5 items in voice subscale | 4-point Likert scale (1–4) | Impairment Swallow Voice Multifield Quality of life |
| Voice Self-Efficacy Questionnaire (VSEQ) | 2011 | To assess subjects' self-efficacy for voice before and after interventions | Individuals with self-declared voice problems | English | 4 items | Visual analog scale (not confident to extremely confident) | |
| University of Rhode Island Change Assessment–Voice (URICA-Voice) | 2011 | To adapt the University of Rhode Island Change Assessment–Voice scale to assess the stages of readiness of patients for adherence in voice treatment | Individuals undergoing treatment for voice disorders | English Portuguese |
32 items in 2 subscales | 5-point Likert scale (strongly disagree to strongly agree) | Behavior Vocal use |
| Thyroidectomy-Related Voice Questionnaire (TVQ) | 2012 | To invent a simple questionnaire and evaluate its usefulness as a prethyroidectomy screening tool | Individuals scheduled to undergo thyroidectomy | English Korean |
20 items | 5-point Likert scale (0–4) | |
| Self-Efficacy in Spasmodic Dysphonia (SE-SD) | 2013 | To study self-efficacy in spasmodic dysphonia patients and to develop a disease-specific self-efficacy spasmodic dysphonia scale | Individuals with spasmodic dysphonia | English | 11 items | 4-point Likert scale (1–4) | |
| Screening Index for Voice Disorders (SIVD) | 2013 | To develop and validate a screening index for voice disorders in teachers | Female teachers | Brazilian Portuguese | 12 items | 4-point Likert scale (1–4) | |
| Work Productivity Activity Impairment Questionnaire–Specific Health Problem–Voice (WPAI-SHP) | 2014 | To ascertain whether existing work productivity tools are regarded by patients as adequate in assessing how the quality and quantity of a person's work is affected by spasmodic dysphonia | Individuals with spasmodic dysphonia | English | 5 items | Variable | |
| Stanford Presenteeism Scale 6 (SPS-6) | 2014 | To ascertain whether work productivity tools are regarded by patients as adequate in assessing how the quality and quantity of a person's work is affected by spasmodic dysphonia | Individuals with spasmodic dysphonia | English | 6 items | 5-point Likert scale (1–5) | |
| Voice-Related Statements (VRS) | 2014 | To determine whether an additional set of researcher-generated voice-related statements are viewed as valuable by individuals with spasmodic dysphonia | Individuals with spasmodic dysphonia | English | 14 items | 5-point Likert scale (1–5) | Time Quality Quantity Personal factors |
| Vocal Fatigue Handicap Questionnaire (VFHQ) | 2015 | To construct and validate a vocal fatigue handicap questionnaire on the basis of strict convergence of distinct conceptual and psychometric criteria with the explicit goal of providing an instrument with a high degree of (a) internal consistency, (b) test–retest reliability, (c) construct and criterion validity, and (d) degree of clinical efficacy or practical relevance | Individuals with voice disorders | Italian English |
30 items in 3 subscales | 5-point Likert scale (0–4) | Emotional Physical Functional |
| Vocal Fatigue Index (VFI) | 2015 | To develop a psychometrically validated self-report tool (a) to generate a cohesive and consensus description of primary vocal fatigue symptoms, (b) to develop a self-report tool that can reliably and validly identify and quantify vocal fatigue symptoms, and (c) to characterize component aspects of chronic vocal fatigue | Individuals with voice complaints | English | 19 items in 3 subscales | 5-point Likert scale (0–4) | Tiredness/avoidance Physical discomfort Improvement with rest |
Language used in initial development; does not refer to later translations.
Table 5.
Patient, setting, and pathology characteristics involved in the development of voice-related patient-reported outcome measures.
| Article | Patient-reported outcome measure | Study population | Setting | N | Distribution of pathology | Age: M (SD), range | Male | Country | |
|---|---|---|---|---|---|---|---|---|---|
| Llewellyn-Thomas et al. (1984) | Linear Analog Scale Assessment of Voice Quality (LASA-VQ) | Laryngeal cancer patients who were undergoing or had undergone radiation therapy | Ontario Cancer Institute | On treatment = 30 Posttreatment = 29 |
Laryngeal cancer (on treatment or posttreatment) TMN: I = 21/17; II = 6/8; III = 1/3; IV = 2/1 |
On treatment: 60.5 (NR) Posttreatment: 60.2 (NR) |
On treatment: 87.00% Posttreatment: 83.00% |
Canada | |
| Carding & Horsley (1992) | Vocal Performance Questionnaire (VPQ) | Dysphonic patients referred to the speech and language therapy clinic by staff otolaryngologists for nonorganic dysphonia | Large regional hospital | 30 | Nonorganic dysphonia = 30 | 44.3 (18.5), 18–76 | 23.30% | Great Britain | |
| Mathieson (1993) | Vocal Tract Discomfort (VTD) | Patients with hyperfunctional dysphonia diagnosed at the voice clinic | Ear, nose, and throat/speech therapy voice clinic, Northwick Park Hospital | 36 | No mucosal changes = 12; mucosal changes = 14; other dysphonia = 10 | 35 (NR), 7–59 | 42.00% | Great Britain | |
| Smith et al. (1996) | Evaluating Voice Disability–Quality of Life Questionnaire (EVD-QOL) | Patients from voice clinics and controls who accompanied patients seeking medical care or others who sought dental treatment or accompanied dental patients | Departments of Otolaryngology, University of Iowa and University of Utah | Cases = 174 Controls = 173 |
Spasmodic dysphonia = 53; neurological/paralysis = 33; nodules = 30; laryngitis = 15; MTD = 10; bowing = 4; laryngeal trauma = 4; vocal fold scar = 3; contact ulcers = 2; miscellaneous = 20 | Age ≤21 22–39 40–65 >65 |
Cases, controls 23.6%, 8.7% 27.0%, 38.2% 27.0%, 38.2% 22.4%, 15% |
Cases: 31.60% Controls: 37.20% |
United States |
| Epstein et al. (1997) | Speech Disability Questionnaire (SDQ) | Patients with adductor spasmodic dysphonia | Middlesex Hospital outpatient department | 40 | Adductor spasmodic dysphonia = 40 | 49.6 (16.5), 20–81 | 42.50% | Great Britain | |
| Jacobson et al. (1997) | Voice Handicap Index (VHI) | Patients seen in the voice clinic with a broad range of voice disorders | Voice clinic, Henry Ford Hospital | 65 | Mass lesion = 21; neurogenic = 17; laryngectomized = 17; MTD = 5; inflammatory = 3; atypical = 2 | 52.3 (16.28), NR | 38.50% | United States | |
| Gliklich et al. (1999) | Voice Outcome Survey (VOS) | Patients with unilateral uncompensated true vocal cord paralysis presenting for medialization thyroplasty and control patients presenting to emergency room with other complaints | Department of Otology and Laryngology, Harvard Medical School | Cases = 61 Controls = 48 |
UVFP = 61 | Cases: 60.5 (18.6), 16–89 Controls: 46.0 (17.7), 21–85 |
Cases: 48.00% Controls: 45.80% |
United States | |
| Hogikyan & Sethuraman (1999) | Voice-Related Quality of Life (V-RQOL) | New patients presenting with a voice complaint and patients presenting for nonvoice complaints | Voice center, University of Michigan | Item refinement: 20 Validation: Cases = 109 Controls = 22 |
NR Inflammatory = 39; neurological = 38; mass lesions = 19; other = 13 |
NR Cases: 51.2 (NR), 19–85 Controls: 49.9 (NR), 19–84 |
NR Cases: 41.30% Controls: 40.90% |
United States | |
| Verdonck-de Leeuw et al. (1999) | Self-Ratings of Vocal Performance (SRVP) | Patients following radiotherapy for early glottic cancer and controls with no known voice defect | Department of Otorhinolaryngology—Head and Neck Surgery, Academic Hospital Vrije Universiteit | Cases = 50 Controls = 20 |
T-stage: 1a = 36; 1b = 14 | Age <65 65–70 70–75 >75 |
Cases, controls 16, 10 20, 6 8, 2 6, 2 |
100.00% | The Netherlands |
| Hill et al. (2000) | Quality of Life in Recurrent Respiratory Papillomatosis (QOL-RRP) | Patients who underwent either outpatient review or surgical clearance of laryngeal papillomas | Royal National Throat, Nose, and Ear Hospital | 26 | RRP = 26 | 42.2 (NR) | 53.80% | Great Britain | |
| Ma & Yiu (2001) | Voice Activity and Participation Profile (VAPP) | Dysphonic subjects with various laryngeal pathologies | Department of Speech and Hearing Sciences, The University of Hong Kong | Item development: 45 SLP = 10 Refinement: 9 SLPs = 10 SLP students = 13 Administration: Cases = 40 Controls = 40 |
NR NR Nodules = 12; polyp = 3; chronic laryngitis = 9; thickened cord = 6; UVFP = 3; miscellaneous = 7 |
NR Cases: 41.33 (13.31), 23–58 Cases: 36.83 (10.04), 20–57 Controls: 35.65 (9.81), 20–55 |
NR Cases: 11.00% Cases: 20.00% Controls: 20.00% |
Hongkong | |
| Lehto et al. (2003) | Voice Symptom Questionnaire (VSQ) | Customer advisors who mainly use the telephone during their work hours at a call center | Telecommunications operator Sonera | 48 | Edema = 3; erythema = 7; incomplete closure = 1; normal = 37 | Female: 29 (NR), 21–40 Male: 26 (NR), 21–38 |
20.80% | Finland | |
| Scott et al. (1997) | Voice Symptom Scale (VoiSS), Phase 1 | Patients referred complaining of hoarseness | Phoniatric clinic, Glasgow Royal Infirmary | 133 | NR | 54 (NR), 18–80 | 32.30% | Great Britain | |
| Deary et al. (2003) | Voice Symptom Scale (VoiSS), Phases 2 and 3 | Typical patients with dysphonia presenting to the ear, nose, and throat department | Voice center, University of Newcastle | Pilot = 168 Refinement = 180 |
NR Functional = 51; vocal cord palsy = 25; laryngitis = 21; “acid” laryngitis = 9; Reinke's edema = 9; asthma = 6; malignancy = 6; RRP = 6; globus/phlegm = 5; nodules = 5; leukoplakia = 4; polyp = 3; granuloma = 3; exophytic lesion = 2; cricoarytenoid joint arthritis = 2; puberphonia = 2; miscellaneous = 6; resolving/normal = 15 |
Male: 49.8 (16.0) Female: 48.4 (13.9) Male: 55.4 (14.0) Female: 53.4 (16.0) |
25.60% 35.00% |
Great Britain | |
| Speyer et al. (2004) | 3-Item Outcome Scale (3-IOS) | Patients with chronic dysphonia diagnosed by phoniatrician | Phoniatric department, University Hospital Utrecht | 77 | MTD = 12; submucosal swelling = 7; nodules = 10; polyps = 6; UVFP = 7; slight vocal fold abnormalities = 24; severe vocal fold abnormalities = 11 | Male: 47 (NR) Female: 40 (NR) Range: 18–76 |
