Abstract
Amblyopia is a developmental disorder of spatial vision that results from abnormal early visual experience usually due to the presence of strabismus, anisometropia, or both strabismus and anisometropia. Amblyopia results in a range of visual deficits that cannot be corrected by optics because the deficits reflect neural abnormalities. Biological motion refers to the motion patterns of living organisms, and is normally displayed as points of lights positioned at the major joints of the body. In this experiment, our goal was twofold. We wished to examine whether the human visual system in people with amblyopia retained the higher-level processing capabilities to extract visual information from the synchronized actions of others, therefore retaining the ability to detect biological motion. Specifically, we wanted to determine if the synchronized interaction of two agents performing a dancing routine allowed the amblyopic observer to use the actions of one agent to predict the expected actions of a second agent. We also wished to establish whether synchronicity sensitivity (detection of synchronized versus desynchronized interactions) is impaired in amblyopic observers relative to normal observers. The two aims are differentiated in that the first aim looks at whether synchronized actions result in improved expected action predictions while the second aim quantitatively compares synchronicity sensitivity, or the ratio of desynchronized to synchronized detection sensitivities, to determine if there is a difference between normal and amblyopic observers. Our results show that the ability to detect biological motion requires more samples in both eyes of amblyopes than in normal control observers. The increased sample threshold is not the result of low-level losses but may reflect losses in feature integration due to undersampling in the amblyopic visual system. However, like normal observers, amblyopes are more sensitive to synchronized versus desynchronized interactions, indicating that higher-level processing of biological motion remains intact. We also found no impairment in synchronicity sensitivity in the amblyopic visual system relative to the normal visual system. Since there is no impairment in synchronicity sensitivity in either the nonamblyopic or amblyopic eye of amblyopes, our results suggest that the higher order processing of biological motion is intact.
Keywords: Amblyopia, Biological motion, Stereopsis, Synchronicity
1. Introduction
Amblyopia is a developmental disorder of spatial vision that results from abnormal early visual experience usually due to the presence of strabismus (an eye turn), anisometropia (a significant and unequal refractive error between the two eyes), or both strabismus and anisometropia (Ciuffreda, Levi, & Selenow, 1991; Levi, 1991; Levi & Carkeet, 1993). Amblyopia results in unilateral visual deficits, without apparent pathology, that cannot be corrected by optics because the deficits reflect neural abnormalities (Kiorpes, 2006; Levi, 2006). The most frequent cause of vision loss in infants and young children, aside from refractive error, amblyopia is clinically diagnosed as a reduction in visual acuity (Ciuffreda, Levi, & Selenow, 1991). In addition, for both types of amblyopia, strabismic and anisometropic, the amblyopic eye exhibits a marked loss of contrast sensitivity (Bradley & Freeman, 1981; Hess & Howell, 1977; Levi & Harwerth, 1977), an increased extent of spatial interference (Levi & Klein, 1985), and deficits in spatial localization (Hess & Holliday, 1992).
Amblyopic neural deficits first appear in the primary visual cortex, V1 (Kiorpes, 2006; Kiorpes & McKee, 1999). More recent studies have reported these amblyopic neural deficits may extend into extrastriate cortical areas (Aaen-Stockdale, Ledgeway, & Hess, 2007; Lerner et al., 2003, 2006; Simmers et al., 2003; Wong, Levi, & McGraw, 2001), and perhaps beyond (Sharma, Levi, & Klein, 2000). And a recent MRI study has found that the LGN may also be affected by amblyopia (Li et al., 2011). However, it is unclear whether these extrastriate cortical regions serve to amplify the losses that occur in V1, or if the downstream losses are simply the reflection of the original losses in V1. Previous studies have demonstrated that higher-level visual processing such as for biological motion, a complex form of structure-from-motion representing humanactions, is unaffected by amblyopia (Neri, Luu, & Levi, 2007).
Biological motion refers to the motion patterns of living organisms, and is normally displayed as points of lights positioned at the major joints of the body. Johansson was the first to use point-light displays to show the perception of biological motion (Johansson, 1973, 1976). Static point-light displays were found to provide no percept of a human agent. Only when the point-lights were in motion could they be organized into the percept of a human agent. Point-light displays carry a wealth of information that provide humanobservers with higher-order information, allowing the observer to identify the figure’s gender (Barclay, Cutting, & Kozlowski, 1978; Cutting, Proffitt, & Kozlowski, 1978), emotional state (Dittrich et al., 1996), identity (Cutting & Kozlowski, 1977; Troje, Westhoff, & Lavrov, 2005), intentions (Bingham, 1987; Runeson & Frykholm, 1983), and even the category of action that the figure is performing (Dittrich, 1993). In this study, we used point-light displays generated from the trajectories of the major joints of two human agents performing a dancing routine (Neri, Luu, & Levi, 2006).
Previous studies investigating the effects of amblyopia on higher-level cognitive functions have found that biological motion detection as processed by global form from motion (Neri, Luu, & Levi, 2007) and local motion information (Thompson et al., 2008) is relatively unaffected. In human observers with normal vision, visual discrimination of a human agent is influenced by the presence of a second agent and by whether the two agents interact in a meaningful and synchronized way, such as during a dancing or fighting routine (Neri, Luu, & Levi, 2006). This kind of synchronized interaction allows the human observer to use the actions of one agent to predict the expected actions of a second agent.
