Abstract
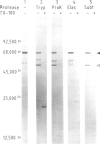
Ribophorins I and II represent proteins that are postulated to be involved in ribosome binding. They are abundant, highly-conserved glycoproteins located exclusively in the membranes of the rough endoplasmic reticulum. As the first step in the further characterization of the structure and function of these proteins, we have isolated and sequenced full-length human cDNA clones encoding ribophorins I and II using probes derived from a human liver expression library cloned into pEX1. The authenticity of the clones was verified by overlaps in the protein sequence of N-terminal and several internal fragments of canine pancreatic ribophorins I and II. The cDNA clones hybridize to mRNA species of 2.5 kb in length, and encode polypeptides of 68.5 and 69.3 kd, respectively. Primary sequence analysis, coupled with biochemical studies on the topology, indicates that both ribophorins are largely luminally disposed, spanning the membrane once and having 150 and 70 amino acid long cytoplasmically disposed C termini, respectively. Both are synthesized as precursors having cleavable signal sequences of 23 (ribophorin I) and 22 (ribophorin II) amino acids. The topology suggested by the primary structure has been confirmed biochemically using proteolytic enzymes and anti-ribophorin antibodies. Proteolysis of intact microsomes with a variety of enzymes resulted in a reduction in the apparent mol. wt of ribophorin I that would correspond to a loss of its 150-amino acid cytoplasmic tail. In the case of ribophorin II, it is completely resistant to such proteolysis which is consistent with its luminal disposition and fairly hydrophobic C terminus.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Todd J. A., Kreibich G. Segregation of the polypeptide translocation apparatus to regions of the endoplasmic reticulum containing ribophorins and ribosomes. I. Functional tests on rat liver microsomal subfractions. J Cell Biol. 1984 Dec;99(6):2247–2253. doi: 10.1083/jcb.99.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Mok W., Kreibich G., Sabatini D. D. Ribosomal-membrane interaction: in vitro binding of ribosomes to microsomal membranes. J Mol Biol. 1974 Sep 25;88(3):559–580. doi: 10.1016/0022-2836(74)90408-2. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Gilmore R., Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A. 1986 Feb;83(3):581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M., Avossa D., Meyer D. I. A structural and functional analysis of the docking protein. Characterization of active domains by proteolysis and specific antibodies. J Biol Chem. 1985 Aug 5;260(16):9137–9145. [PubMed] [Google Scholar]
- Hortsch M., Avossa D., Meyer D. I. Characterization of secretory protein translocation: ribosome-membrane interaction in endoplasmic reticulum. J Cell Biol. 1986 Jul;103(1):241–253. doi: 10.1083/jcb.103.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M., Meyer D. I. Immunochemical analysis of rough and smooth microsomes from rat liver. Segregation of docking protein in rough membranes. Eur J Biochem. 1985 Aug 1;150(3):559–564. doi: 10.1111/j.1432-1033.1985.tb09057.x. [DOI] [PubMed] [Google Scholar]
- Knowles W. J., Bologna M. L. Isolation of the chemical domains of human erythrocyte spectrin. Methods Enzymol. 1983;96:305–313. doi: 10.1016/s0076-6879(83)96028-7. [DOI] [PubMed] [Google Scholar]
- Kreibich G., Czakó-Graham M., Grebenau R., Mok W., Rodriguez-Boulan E., Sabatini D. D. Characterization of the ribosomal binding site in rat liver rough microsomes: ribophorins I and II, two integral membrane proteins related to ribosome binding. J Supramol Struct. 1978;8(3):279–302. doi: 10.1002/jss.400080307. [DOI] [PubMed] [Google Scholar]
- Kreibich G., Freienstein C. M., Pereyra B. N., Ulrich B. L., Sabatini D. D. Proteins of rough microsomal membranes related to ribosome binding. II. Cross-linking of bound ribosomes to specific membrane proteins exposed at the binding sites. J Cell Biol. 1978 May;77(2):488–506. doi: 10.1083/jcb.77.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Ulrich B. L., Sabatini D. D. Proteins of rough microsomal membranes related to ribosome binding. I. Identification of ribophorins I and II, membrane proteins characteristics of rough microsomes. J Cell Biol. 1978 May;77(2):464–487. doi: 10.1083/jcb.77.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M. Methods for the preparation of messenger RNA. Methods Enzymol. 1983;96:24–38. doi: 10.1016/s0076-6879(83)96006-8. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Amar-Costesec A., Kreibich G. Segregation of the polypeptide translocation apparatus to regions of the endoplasmic reticulum containing ribophorins and ribosomes. II. Rat liver microsomal subfractions contain equimolar amounts of ribophorins and ribosomes. J Cell Biol. 1984 Dec;99(6):2254–2259. doi: 10.1083/jcb.99.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A small particulate component of the cytoplasm. J Biophys Biochem Cytol. 1955 Jan;1(1):59–68. doi: 10.1083/jcb.1.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Rosenfeld M. G., Marcantonio E. E., Hakimi J., Ort V. M., Atkinson P. H., Sabatini D., Kreibich G. Biosynthesis and processing of ribophorins in the endoplasmic reticulum. J Cell Biol. 1984 Sep;99(3):1076–1082. doi: 10.1083/jcb.99.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires T. K., Narurkar L., Pitot H. C. The association in vitro of polyribosomes with ribonuclease-treated derivatives of hepatic rough endoplasmic reticulum. Characteristics of the membrane binding sites and factors influencing association. Biochem J. 1971 Nov;125(1):67–79. doi: 10.1042/bj1250067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K. Solubilization and immune-detection of beta-galactosidase hybrid proteins carrying foreign antigenic determinants. Nucleic Acids Res. 1983 Jun 25;11(12):4077–4092. doi: 10.1093/nar/11.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Huth A., Rapoport T. A. Xenopus oocytes can secrete bacterial beta-lactamase. Nature. 1984 Jun 14;309(5969):637–639. doi: 10.1038/309637a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984 Oct;3(10):2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]