44.20% | The Netherlands | |
| Rosen et al. (2004) | Voice Handicap Index-10 (VHI-10) | Patients with dysphonia presenting to the voice clinic and volunteers consisting of family members of patients visiting the department | Laryngology clinic, University of Pittsburgh | Item analysis: Cases = 100 Controls = 159 Longitudinal = 59 Comparative: Cases = 819 Controls = 173 |
Functional = 5; allergic laryngitis = 2; LPR = 13; MTD = 11; neurologic, other = 2; Parkinson's = 2; paradoxical vocal fold motion = 1; psychologic disease = 1; puberphonia = 1; Reinke's edema = 7; RRP = 3; abductor spasmodic dysphonia = 1; adductor spasmodic dysphonia = 2; subglottic stenosis = 1; atrophy = 3; cancer = 1; cyst = 10; vocal process granuloma = 1; nodules = 7; UVFP = 10; paresis = 1; polyp = 9; scar = 5 Functional = 1; MTD = 13; paradoxical vocal fold motion = 3; Reinke's edema = 3; cancer = 1; cyst = 4; granuloma = 1; nodules = 2; UVFP = 15; paresis = 2; polyp = 10; scar = 4 UVFP = 104; paresis/atrophy = 90; MTD = 147; polyp/cyst/nodule = 166; paradoxical vocal fold motion = 87; functional = 32; reflux = 37; scar = 54; neurologic = 52; Reinke's edema = 27; adductor spasmodic dysphonia = 23 |
NR NR NR |
NR NR NR |
United States | |
| Arffa et al. (2012) | Voice Handicap Index-10 (VHI-10), normative | Family members of otolaryngology patients without voice complaints | Laryngology clinic, University of Pittsburgh | 156 | Healthy controls = 156 | NR | 32.00% | United States | |
| van Gogh et al. (2005) | 5-Item Screening Questionnaire (5-ISQ) | Patients visiting the outpatient clinic for follow-up visit after initial radiation or endoscopic surgery for early glottic cancer and controls without voice complaints | Department of Otorhinolaryngology—Head and Neck Surgery, Vrije University Medical Center | Cases = 177 (radiation = 126; surgery = 51) Controls = 110 |
Laryngeal cancer (dysplasia to T2) | Cases: Radiation = 66 (NR), 39–80 Surgery = 66 (NR), 40–81 Controls: 61(NR), 40–80 |
Cases: Radiation = 92.90% Surgery = 88.20% Controls = 50.00% |
The Netherlands | |
| Bach et al. (2005) | Glottal Function Index (GFI) | Patients presenting with dysphonia resulting from a variety of clinical entities and following treatment thereof | Center for Voice Disorders, Wake Forest University | Item development = NR Responsiveness = 40 Specificity: Cases = 120 Controls = 40 |
NA Glottic insufficiency = 40 Nodules = 40; adductor spasmodic dysphonia = 40; granuloma = 40 |
NA Median: 49 Cases: NR Controls: median = 39 |
NA 37.00% Cases: NR Controls: 50.00% |
United States | |
| Laukkanen et al. (2009) | Self-Evaluation of Voice as Treatment Outcome Measure (SEVTOM) | Female primary school teachers with functionally healthy voices | Recruited via Internet questionnaire | 90 | Not evaluated/functionally normal = 90 | 41.1 (8.5) | 0.00% | Finland | |
| Baylor et al. (2009) | Communicative Participation Item Bank (CPIB) | Adults with spasmodic dysphonia | Recruited through multiple sources: NSDA, ASHA SIG 3, and local voice clinics | 208 | Spasmodic dysphonia = 208 | 55.4 (11.0), 27–83 | 22.10% | United States | |
| Baylor et al. (2013) | Communicative Participation Item Bank (CPIB) | Adults with multiple sclerosis, Parkinson's disease, amyotrophic lateral sclerosis, and head and neck cancer | Recruited through multiple sources: Internet listserv, support groups, and local disease registries | 701 | Multiple sclerosis = 216; Parkinson's disease = 218; amyotrophic lateral sclerosis = 70; head and neck cancer = 197 | 58.8 (12.4), 24–99 | 45.70% | United States | |
| Epstein et al. (2009) | Voice Disability Coping Questionnaire (VDCQ) | Voice clinic referrals at one hospital | Voice clinic, Royal National Throat, Nose, and Ear Hospital and Ear Institute | 80 | Adductor spasmodic dysphonia = 40; MTD = 40 | Overall: 45.4 (NR) Adductor spasmodic dysphonia: 49.70 (16.281) MTD: 41.31 (19.569) |
35.00% | Great Britain | |
| Konnai et al. (2010) | Voice Disorder Outcome Profile (Voice-DOP) | Individuals with current dysphonia and age-matched controls | All India Institute of Speech and Hearing, Mysore; St. Johns Medical College and Hospital, Bangalore; Government ENT Hospital, Hyderabad | Development: SLPs = 10 SLP students = 10 People with dysphonia = 5 Refinement: SLPs = 10 SLP students = 5 Administration: Cases = 42 Controls = 30 |
Different vocal pathologies = 5 NA Nodules = 6; glottic chink = 6; carcinoma = 6; gastroesophageal reflux disease = 6; puberphonia = 7; UVFP = 4; laryngitis = 2; atypical = 5 |
NR NA 3. 34 (NR), 18–60 |
NR NA 3.83% |
India | |
| Vitali et al. (2010) | Scleroderma Logopedic Scale (SLS-Voice) | Patients with laboratory-defined systemic sclerosis disease | Ospedale Maggiore, Immunology Clinical Unit | Focus group = NR Pilot = 28 Phase 2 = 16 Phase 3 = 15 Administration: Cases = 86 Controls = 40 |
Systemic sclerosis N = 28 N = 16 N = 15 N = 86 |
NR NR NR Cases: 57.0 (12.7) Controls: 56.1 (7.5) |
NR NR NR Cases: 19.00% Controls: 20.00% |
Italy | |
| Gillespie & Abbott (2011) | Voice Self-Efficacy Questionnaire (VSEQ) | Teachers from the Pittsburgh Public School District who reported current or past self-identified voice problem | University of Pittsburgh Voice Center | Item development: SLPs = 2 Administration = 14 |
NR | NR | 14.00% | United States | |
| Teixeira et al. (2013) | University of Rhode Island Change Assessment–Voice (URICA-Voice) | Patients receiving voice therapy at the university hospital at two institutions | Department of Speech-Language Pathology and Audiology, Hospital of the Universidade Federal de Minas Gerais and Hospital San Paulo, Universidade Federal de Sao Paulo | 66 | Behavioral dysphonia = 60 Nonbehavioral dysphonia = 6 |
42.27 (NR), 18–68 | 12.10% | Brazil | |
| Nam et al. (2012) | Thyroidectomy-Related Voice Questionnaire (TVQ) | Patients scheduled to undergo thyroidectomy | Department of Otolaryngology and Surgery, The Catholic University of Korea | 500 | LPR = 136; nodules = 24; polyp = 9; vocal fold palsy = 6; Reinke's edema = 2; cyst = 1; vocal sulcus = 1; normal = 321 | 45.5 (11.97), 16–76 | 17.60% | Korea | |
| Hu et al. (2013) | Self Efficacy in Spasmodic Dysphonia (SE-SD) | Spasmodic dysphonia patients | Department of Otolaryngology, University of Washington | Item development: Laryngologists = 3 Fellow = 1 SLP = 3 Patients = 2 Administration = 145 |
NR Adductor spasmodic dysphonia = 139; abductor spasmodic dysphonia = 6 |
NR 59.5 (13.6) |
NR 24.80% |
United States | |
| Ghirardi et al. (2013) | Screening Index for Voice Disorders (SIVD) | Current female teachers from the public school system in San Paulo, Brazil | Pontifical Catholic University of San Paulo and School of Public Health at University of San Paulo | Item development = 252 Internal validation = 130 External validation = 122 |
NR NR Voice disorder = 73; no voice disorder = 49 |
NR 40.6 (NR) 39.4 (NR) |
0.00% | Brazil | |
| Isetti & Meyer (2014) | Work Productivity Activity Impairment Questionnaire–Specific Health Problem–Voice (WPAI-SHP) | Patients diagnosed with spasmodic dysphonia getting treatment at the academic voice center | Department of Otolaryngology, University of Washington | 9 | Spasmodic dysphonia = 9 | 51 (13.2), 28–71 | 33.00% | United States | |
| Isetti & Meyer (2014) | Stanford Presenteeism Scale 6 (SPS-6) | Patients diagnosed with spasmodic dysphonia getting treatment at the academic voice center | Department of Otolaryngology, University of Washington | 9 | Spasmodic dysphonia = 9 | 51 (13.2), 28–71 | 33.00% | United States | |
| Isetti & Meyer (2014) | Voice-Related Statements (VRS) | Patients diagnosed with spasmodic dysphonia getting treatment at the academic voice center | Department of Otolaryngology, University of Washington | 9 | Spasmodic dysphonia = 9 | 51 (13.2), 28–71 | 33.00% | United States | |
| Paolillo & Pantaleo,(2015) | Vocal Fatigue Handicap Questionnaire (VFHQ) | Patients with voice disorders at an academic medical center | San Leopoldo Mandic Hospital | Item development: Laryngologists = 3 SLP = 1 Patients = 20 Item reduction = 30 Validation = 87 |
Neurogenic = 2; structural = 9; inflammatory = 4; functional = 5 Neurogenic = 2; structural = 15; inflammatory = 7; functional = 6 Neurogenic = 6; structural = 44; inflammatory = 19; functional = 18 |
44.28 (15.04), NR 43.82 (12.4), NR 43.33 (16.83), NR |
40.00% 30.00% 28.00% |
Italy | |
| Nanjundeswaran et al. (2015) | Vocal Fatigue Index (VFI) | Voice clinic referrals at two academic voice centers | University of Pittsburgh Voice Clinic, Vanderbilt Voice Center | Item development: Laryngologists = 4 SLPs = 6 Initial testing = 197 Validation: Cases = 98 Controls = 70 |
NA Atrophy = 29; membranous lesion = 46; MTD/dysphonia = 48; granuloma = 3; paralysis = 18; scar = 6; ADSD = 16; laryngitis = 4; cancer = 4; tremor = 2; RRP = 3; leukoplakia = 9; paresis = 1; LPR = 1; hemorrhage = 1; chondroma = 1; NOS = 3 Scar = 6; paralysis = 14; atrophy = 15; paresis = 7; membranous lesion = 24; granuloma = 1; MTD/dysphonia = 17; RRP = 2; LPR = 2; edema = 2; hemorrhage = 2; subepithelial mass = 1; leukoplakia = 3; laryngitis = 1; NOS = 8 |
NA UPVC: 51.76 (19.54) VVC: 50.94 (16.01) Controls: 39 (15) Cases: NR |
NA 37.00% Controls: 30.00% Cases: NR |
United States | |
Note. TNM =cancer stage (T = tumor size, N = nodal status, M = metastases); NR = not recorded; MTD = muscle tension dysphonia; UVFP = unilateral vocal fold paralysis; SLP = speech-language pathologist; LPR = laryngopharyngeal reflux; RRP = recurrent respiratory papillomatosis; NSDA = National Spasmodic Dysphonia Association; ASHA = American Speech-Language-Hearing Association; SIG 3 = American Speech-Language-Hearing Association Special Interest Group 3; ADSD = adductor spasmodic dysphonia; NOS = not otherwise specified; UPVC = University of Pittsburgh Voice Center; VVC = Vanderbilt Voice Center.
Publication year ranged from 1984 (Linear Analog Scale Assessment of Voice Quality [LASA-VQ]; Llewellyn-Thomas et al., 1984) to 2015 (Vocal Fatigue Index [VFI]; Nanjundeswaran, Jacobson, Gartner-Schmidt, & Verdolini Abbott, 2015), with increasingly more instruments being introduced over time (see Figure 3). In order of frequency, PRO measures were developed in the United States (13), Great Britain (7), the Netherlands (3), Brazil (2), Italy (2), Canada (1), Hong Kong (1), Finland (1), India (1), and South Korea (1; see Table 5). Development of each instrument occurred at an academic center. Sample size used in the instrument development process varied from nine to 1,310 subjects (see Table 5). One measure was a subscale within a broader instrument: Scleroderma Logopedic Scale (SLS-Voice; Vitali et al., 2010). Ten studies did not report the age and/or gender distribution of respondents (cases and controls, when applicable) used in each step of PRO measure development: Voice-Related Quality of Life (V-RQOL; Hogikyan & Sethuraman, 1999), Voice Activity and Participation Profile (VAPP; Ma & Yiu, 2007), Glottal Function Index (GFI; Bach, Belafsky, Wasylik, Postma, & Koufman, 2005), Voice Handicap Index-10 (VHI-10; Arffa, Krishna, Gartner-Schmidt, & Rosen, 2012; Rosen, Lee, Osborne, Zullo, & Murry, 2004), Voice Self-Efficacy Questionnaire (VSEQ; Gillespie & Abbott, 2011), VFI, Screening Index for Voice Disorders (SIVD; Ghirardi, Ferreira, Giannini, & Latorre Mdo, 2013), Self-Efficacy in Spasmodic Dysphonia (SE-SD; Hu et al., 2013), SLS-Voice, and Voice Disorder Outcome Profile (Voice-DOP; Konnai, Jayaram, & Scherer, 2010). Distribution of pathology among respondents differed by PRO measure. Among voice-related PRO measures, only one of 32 used item response theory psychometric techniques (Communicative Participation Item Bank [CPIB]; Baylor et al., 2013, 2014; Baylor, Yorkston, Eadie, Miller, & Amtmann, 2009; Eadie et al., 2014), whereas the remainder applied clinimetric (Feinstein, 1983) or classical test theory (Anastasi, 1988) methodology.
Figure 3.
Disposition of studies identified for this review. PRO = patient-reported outcome.
Constructs Measured
The constructs measured were heterogeneous (see Table 4) and included the following:
Coping: Voice Disability Coping Questionnaire (VDCQ; Epstein, Hirani, Stygall, & Newman, 2009)
Quality of life: Voice Outcome Survey (VOS; Gliklich, Glovsky, & Montgomery, 1999), V-RQOL, Voice-DOP, Evaluating Voice Disability–Quality of Life Questionnaire (EVD-QOL; Smith et al., 1996), 3-Item Outcome Scale (3-IOS; Speyer, Wieneke, & Dejonckere, 2004)
Handicap: Voice Handicap Index (VHI; Jacobson et al., 1997; Rosen, Murry, Zinn, Zullo, & Sonbolian, 2000), VHI-10, CPIB
Vocal performance: Vocal Performance Questionnaire (VPQ; Carding & Horsley, 1992; Carding, Horsley, & Docherty, 1998)
Vocal impairment: Self-Ratings of Vocal Performance (SRVP; Verdonck-de Leeuw et al., 1999)
Vocal fatigue: Self-Evaluation of Voice as Treatment Outcome Measure (SEVTOM; Laukkanen, Leppanen, & Ilomaki, 2009), VFI, Vocal Fatigue Handicap Questionnaire (VFHQ; Paolillo & Pantaleo, 2015)
Voice quality: LASA-VQ, Thyroidectomy-Related Voice Questionnaire (TVQ; Nam et al., 2012)
Self-efficacy: SE-SD, VSEQ
Work productivity: Work Productivity Activity Impairment Questionnaire–Specific Health Problem–Voice (WPAI-SHP; Isetti & Meyer, 2014), Stanford Presenteeism Scale 6 (SPS-6; Isetti & Meyer, 2014), Voice-Related Statements (VRS; Isetti & Meyer, 2014)
Activity limitation: VAPP
Disability: Speech Disability Questionnaire (SDQ; Epstein, Stygall, & Newman, 1997)
Burden of disease: Quality of Life in Recurrent Respiratory Papillomatosis (QOL-RRP; Hill, Akhtar, Corroll, & Croft, 2000)
Voice symptoms: GFI, Voice Symptom Scale (VoiSS; Deary, Wilson, Carding, & MacKenzie, 2003; Scott, Robinson, Wilson, & Mackenzie, 1997), Voice Symptom Questionnaire (VSQ; Lehto, Rantala, Vilkman, Alku, & Backstrom, 2003), SLS-voice
Vocal tract discomfort: Vocal Tract Discomfort (VTD; Mathieson, 1993)
Adherence to voice therapy: University of Rhode Island Change Assessment–Voice (URICA-Voice; Teixeira et al., 2013)
Screening for voice disorders: SIVD, 5-Item Screening Questionnaire (5-ISQ; van Gogh et al., 2005)
Disease-Specific and General Voice-Related PRO Measures
Instruments were divided into condition-specific and general voice PRO measures (see Table 4). Diseases or conditions specifically targeted included laryngeal cancer (LASA-VQ, 5-ISQ, SRVP), nonorganic or hyperfunctional dysphonia (VPQ), spasmodic dysphonia (VDCQ, SDQ, SE-SD, WPAI-SHP/SPS-6/VRS), unilateral vocal fold paralysis (VOS), recurrent respiratory papillomatosis (QOL-RRP), scleroderma (SLS-Voice), and patients undergoing thyroidectomy (TVQ). Three focused on occupational voice use, including two specific to teachers (SEVTOM, SIVD) and one for telephone customer advisors (VSQ). The remaining PRO measures were aimed at a more general respondent population that may either be at risk for or currently have various voice-related or communication-impairing conditions.
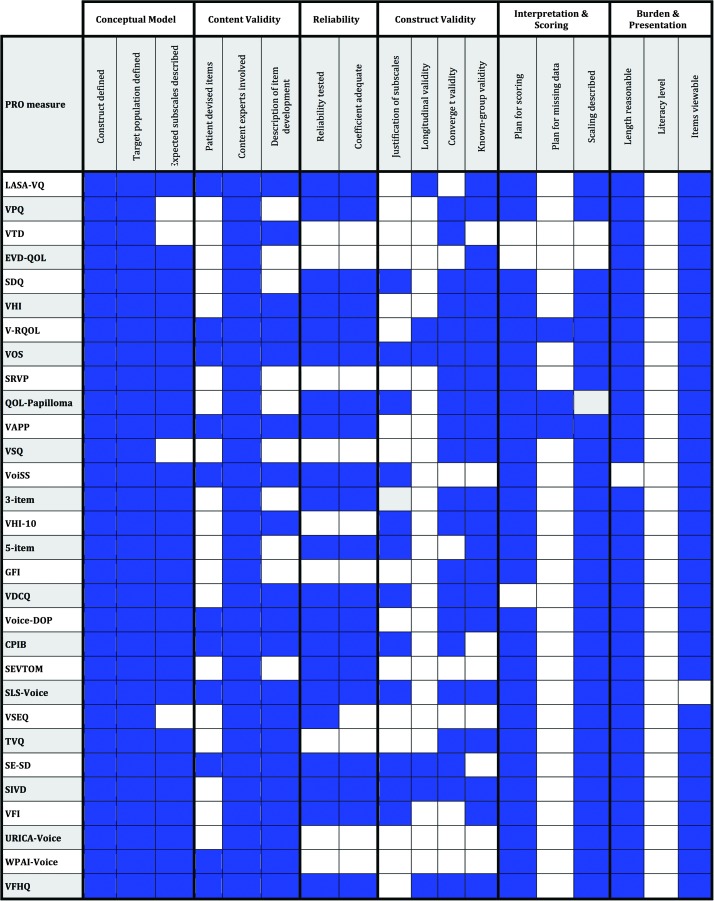
Assessment of Measurement Characteristics
Figure 4 provides an itemized, schematic overview of the measurement characteristics and utility for the 32 identified voice-related PRO measures. Predominant patterns among these instruments with domain-specific examples are described below. The VHI and VHI-10 are the most commonly used voice-related PRO measures and the most familiar to practitioners and clinical researchers. Because of their familiarity and common use, we chose to use these instruments as exemplars when possible.
Figure 4.
Summary comparison of measurement properties among identified patient-reported outcome (PRO) measures. LASA-VQ = Linear Analog Scale Assessment of Voice Quality; VPQ = Vocal Performance Questionnaire; VTD = Vocal Tract Discomfort; EVD-QOL = Evaluating Voice Disability–Quality of Life Questionnaire; SDQ = Speech Disability Questionnaire; VHI = Voice Handicap Index; V-RQOL = Voice-Related Quality of Life; VOS = Voice Outcome Survey; SRVP = Self-Ratings of Vocal Performance; QOL-RRP = Quality of Life in Recurrent Respiratory Papillomatosis; VAPP = Voice Activity and Participation Profile; VSQ = Voice Symptom Questionnaire; VoiSS = Voice Symptom Scale; 3-IOS = 3-Item Outcome Scale; VHI-10 = Voice Handicap Index-10; 5-ISQ = 5-Item Screening Questionnaire; GFI = Glottal Function Index; VDCQ = Voice Disability Coping Questionnaire; Voice-DOP = Voice Disorder Outcome Profile; CPIB = Communicative Participation Item Bank; SEVTOM = Self-Evaluation of Voice as Treatment Outcome Measure; SLS-Voice = Scleroderma Logopedic Scale; VSEQ = Voice Self-Efficacy Questionnaire; TVQ = Thyroidectomy-Related Voice Questionnaire; SE-SD = Self-Efficacy in Spasmodic Dysphonia; SIVD = Screening Index for Voice Disorders; VFI = Vocal Fatigue Index; URICA-Voice = University of Rhode Island Change Assessment–Voice; WPAI-SHP = Work Productivity Activity Impairment Questionnaire–Specific Health Problem–Voice; VFHQ = Vocal Fatigue Handicap Questionnaire.
Conceptual Model
Development of the PRO measures varied. All included some description of the respective conceptual model. Each defined the PRO construct to be measured and identified the intended respondent population. Deficiencies related to failure to address whether the tool was expected to have a single scale or multiple subscales were noted in four of 32 PRO measures: VPQ, VTD, VSQ, and VSEQ (see Figure 4).
Content Validity
All measures demonstrated some degree of content validity, albeit with considerable inconsistency and variable methodological rigor. Most (22/32) provided some description of the process used to derive included items in their respective PRO measure (see Figure 4). In contrast, few PRO measures included subjects in the item development process. Ten (41%) sought direct patient input (e.g., focus groups, interviews) to inform the content of items included in the respective measures: LASA-VQ, VOS, V-RQOL, VAPP, VoiSS, CPIB, Voice-DOP, SLS-Voice, WPAI-SHP/SPS-6/VRS, and VFHQ. In contrast, all 32 included content experts (e.g., voice practitioners, speech-language pathologists, laryngologists, otolaryngologists) in the instrument construction process.
Reliability
Reliability (e.g., test–retest, internal consistency) of the final proposed PRO measure was not tested in nine of 32 identified: VTD, EVD-QOL, SRVP, VSQ, VHI-10, GFI, URICA-Voice, TVQ, and WPAI-SHP/SPS-6/VRS (see Figure 4). Some studies performed reliability testing, but not on the final PRO measure items. For instance, in developing the VHI-10, initial item-analysis reliability testing identified nine items (VHI-9) that had combined coefficient alpha of 0.90. After reliability testing, a clinical consensus conference of content experts convened to determine which of the original 30 VHI items were most clinically relevant. Only five items identified by the experts overlapped with the reliability-tested VHI-9. Thus, five reliability-tested items were combined with five items deemed most clinically relevant. The final combined 10 items in the VHI-10 were not retested for reliability. Among the PRO measures that appropriately tested reliability, 20 of 21 reported adequate reliability indices (r ≥ .70) or justified lower values (Aaronson et al., 2002; Reeve et al., 2013).
Construct Validity
The 32 studies included were less consistent in demonstrating various forms of construct validity, one of which evaluated whether a measure's factor or subscale structure was empirically justified. Overall, 12 of 32 voice-related PRO measures statistically verified the existence of either a single scale (e.g., common factor) or discrete subscales: SDQ, VOS, QOL-RRP, VoiSS, VHI-10, 5-ISQ, CPIB, VDCQ, SLS-Voice, SE-SD, SIVD, and VFI (see Figure 4). Others, including the VHI, did not provide a statistical basis for their subscale structure (i.e., emotional, functional, and physical).
A second characteristic considered in this category was longitudinal validity, defined as both demonstrated test–retest reliability (e.g., stability in the absence of any known change) and responsiveness to change. This criterion was alternatively met if the PRO measure was designed for screening purposes and this was explicitly stated and/or the measure was not intended to track change in its construct over time. Only three studies adequately demonstrated evidence of longitudinal validity (VOS, V-RQOL, LASA-VQ), and an additional three fulfilled the criterion as they were designed for screening purposes and/or did not intend at the time of publication to track their construct's change over time (SE-SD, SIVD, VFHQ).
Convergent validity is a third form of construct validity evaluated. It exists when the candidate PRO measure shows an expected association with other existing PRO measures that focus on similar construct or relevant clinical correlates (e.g., objective, physiologic data). Overall, 21 of 32 studies showed evidence of this form of validity: VPQ, VTD, SDQ, VHI, VOS, V-RQOL, SRVP, QOL-RRP, VAPP, VSQ, 3-IOS, VHI-10, GFI, CPIB, VDCQ, Voice-DOP, SLS-Voice, TVQ, SE-SD, SIVD, and VFHQ (see Figure 4). A fourth form of construct validity, known-group validity, refers to expected differences between groups known to differ in the degree of the construct (e.g., cases and controls). In all, 22 of 32 PRO measures met this criterion: LASA-VQ, VPQ, EVD-QOL, SDQ, VHI, VOS, V-RQOL, SRVP, QOL-RRP, VAPP, VSQ, 3-IOS, VHI-10, 5-ISQ, GFI, VDCQ, Voice-DOP, SLS-Voice, TVQ, SIVD, VFHQ, and VFI (see Figure 4).
Scoring and Interpretation
Each study provided some description of either how to score the measure or a means to interpret the scores. Although the majority provided both, three PRO measures did not describe the proposed scoring approach or algorithm: VDCQ, VTD, and EVD-QOL. Among those that did, the most common scoring method was simple summation. No PRO measure addressed missing data. In other words, none offered a plan for managing or interpreting PRO measures that had missing responses. A third parameter assessed whether an explanation of how to interpret the PRO measure score was provided. Most (29/32) stated that higher scores were worse without offering mathematically justified scaling (see Figure 4). In fact, no PRO measures offered statistically justified anchors to help interpret what a particular severity score indicated.
Respondent Burden and Presentation
Current reviewers felt the time needed to complete the items was reasonable in 31 of 32 voice-related PRO measures (see Figure 4). All but one instrument presented their full set of questions in a published article or referenced an accessible source (Vitali et al., 2010). No study described the literacy level for their respective PRO measure.
Discussion
Growing emphasis on patient-centered outcomes research and comparative effectiveness research has increased the need to capture accurate, patient-centered data to evaluate the effectiveness of treatment, management, and direct decision making. PRO measures are the predominant method used to systematically collect patient perspective and experience. They can be designed to quantify relatively qualitative phenomena for which objective measures are lacking or inadequate. This task is particularly applicable and relevant to voice disorders wherein the patient's perspective contextualizes its severity on disability or quality of life.
Recognition of the importance of PRO measures in voice disorders has sparked rapid growth in their number and construct diversity (e.g., quality of life, coping) since the first PRO measure was introduced into this field in 1984 (LASA-VQ). Questionnaires also differ in applicability. Some voice-related PRO measures are disease specific (e.g., spasmodic dysphonia, unilateral vocal fold paralysis), whereas others are designed to assess a broader, diverse population. This expansion unfortunately has also led to wide variability in the methodological rigor of their development. Identifying the appropriate PRO measure for a given purpose requires nuanced understanding of a particular measure's underlying conceptual model and methodological properties (e.g., clinimetrics, psychometrics).
This systematic review extracted existing voice-related PRO measures and reviewed their content, measurement characteristics, and applicability. A total of 32 voice-related instruments were identified and analyzed. Catchment was higher than prior systematic reviews (Biddle et al., 2002; Branski et al., 2010; Franic et al., 2005) on the basis of the current review's contemporariness, broad inclusion criteria, and exhaustive systematic literature search.
As expected, psychometric rigor was quite disparate among identified PRO measures. The range of target individuals involved in the development and/or validation of these measures varied significantly from nine to more than 1,300 (Isetti & Meyer, 2014; Rosen et al., 2004). It is generally recommended that variable and subject sampling are optimized for factor/principal components analysis–based methods and/or that there be more than 100 subjects involved in validation (Terwee et al., 2007; Velicer & Fava, 1998). Few studies achieved this standard. Adequacy and applicability of measures that include few individuals from the target population in development should be questioned. Three PRO measures identified were developed for use in another population and were being adapted and tested post hoc for application in individuals with voice problems (Isetti & Meyer, 2014; Teixeira et al., 2013). Revalidating a measure before applying it to a new target population is an often overlooked and important step. Distorted results are a risk when end users implement implement PRO measures designed for a specific target population into a new application or population.. Outcomes that are based on responses to a measure that is not designed for a particular population may not be valid. Thus, it is important for PRO measure developers to fully describe the population used in their creation (e.g., age, gender, race, disorder). Many voice-related tools did not provide this information.
Lack of Patient Centeredness
Only 41% of PRO measures directly engaged patients in the item development stage (i.e., devising items) despite claiming to be patient centric. Lack of empiric content data from patients significantly limits content validity. The foundation for PRO measures is the target population perspective and experience. Thus, omitting patients at this stage compromises the validity of scores and creates a condition in which patients answer questions that are designed by and based on the experience and opinions of content experts who do not live with their particular condition. This concern was highlighted in a prior systematic review regarding content validity of voice-related PRO measures (Branski et al., 2010). The report found that five of nine PRO measures did not include patients in item development. It is interesting to note that the VHI was one measure found to have included patients in this process. Careful re-evaluation of the original VHI article and correspondence with authors reveals that items were derived from case history in chart reviews, not from empiric patient interviews or focus groups. Case histories rely on provider recollection and therefore are a provider's interpretation of the patient's perspective. This deficiency was not recognized by prior systematic reviews (Biddle et al., 2002; Branski et al., 2010; Franic et al., 2005). As a consequence, the content validity of the VHI and, transitively, the VHI-10 may be overestimated.
Development Characteristics
This analysis was not designed to evaluate every study that used or translated voice-related PRO measures. Rather, it focused on identifying and evaluating the strengths and weaknesses of measurement properties. Most published measures purported reliability and validity. This simple statement is often considered sufficient legitimization of a PRO measure's quality by end users. It is important to recognize that reliability and validity are not discrete concepts and exist on a spectrum (Newton & Shaw, 2013).
Half of voice PRO measures (50%) met at least one criteria in each measurement domain assessed, and none met all criteria. Reasons for deficiencies are multifactorial. Some may be due to lack of understanding of the complex underlying psychometric methodology and the time intensity necessary to create a high-quality PRO measure. As an alternative, other instruments still may be under development. Often, PRO measures are devised and published in stages, as exemplified by the VoiSS, VHI-10, and CPIB.
Construct Validity
PRO measure development was scrutinized for quantitative justification of proposed subscales such as factor analysis or item response theory techniques (Hambleton & Swaminathan, 1985). The VHI and 13 other instruments cited subscales (e.g., emotional, physical, and functional) without evaluating their empirical basis using item-level analysis (see Figure 4). Without proven, discrete subscales, all items may measure the same overall construct. For instance, an analysis of VHI items during the development of the VHI-10 found a common factor: All VHI questions, despite assignment to the social, emotional, or functional subscales, measure aspects of the same construct. However, a separate factor analysis performed as part of cross-cultural adaption of the VHI and VHI-10 was able to demonstrate the VHI's three-factor solution (Lam et al., 2006). In contrast, two studies evaluating the factor structure (one using Rasch analysis) found only two unidimensional constructs or factors (Bogaardt, Hakkesteegt, Grolman, & Lindeboom, 2007; Wilson et al., 2004). Thus, controversy remains over what discrete construct(s) are empirically measured by the VHI. This issue may also exist for the V-RQOL and other measures that use distinct subscales without statistical justification.
We also evaluated responsiveness to change (longitudinal validity). Instruments that aim to measure change over time should demonstrate stability in score when no change is expected. Otherwise, distinguishing “real” from random or “chance” differences becomes difficult. Test–retest reliability is used to evaluate score stability. Scores should also show meaningful change in an expected direction after an intervention (responsiveness to change). This property is a claimed attribute of most voice-related PRO measures; that is, they should be able to track change in the construct (e.g., dysphonia severity) over time. However, in this analysis, only three of 32 instruments met the criterion: VOS, V-RQOL, and LASA-VQ. Some measures have demonstrated responsiveness to change in subsequent studies since their initial development (e.g., VHI, VPQ). Nonetheless, on the basis of their initial development, many voice-related PRO measures may not be appropriate for and give spurious results in clinical trials and other comparative effectiveness studies.
Two other forms of construct validity evaluated in this review were convergent validity and known-group validity. The former relates to whether the proposed PRO measure correlates in an expected way with either an existing PRO measure(s) or clinical data that quantify the same concept. The latter is the ability of the instrument to distinguish among those respondents who are expected to differ. Most voice-related PRO measures (25/32) met at least one of these assessed variants of construct validity.
Scoring and Interpretation
Most voice-related PRO measures (26/32) provided some degree of scoring instructions. Most also provided some information on score interpretation, typically indicating that higher scores correlate with greater disease burden. Interpreting scores derived from summation is a common problem faced by end users. It is not always clear what a score means or when a minimally important clinical difference exists (Guyatt et al., 2002). A clinically important change should be a change in score that patients consider to be important. Many strategies have been proposed and depend on whether the intended use is within-person or population-based analysis (Guyatt et al., 2002). Estimation of this value for a particular measure is important to its interpretability. Its omission in the validation process represents a critical weakness in currently available PRO measures and limits their usefulness in decision making and implementation in clinical trials.
Incomplete questionnaires are also common occurrences in both clinical practice and research applications. Implications of missing PRO measure data can be significant, particularly if answers are missing systematically, thus introducing bias. For example, incomplete data could result from the reading level of the instrument or to questions that may not pertain to all respondents (e.g., occupational questions in retired individuals). A strategy for accommodating missing data is important to consider when developing a PRO measure. Many techniques for dealing with incomplete data exist. However, in contrast to scoring information, only three of the 32 identified PRO measures mentioned management of incompletely scored questionnaires. In fact, the only strategy cited was to omit incomplete questionnaires (Hill et al., 2000; Hogikyan & Sethuraman, 1999; Ma & Yiu, 2001).
Respondent Burden and Presentation
PRO measures inherently place burden on respondents in terms of length and complexity. Reasonable length depends on the intended respondent population and the setting in which it is being administered (e.g., clinic vs. research laboratory). With few exceptions, identified PRO measures were considered to have reasonable time to complete, ranging in length from three to 32 items.
No instrument described its literacy level. Some instruments identified in the current study overlapped with those identified in a prior review that focused on readability among voice-related PRO measures (Zraick & Atcherson, 2012). In all, the seven overlapping PRO measures had Flesch-Kincaid Reading Ease scores (Kincaid, Fishburne, Rogers, & Chissom, 1975) ranging from 89 (fifth-grade reading level) to 66 (10th-grade reading level). The VHI, VHI-10, VPQ, VoiSS, and VOS all met the fifth-grade reading level recommended by health literacy experts. In contrast, the VAPP had a 10th-grade reading level.
This last point highlights that perceived deficiencies in initial development are often evaluated in subsequent studies. Many of these PRO measures are used worldwide and have undergone post hoc reliability and validity testing in several languages. Some are now staples in the battery of tests used to screen for, assess, and track improvement in voice-related conditions. Nonetheless, end users must be careful to consider the underlying measurement characteristics of these measures before applying them for a particular purpose. A poorly selected outcome measure can distort clinical or research data, leading to spurious conclusions. In addition, end users must be able to access and personally assess a PRO measure and its items prior to implementation. Nearly all published articles describing the development and validation process (31/32) either listed or provided a link to view the specific items incorporated into the respective PRO measure.
Limitations
Despite the careful design, the search may not have captured all available literature, as poorly indexed literature is often difficult to identify. Hand searches were used to mitigate this limitation. We also limited our search to voice-related PRO measures published in English; those published in other languages were not captured. There is also the risk of subjectivity in the scoring of PRO measure characteristics. Every effort was made to minimize this risk by using three independent reviewers for each instrument considered. Moreover, not all psychometric and applicability parameters were evaluated (e.g., translatability, alternate form reliability [comparability of paper- and Internet-based administrations]). Reviewers were not blinded to the authors of the PRO measures they were evaluating. This could potentially introduce bias; however, blinding of authors is not common practice either by the Cochrane Collaboration or in Agency for Healthcare Research and Quality systematic reviews, which are considered the standards of excellence in systematic review methodology. Last, it is incumbent on the end user to understand the measurement and applicability characteristics of any candidate measure and to select a measure developed for a specified targeted construct in a similar population that corresponds to their specific clinical or research needs and aims.
Clinical Implications
This systematic review has important clinical and research implications. First, it highlights that voice-related PRO measures are varied in psychometric properties. This is critically important because a clinician or researcher considering using a particular instrument should be aware of whether it has been developed and validated properly for the proposed application. For example, a measure that lacks demonstrable responsiveness to change should not be used to track treatment outcomes. The glossary shown in Table 2 describes those parameters that clinicians and researchers should consider when deciding which PRO measure to implement into their practice or research. A poorly designed PRO measure could simply give the wrong answers. Clinicians and researchers rely on these tools to guide care, and they should be able to have faith that they are measuring what they think they are. Many instruments exist, and this systematic review provides some guidance regarding the respective strengths and limitations of available voice-related measures. This review also highlights methodological deficiencies in current measures that may be addressable in future studies.
Conclusions
PRO measures are currently the principal means of evaluating treatment effectiveness in voice-related conditions. Despite their prominence, available PRO measures have disparate psychometric rigor. Two important thematic deficiencies in current voice-related PRO measures are the lack of patient involvement in the item development process and lack of strong construct validity. Issues related to response burden and scoring and interpretation were also highlighted. Care must be taken to understand the measurement properties and utility of PRO measures before selecting and advocating their use for either research or clinical applications.
Acknowledgment
Funding was provided by National Institute on Deafness and Other Communication Disorders Grants K23DC013559 and L30DC012687 to the first author.
Appendix A
MEDLINE Search Strategies: Dysphonia (PubMed Platform)
| Search terms | Search results | |
|---|---|---|
| #1 | “voice disorder”[tiab] OR “voice disorders”[tiab] OR “voice disordered”[tiab] OR “voice handicap”[tiab] OR “vocal handicap”[tiab] OR “vocally handicapped”[tiab] OR “voice handicap”[tiab] OR “voice handicaps”[tiab] OR “voice handicapped”[tiab] OR “vocal disorder”[tiab] OR “vocal disorders”[tiab] OR “vocally disordered”[tiab] OR “vocal disability”[tiab] OR “vocal disabilities”[tiab] OR “voice disability”[tiab] OR “voice disabilities”[tiab] OR dysphonia*[tiab] OR dysphonic[tiab] OR aphonia*[tiab] OR aphonic[tiab] OR “Voice Disorders”[MeSH] OR “Vocal Cords/pathology”[MeSH] OR “Vocal Cord Paralysis”[MeSH] OR “vocal cord paralysis”[tiab] OR “vocal fold paralysis”[tiab] OR hoarse[tiab] OR hoarseness[tiab] OR “speech disability”[tiab] OR “speech disabilities”[tiab] OR “voice symptom”[tiab] OR “voice symptoms”[tiab] OR “vocal symptom”[tiab] OR “vocal symptoms” OR “vocal roughness”[tiab] OR “voice activity”[tiab] OR “vocal fatigue”[tiab] OR “resonance disorder”[tiab] OR “resonance disorders”[tiab] OR phonation[tiab] | 18,698 |
| #2 | “Psychometrics”[MeSH] OR psychometric*[tiab] OR scale*[tiab] OR score*[tiab] OR inventory[tiab] OR inventories[tiab] OR questionnaire*[tiab] OR Questionnaires[MeSH] OR inventories[tiab] OR index[tiab] OR indices[tiab] OR instrument*[tiab] OR outcome measure*[tiab] OR measurement[tiab] OR “Patient Satisfaction”[MeSH] OR “quality of life”[tiab] OR “Qualitative Research”[MeSH] OR validation studies[pt] OR “Treatment Outcome”[MeSH] OR “Disability Evaluation”[MeSH] OR “Health Surveys”[MeSH] OR “Reproducibility of Results”[MeSH] OR “Severity of Illness Index”[MeSH] | 2,991,677 |
| #3 | #1 AND #2 AND English[lang] AND Humans[MeSH] | 3,878 |
| #4 | newspaper article[pt] OR letter[pt] OR comment[pt] OR case reports[pt] OR review[pt] OR practice guideline[pt] OR news[pt] OR editorial[pt] OR historical article[pt] OR meta-analysis[pt] OR legal cases[pt] OR jsubsetk | 4,930,194 |
| #5 | #3 NOT #4 | 3,116 |
Note. [tiab] = title/abstract word; [MeSH] = medical subject heading; [pt] = publication type; [lang] = language; jsubsetk = consumer health literature.
Appendix B
Cumulative Index of Nursing and Allied Health Literature Search Strategies: Dysphonia (EbscoHost Platform)
| Search terms | Search results | |
|---|---|---|
| #1 | (MH “Voice Disorders”) OR “voice disorders” OR “voice disorder” OR “voice disordered” OR “voice handicap” OR “vocal handicap” OR “vocally handicapped” OR “voice handicaps” OR “voice handicapped” OR “vocal disorder” OR “vocal disorders” OR “vocally disordered” OR “vocal disability” OR “vocal disabilities” OR “voice disability” OR “voice disabilities” OR “dysphonia*” OR “dysphonic” OR (MH “Aphonia”) OR “aphonia*” OR “aphonic” OR (MH “Vocal Cords/PA”) OR (MH “Vocal Cord Paralysis”) OR “vocal cord paralysis” OR “vocal fold paralysis” OR (MH “Hoarseness”) OR “hoarse” OR “speech disability” OR “speech disabilities” OR “voice symptom” OR “voice symptoms” OR “vocal symptom” OR “vocal symptoms” OR “vocal roughness” OR “voice activity” OR “vocal fatigue” OR “resonance disorder” OR “resonance disorders” OR (MH “Phonation”) OR “phonation” | 3,946 |
| #2 | (MH “Psychometrics”) OR “psychometric*” OR (MH “Scales”) OR “scale*” OR “score*” OR (MH “Inventories”) OR “inventories” OR “inventory” OR (MH “Questionnaires”) OR “questionnaire*” OR “index” OR “indices” OR “instrument*” OR (MH “Instrument Validation”) OR (MH “Outcome Assessment”) OR “outcome measure*” OR (MH “Treatment Outcomes”) OR “measurement” OR (MH “Patient Satisfaction”) OR (MH “Quality of Life”) OR “quality of life” OR (MH “Qualitative Studies”) OR (MH “Validation Studies”) OR (MH “Disability Evaluation”) OR (MH “Surveys”) OR (MH “Reproducibility of Results”) OR (MH “Severity of Illness Indices”) | 627,136 |
| #3 | #1 AND #2 Limiters - Exclude MEDLINE records; Human; Language: English | 142 |
Note. MH = medical subject heading.
Appendix C
Health and Psychosocial Instrument Search Strategies: Dysphonia (Ovid Platform)
| Search terms | Search results | |
|---|---|---|
| #1 | (“voice disorder$” or “voice handicap$” or “vocal$ handicap$” or “vocal disorder$” or dysphoni$ OR aphoni$ or “vocal cord paralysis” or hoarse$ or “vocal disability$” or “speech disability$” or “voice disabilit$” or “vocal fatigue” or “voice symptom$” or “vocal symptom*” or “vocal roughness”).mp. | 17 |
Note. mp = title, acronym, descriptors, measure descriptors, sample descriptors, abstract, source.
Funding Statement
Funding was provided by National Institute on Deafness and Other Communication Disorders Grants K23DC013559 and L30DC012687 to the first author.
References
- Aaronson N., Alonso J., Burnam A., Lohr K. N., Patrick D. L., Perrin E., & Stein R. E. (2002). Assessing health status and quality-of-life instruments: Attributes and review criteria. Quality of Life Research, 11, 193–205. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Berzon R. A., Revicki D. A., Lenderking W. R., Moinpour C. M., Basch E., … International Society for Quality of Life Research. (2012). The use of patient-reported outcomes (PRO) within comparative effectiveness research: Implications for clinical practice and health care policy. Medical Care, 50, 1060–1070. doi:10.1097/MLR.0b013e318268aaff [DOI] [PubMed] [Google Scholar]
- Anastasi A. (1988). Psychological testing. New York, NY: Macmillan. [Google Scholar]
- Andrae D. A., Patrick D. L., Drossman D. A., & Covington P. S. (2013). Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health and Quality of Life Outcomes, 11, 208 doi:10.1186/1477-7525-11-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arffa R. E., Krishna P., Gartner-Schmidt J., & Rosen C. A. (2012). Normative values for the Voice Handicap Index-10. Journal of Voice, 26, 462–465. doi:10.1016/j.jvoice.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Bach K. K., Belafsky P. C., Wasylik K., Postma G. N., & Koufman J. A. (2005). Validity and reliability of the Glottal Function Index. Archives of Otolaryngology—Head & Neck Surgery, 131, 961–964. doi:10.1001/archotol.131.11.961 [DOI] [PubMed] [Google Scholar]
- Baylor C., McAuliffe M. J., Hughes L. E., Yorkston K., Anderson T., Kim J., & Amtmann D. (2014). A differential item functioning (DIF) analysis of the Communicative Participation Item Bank (CPIB): Comparing individuals with Parkinson's disease from the United States and New Zealand. Journal of Speech, Language, and Hearing Research, 57, 90–95. doi:10.1044/1092-4388(2013/12-0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor C., Yorkston K., Eadie T., Kim J., Chung H., & Amtmann D. (2013). The Communicative Participation Item Bank (CPIB): Item bank calibration and development of a disorder-generic short form. Journal of Speech, Language, and Hearing Research, 56, 1190–1208. doi:10.1044/1092-4388(2012/12-0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor C. R., Yorkston K. M., Eadie T. L., Miller R. M., & Amtmann D. (2009). Developing the Communicative Participation Item Bank: Rasch analysis results from a spasmodic dysphonia sample. Journal of Speech, Language, and Hearing Research, 52, 1302–1320. doi:10.1044/1092-4388(2009/07-0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belafsky P. C., Postma G. N., & Koufman J. A. (2002). Validity and reliability of the Reflux Symptom Index (RSI). Journal of Voice, 16, 274–277. [DOI] [PubMed] [Google Scholar]
- Benninger M. S., Ahuja A. S., Gardner G., & Grywalski C. (1998). Assessing outcomes for dysphonic patients. Journal of Voice, 12, 540–550. [DOI] [PubMed] [Google Scholar]
- Biddle A. K., Watson L. R., Hooper C. R., Lohr K. N., & Sutton S. F. (2002). Criteria for determining disability in speech-language disorders (Summary, Evidence Report/Technology Assessment: Number 52). Retrieved from Agency for Healthcare Research and Quality (AHRQ) Evidence Report Summaries, https://www.ncbi.nlm.nih.gov/books/NBK11866/ [PMC free article] [PubMed] [Google Scholar]
- Bogaardt H. C., Hakkesteegt M. M., Grolman W., & Lindeboom R. (2007). Validation of the Voice Handicap Index using Rasch analysis. Journal of Voice, 21, 337–344. doi:10.1016/j.jvoice.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Branski R. C., Cukier-Blaj S., Pusic A., Cano S. J., Klassen A., Mener D., … Kraus D. H. (2010). Measuring quality of life in dysphonic patients: A systematic review of content development in patient-reported outcomes measures. Journal of Voice, 24, 193–198. doi:10.1016/j.jvoice.2008.05.006 [DOI] [PubMed] [Google Scholar]
- Carding P. N., & Horsley I. A. (1992). An evaluation study of voice therapy in non-organic dysphonia. European Journal of Disorders of Communication, 27, 137–158. [DOI] [PubMed] [Google Scholar]
- Carding P. N., Horsley I. A., & Docherty G. J. (1998). The effectiveness of voice therapy for patients with non-organic dysphonia. Clinical Otolaryngology and Allied Sciences, 23, 310–318. [DOI] [PubMed] [Google Scholar]
- Cheng J., & Woo P. (2010). Correlation between the Voice Handicap Index and voice laboratory measurements after phonosurgery. Ear, Nose and Throat Journal, 89, 183–188. [PubMed] [Google Scholar]
- Cohen S. M., Kim J., Roy N., Asche C., & Courey M. (2012a). Direct health care costs of laryngeal diseases and disorders. Laryngoscope, 122, 1582–1588. doi:10.1002/lary.23189 [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Kim J., Roy N., Asche C., & Courey M. (2012b). Prevalence and causes of dysphonia in a large treatment-seeking population. Laryngoscope, 122, 343–348. doi:10.1002/lary.22426 [DOI] [PubMed] [Google Scholar]
- Deary I. J., Wilson J. A., Carding P. N., & MacKenzie K. (2003). VoiSS: A patient-derived voice symptom scale. Journal of Psychosomatic Research, 54, 483–489. [DOI] [PubMed] [Google Scholar]
- Dew K., Keefe V., & Small K. (2005). “Choosing” to work when sick: Workplace presenteeism. Social Science and Medicine, 60, 2273–2282. doi:10.1016/j.socscimed.2004.10.022 [DOI] [PubMed] [Google Scholar]
- Eadie T. L., Lamvik K., Baylor C. R., Yorkston K. M., Kim J., & Amtmann D. (2014). Communicative participation and quality of life in head and neck cancer. Annals of Otology, Rhinology & Laryngology, 123, 257–264. doi:10.1177/0003489414525020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R., Hirani S. P., Stygall J., & Newman S. P. (2009). How do individuals cope with voice disorders? Introducing the Voice Disability Coping Questionnaire. Journal of Voice, 23, 209–217. doi:10.1016/j.jvoice.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Epstein R., Stygall J., & Newman S. (1997). The short-term impact of Botox injections on speech disability in adductor spasmodic dysphonia. Disability and Rehabilitation, 19, 20–25. [DOI] [PubMed] [Google Scholar]
- Feeny D. H., Eckstrom E., Whitlock E. P., & Perdue L. A. (2013). A primer for systematic reviewers on the measurement of functional status and health-related quality of life in older adults (AHRQ Publication No. 13-EHC128-EF). Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Feeny D., Furlong W., Boyle M., & Torrance G. W. (1995). Multi-attribute health status classification systems. Health Utilities Index. Pharmacoeconomics, 7, 490–502. [DOI] [PubMed] [Google Scholar]
- Feinstein A. R. (1983). An additional basic science for clinical medicine: IV. The development of clinimetrics. Annals of Internal Medicine, 99, 843–848. [DOI] [PubMed] [Google Scholar]
- Francis D. O., McPheeters M. L., Noud M., Penson D. F., & Feurer I. D. (2016). Checklist to operationalize measurement characteristics of patient-reported outcome measures. Systematic Reviews, 5, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franic D. M., Bramlett R. E., & Bothe A. C. (2005). Psychometric evaluation of disease specific quality of life instruments in voice disorders. Journal of Voice, 19, 300–315. doi:10.1016/j.jvoice.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Garrow A. P., Khan N., Tyson S., Vestbo J., Singh D., & Yorke J. (2015). The development and first validation of the Manchester Early Morning Symptoms Index (MEMSI) for patients with COPD. Thorax, 70, 757–763. doi:10.1136/thoraxjnl-2014-206410 [DOI] [PubMed] [Google Scholar]
- Ghirardi A. C., Ferreira L. P., Giannini S. P., & Latorre Mdo R. (2013). Screening Index for Voice Disorder (SIVD): Development and validation. Journal of Voice, 27, 195–200. doi:10.1016/j.jvoice.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Gillespie A. I., & Abbott K. V. (2011). The influence of clinical terminology on self-efficacy for voice. Logopedics, Phoniatrics, Vocology, 36, 91–99. doi:10.3109/14015439.2010.539259 [DOI] [PubMed] [Google Scholar]
- Gliklich R. E., Glovsky R. M., & Montgomery W. W. (1999). Validation of a voice outcome survey for unilateral vocal cord paralysis. Otolaryngology—Head & Neck Surgery, 120, 153–158. [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Osoba D., Wu A. W., Wyrwich K. W., Norman G. R., & Clinical Significance Consensus Meeting Group. (2002). Methods to explain the clinical significance of health status measures. Mayo Clinic Proceedings, 77, 371–383. doi:10.1016/S0025-6196(11)61793-X [DOI] [PubMed] [Google Scholar]
- Guyatt G., & Schunemann H. (2007). How can quality of life researchers make their work more useful to health workers and their patients? Quality of Life Research, 16, 1097–1105. doi:10.1007/s11136-007-9223-3 [DOI] [PubMed] [Google Scholar]
- Hambleton R. K., & Swaminathan H. (1985). Item response theory: Principles and applications. Boston, MA: Kluwer. [Google Scholar]
- Hill D. S., Akhtar S., Corroll A., & Croft C. B. (2000). Quality of life issues in recurrent respiratory papillomatosis. Clinical Otolaryngology and Allied Sciences, 25, 153–160. [DOI] [PubMed] [Google Scholar]
- Hirano M. (1981). Clinical examination of voice. New York, NY: Springer-Verlag. [Google Scholar]
- Hogikyan N. D., & Sethuraman G. (1999). Validation of an instrument to measure voice-related quality of life (V-RQOL). Journal of Voice, 13, 557–569. [DOI] [PubMed] [Google Scholar]
- Hopkins C., Yousaf U., & Pedersen M. (2006). Acid reflux treatment for hoarseness (Art. No. CD005054). Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD005054.pub2 [DOI] [PubMed] [Google Scholar]
- Hsiung M. W., Pai L., & Wang H. W. (2002). Correlation between Voice Handicap Index and voice laboratory measurements in dysphonic patients. European Archives of Oto-Rhino-Laryngology, 259, 97–99. [DOI] [PubMed] [Google Scholar]
- Hu A., Isetti D., Hillel A. D., Waugh P., Comstock B., & Meyer T. K. (2013). Disease-specific self-efficacy in spasmodic dysphonia patients. Otolaryngology—Head & Neck Surgery, 148, 450–455. doi:10.1177/0194599812472319 [DOI] [PubMed] [Google Scholar]
- Hutchings H. A., Cheung W. Y., Russell I. T., Durai D., Alrubaiy L., & Williams J. G. (2015). Psychometric development of the Gastrointestinal Symptom Rating Questionnaire (GSRQ) demonstrated good validity. Journal of Clinical Epidemiology, 68, 1176–1183. doi:10.1016/j.jclinepi.2015.03.019 [DOI] [PubMed] [Google Scholar]
- Isetti D., & Meyer T. (2014). Workplace productivity and voice disorders: A cognitive interviewing study on presenteeism in individuals with spasmodic dysphonia. Journal of Voice, 28, 700–710. doi:10.1016/j.jvoice.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Jacobson B., Johnson A., Grywalski C., Silbergleit A., Jacobson G., & Benninger M. S. (1997). The Voice Handicap Index (VHI): Development and validation. American Journal of Speech-Language Pathology, 6, 66–70. [Google Scholar]
- Johnston B. C., Ebrahim S., Carrasco-Labra A., Furukawa T. A., Patrick D. L., Crawford M. W., … Nesrallah G. (2015). Minimally important difference estimates and methods: A protocol. BMJ Open, 5(10), e007953 doi:10.1136/bmjopen-2015-007953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempster G. B., Gerratt B. R., Verdolini Abbott K., Barkmeier-Kraemer J., & Hillman R. E. (2009). Consensus auditory-perceptual evaluation of voice: Development of a standardized clinical protocol. American Journal of Speech-Language Pathology, 18, 124–132. doi:10.1044/1058-0360(2008/08-0017) [DOI] [PubMed] [Google Scholar]
- Kincaid J. P., Fishburne R. P., Rogers R. L., & Chissom B. S. (1975). Derivation of new readability formulas (Automated Readability Index, Fog Count, and Flesch Reading Ease Formula) for Navy enlisted personnel. Springfield, VA: National Technical Information Service. [Google Scholar]
- Konnai R. M., Jayaram M., & Scherer R. C. (2010). Development and validation of a voice disorder outcome profile for an Indian population. Journal of Voice, 24, 206–220. doi:10.1016/j.jvoice.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Lam P. K., Chan K. M., Ho W. K., Kwong E., Yiu E. M., & Wei W. I. (2006). Cross-cultural adaptation and validation of the Chinese Voice Handicap Index-10. Laryngoscope, 116, 1192–1198. doi:10.1097/01.mlg.0000224539.41003.93 [DOI] [PubMed] [Google Scholar]
- Laukkanen A. M., Leppanen K., & Ilomaki I. (2009). Self-evaluation of voice as a treatment outcome measure. Folia Phoniatrica et Logopaedica, 61, 57–65. doi:10.1159/000201000 [DOI] [PubMed] [Google Scholar]
- Lehto L., Rantala L., Vilkman E., Alku P., & Backstrom T. (2003). Experiences of a short vocal training course for call-centre customer service advisors. Folia Phoniatrica et Logopaedica, 55, 163–176. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Thomas H. A., Sutherland H. J., Hogg S. A., Ciampi A., Harwood A. R., Keane T. J., … Boyd N. F. (1984). Linear analogue self-assessment of voice quality in laryngeal cancer. Journal of Chronic Diseases, 37, 917–924. [DOI] [PubMed] [Google Scholar]
- Ma E. P., & Yiu E. M. (2001). Voice activity and participation profile: Assessing the impact of voice disorders on daily activities. Journal of Speech, Language, and Hearing Research, 44, 511–524. [DOI] [PubMed] [Google Scholar]
- Ma E. P., & Yiu E. M. (2007). Scaling voice activity limitation and participation restriction in dysphonic individuals. Folia Phoniatrica et Logopaedica, 59, 74–82. doi:10.1159/000098340 [DOI] [PubMed] [Google Scholar]
- Mathieson L. (1993). Vocal tract discomfort in hyperfunctional dysphonia. Voice, 2, 40–48. [Google Scholar]
- McHorney C. A., Ware J. E. Jr., & Raczek A. E. (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31, 247–263. [DOI] [PubMed] [Google Scholar]
- Mokkink L. B., Terwee C. B., Patrick D. L., Alonso J., Stratford P. W., Knol D. L., … de Vet H. C. (2010). The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Quality of Life Research, 19(4), 539–549. doi:10.1007/s11136-010-9606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokkink L. B., Terwee C. B., Stratford P. W., Alonso J., Patrick D. L., Riphagen I., … de Vet H. C. (2009). Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Quality of Life Research, 18(3), 313–333. doi:10.1007/s11136-009-9451-9 [DOI] [PubMed] [Google Scholar]
- Monk M. (1981). Blood pressure awareness and psychological well-being in the health and nutrition examination survey. Clinical and Investigative Medicine, 4, 183–189. [PubMed] [Google Scholar]
- Nam I. C., Bae J. S., Shim M. R., Hwang Y. S., Kim M. S., & Sun D. I. (2012). The importance of preoperative laryngeal examination before thyroidectomy and the usefulness of a voice questionnaire in screening. World Journal of Surgery, 36, 303–309. doi:10.1007/s00268-011-1347-5 [DOI] [PubMed] [Google Scholar]
- Nanjundeswaran C., Jacobson B. H., Gartner-Schmidt J., & Verdolini Abbott K. (2015). Vocal Fatigue Index (VFI): Development and validation. Journal of Voice, 29, 433–440. doi:10.1016/j.jvoice.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Newton P. E., & Shaw S. D. (2013). Standards for talking and thinking about validity. Psychological Methods, 18, 301–319. [DOI] [PubMed] [Google Scholar]
- Paolillo N. P., & Pantaleo G. (2015). Development and validation of the Voice Fatigue Handicap Questionnaire (VFHQ): Clinical, psychometric, and psychosocial facets. Journal of Voice, 29, 91–100. doi:10.1016/j.jvoice.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Patient-Centered Outcomes Research Institute. (2014). Working definition of patient-centered outcomes research. Retrieved from http://www.pcori.org/images/PCOR_Rationale.pdf
- Patrick D. L., Burke L. B., Powers J. H., Scott J. A., Rock E. P., Dawisha S., … Kennedy D. L. (2007). Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health, 10(Suppl. 2), S125–S137. doi:10.1111/j.1524-4733.2007.00275.x [DOI] [PubMed] [Google Scholar]
- Penson D. F., Litwin M. S., & Aaronson N. K. (2003). Health related quality of life in men with prostate cancer. Journal of Urology, 169, 1653–1661. doi:10.1097/01.ju.0000061964.49961.55 [DOI] [PubMed] [Google Scholar]
- Pleil A. M., Coyne K. S., Reese P. R., Jumadilova Z., Rovner E. S., & Kelleher C. J. (2005). The validation of patient-rated global assessments of treatment benefit, satisfaction, and willingness to continue—The BSW. Value Health, 8(Suppl. 1), S25–S34. doi:10.1111/j.1524-4733.2005.00069.x [DOI] [PubMed] [Google Scholar]
- Pouwer F., Snoek F. J., van der Ploeg H. M., Ader H. J., & Heine R. J. (2000). The well-being questionnaire: Evidence for a three-factor structure with 12 items (W-BQ12). Psychological Medicine, 30, 455–462. [DOI] [PubMed] [Google Scholar]
- Reeve B. B., Wyrwich K. W., Wu A. W., Velikova G., Terwee C. B., Snyder C. F., … Butt Z. (2013). ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Quality of Life Research, 22, 1889–1905. doi:10.1007/s11136-012-0344-y [DOI] [PubMed] [Google Scholar]
- Regnault A., Hamel J. F., & Patrick D. L. (2015). Pooling of cross-cultural PRO data in multinational clinical trials: How much can poor measurement affect statistical power? Quality of Life Research, 24(2), 273–277. doi:10.1007/s11136-014-0765-x [DOI] [PubMed] [Google Scholar]
- Riehm K. E., Kwakkenbos L., Carrier M. E., Bartlett S. J., Malcarne V. L., Mouthon L., … Investigators, Scleroderma Patient-Centered Intervention Network . (2016). Validation of the Self-Efficacy for Managing Chronic Disease (SEMCD) scale: A Scleroderma Patient-Centered Intervention Network (SPIN) cohort study. Arthritis Care and Research, 68, 1195–1200. doi:10.1002/acr.22807 [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Lee A. S., Osborne J., Zullo T., & Murry T. (2004). Development and validation of the Voice Handicap Index-10. Laryngoscope, 114, 1549–1556. doi:10.1097/00005537-200409000-00009 [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Murry T., Zinn A., Zullo T., & Sonbolian M. (2000). Voice Handicap Index change following treatment of voice disorders. Journal of Voice, 14, 619–623. [DOI] [PubMed] [Google Scholar]
- Roy N., Barkmeier-Kraemer J., Eadie T., Sivasankar M. P., Mehta D., Paul D., & Hillman R. (2013). Evidence-based clinical voice assessment: A systematic review. American Journal of Speech-Language Pathology, 22, 212–226. doi:10.1044/1058-0360(2012/12-0014) [DOI] [PubMed] [Google Scholar]
- Roy N., Merrill R. M., Gray S. D., & Smith E. M. (2005). Voice disorders in the general population: Prevalence, risk factors, and occupational impact. Laryngoscope, 115, 1988–1995. doi:10.1097/01.mlg.0000179174.32345.41 [DOI] [PubMed] [Google Scholar]
- Ruotsalainen J. H., Sellman J., Lehto L., Jauhiainen M., & Verbeek J. H. (2007a). Interventions for preventing voice disorders in adults (Art. No. CD006372). Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD006372.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen J. H., Sellman J., Lehto L., Jauhiainen M., & Verbeek J. H. (2007b). Interventions for treating functional dysphonia in adults (Art. No. CD006373). Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD006373.pub2 [DOI] [PubMed] [Google Scholar]
- Scott S., Robinson K., Wilson J. A., & Mackenzie K. (1997). Patient-reported problems associated with dysphonia. Clinical Otolaryngology and Allied Sciences, 22, 37–40. [DOI] [PubMed] [Google Scholar]
- Sherbourne C. D., & Stewart A. L. (1991). The MOS Social Support survey. Social Science and Medicine, 32, 705–714. [DOI] [PubMed] [Google Scholar]
- Smith E., Verdolini K., Gray S., Nichols S., Lemke J., Barkmeier J., … Hoffman H. (1996). Effect of voice disorders on quality of life. Journal of Medical Speech-Language Pathology, 4, 223–244. [Google Scholar]
- Snyder C. F., Jensen R. E., Segal J. B., & Wu A. W. (2013). Patient-reported outcomes (PROs): Putting the patient perspective in patient-centered outcomes research. Medical Care, 51(8 Suppl. 3), S73–S79. doi:10.1097/MLR.0b013e31829b1d84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer R., Wieneke G. H., & Dejonckere P. H. (2004). Self-assessment of voice therapy for chronic dysphonia. Clinical Otolaryngology and Allied Sciences, 29, 66–74. [DOI] [PubMed] [Google Scholar]
- Teixeira L. C., Rodrigues A. L., Silva A. F., Azevedo R., Gama A. C., & Behlau M. (2013). The use of the URICA-Voice questionnaire to identify the stages of adherence to voice treatment. Codas, 25, 8–15. [DOI] [PubMed] [Google Scholar]
- Terwee C. B., Bot S. D., de Boer M. R., van der Windt D. A., Knol D. L., Dekker J., … de Vet H. C. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology, 60, 34–42. doi:10.1016/j.jclinepi.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Torrance G. W., Furlong W., Feeny D., & Boyle M. (1995). Multi-attribute preference functions. Health Utilities Index. Pharmacoeconomics, 7, 503–520. [DOI] [PubMed] [Google Scholar]
- van Gogh C. D., Verdonck-de Leeuw I. M., Boon-Kamma B. A., Langendijk J. A., Kuik D. J., & Mahieu H. F. (2005). A screening questionnaire for voice problems after treatment of early glottic cancer. International Journal of Radiation Oncology, Biology, Physics, 62, 700–705. doi:10.1016/j.ijrobp.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Velicer W. F., & Fava J. L. (1998). Effects of variable and subject sampling on factor pattern recovery. Psychological Methods, 3, 231–251. [Google Scholar]
- Verdonck-de Leeuw I. M., Keus R. B., Hilgers F. J., Koopmans-van Beinum F. J., Greven A. J., de Jong J. M., … Bartelink H. (1999). Consequences of voice impairment in daily life for patients following radiotherapy for early glottic cancer: Voice quality, vocal function, and vocal performance. International Journal of Radiation Oncology, Biology, Physics, 44, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Vitali C., Borghi E., Napoletano A., Polini F., Caronni M., Ammenti P., & Cattaneo D. (2010). Oropharyngolaryngeal disorders in scleroderma: Development and validation of the SLS scale. Dysphagia, 25, 127–138. doi:10.1007/s00455-009-9235-y [DOI] [PubMed] [Google Scholar]
- Ware J. E. Jr., & Sherbourne C. D. (1992). The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30, 473–483. [PubMed] [Google Scholar]
- Wegener S. T., Castillo R. C., Heins S. E., Bradford A. N., Newell M. Z., Pollak A. N., & MacKenzie E. J. (2014). The development and validation of the Readiness to Engage in Self-Management after Acute Traumatic Injury questionnaire. Rehabilitation Psychology, 59, 203–210. doi:10.1037/a0035693 [DOI] [PubMed] [Google Scholar]
- Wheeler K. M., Collins S. P., & Sapienza C. M. (2006). The relationship between VHI scores and specific acoustic measures of mildly disordered voice production. Journal of Voice, 20, 308–317. doi:10.1016/j.jvoice.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Wilson J. A., Webb A., Carding P. N., Steen I. N., MacKenzie K., & Deary I. J. (2004). The Voice Symptom Scale (VoiSS) and the Vocal Handicap Index (VHI): A comparison of structure and content. Clinical Otolaryngology and Allied Sciences, 29, 169–174. doi:10.1111/j.0307-7772.2004.00775.x [DOI] [PubMed] [Google Scholar]
- Woisard V., Bodin S., Yardeni E., & Puech M. (2007). The Voice Handicap Index: Correlation between subjective patient response and quantitative assessment of voice. Journal of Voice, 21, 623–631. doi:10.1016/j.jvoice.2006.04.005 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2001). The International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: Author; Retrieved from http://who.int/classifications/icf/en/ [Google Scholar]
- Zraick R. I., & Atcherson S. R. (2012). Readability of patient-reported outcome questionnaires for use with persons with dysphonia. Journal of Voice, 26, 635–641. doi:10.1016/j.jvoice.2011.01.009 [DOI] [PubMed] [Google Scholar]