In this study, our goal was twofold. We wished to examine whether the human visual system in people with amblyopia retained the higher-level processing capabilities to extract visual information from the synchronized actions of others, therefore retaining the ability to detect biological motion. Specifically, we wanted to determine if the synchronized interaction of two agents performing a dancing routine allowed the amblyopic observer to use the actions of one agent to predict the expected actions of a second agent. We also wished to establish whether synchronicity sensitivity (detection of synchronized versus desynchronized interactions) is impaired in amblyopic observers relative to normal observers. The two aims are differentiated in that the first aim looks at whether synchronized actions result in improved expected action predictions while the second aim quantitatively compares synchronicity sensitivity, or the ratio of desynchronized to synchronized detection sensitivities, to determine if there is a difference between normal and amblyopic observers.
There is reason to suspect that synchronicity sensitivity may be impaired in amblyopic observers. The synchronous firing of spatially separate neurons is thought to be involved in the temporal processing of visual information (Asper, Crewther, & Crewther, 2000; Engel, Konig, & Singer, 1991; Roelfsema et al., 1994; but see Shadlen & Movshon, 1999 for a different view). However, the synchronicity of firing is reduced in cortical neurons driven by the amblyopic eye of strabismic cats, in comparison to the synchronous firing in both eyes of normal cats and the non-amblyopic eyes of strabismic cats (Roelfsema et al., 1994). Moreover, previous studies have reported that spatio-temporal processing may be impaired in humans with amblyopia (Asper, Crewther, & Crewther, 2000; Popple & Levi, 2008). These findings suggest that since synchronization of neural firing may be impaired in the amblyopic eye, synchronicity sensitivity will also be impaired in the amblyopic eye.
Our results show that the ability to detect biological motion requires more samples (dot trajectories) in both eyes of amblyopes than in normal control observers. The increased sample threshold is not the result of low-level losses (dot trajectories were highly visible) but may reflect losses in feature integration due to undersampling in the amblyopic visual system (Levi & Klein, 1986; Levi, Klein, & Sharma, 1999; Levi, Klein, & Yap, 1987). However, like normal observers, amblyopes are more sensitive to synchronized versus desynchronized interactions, indicating that higher-level processing of biological motion remains intact, as previously reported (Neri, Luu, & Levi, 2007; Thompson et al., 2008). Similar to normal vision, in amblyopia the difference in biological motion perception between synchronized and desynchronized stimuli is due to the disruptive effect of desynchronization on the perception of biological motion. We also found no impairment in synchronicity sensitivity in the amblyopic visual system relative to the normal visual system. This suggests that higher order processing of biological motion remains intact in the amblyopic visual system.
2. Material and methods
2.1. Observers
Seven amblyopic observers participated in our study (see Table 1 for the visual characteristics of these observers). Of the seven amblyopic observers, three were strabismic (SS1–SS3), two were both strabismic and anisometropic (SB1 and SB2), and two were anisometropic (SA1 and SA2). In the figures, the amblyopic results are colored according to the type of amblyopia (strabismic – red; strabismic and anisometropic – blue; non-strabismic anisometropic – green). All amblyopic observers wore their best optical correction whenperforming the study. Five observers with normal, or corrected to normal, visual acuity and stereoacuity participated as controls in our study. All observers, except for one author, were naïve observers.
Table 1.
Visual characteristics of amblyopic observers. Visual acuities were determined using a Bailey–Lovie chart. Stereopsis was measured using the Randot “Circles” Stereotest (Stereo Optical Co., Chicago, IL). Anisometropia was defined as a spherical equivalent difference greater than 1.50 diopters.
| Observer | Age (years) | Gender | Strabismus (at 6 m) | Eye | Refractive error (diopters) | Line letter VA (single letter VA) | Stereopsis |
|---|---|---|---|---|---|---|---|
| Strabismic | |||||||
| SS1 | 23 | M | L EsoT 3–4Δ | R | +5.50 − 2.25 × 005 | 20/16+2 | 400″ |
| L | +5.50 − 1.50 × 175 | 20/50−2 (20/25−2) | |||||
| SS2 | 20 | F | L EsoT 4Δ | R | −0.50 − 0.75 × 095 | 20/16−1 | Fail |
| L | −0.25 − 0.50 × 050 | 20/32+1 (20/25+1) | |||||
| SS3 | 27 | F | L EsoT 20–25Δ | R | −0.50 − 3.75 × 150 | 20/80−2 (20/25−2) | Fail |
| L | −2.00 − 3.50 × 025 | 20/20−2 | |||||
| Strabismic and anisometropic | |||||||
| SB1 | 26 | F | R EsoT 4–6Δ and HypoT 4Δ | R | +2.75 − 1.00 × 160 | 20/80−1 (20/50−1) | Fail |
| L | −1.00 − 0.50 × 180 | 20/16−1 | |||||
| SB2 | 24 | M | R EsoT 4–5Δ | R | +3.50 − 1.00 × 100 | 20/63+1 (20/40−1) | Fail |
| L | Plano | 20/16−1 | |||||
| Non-strabismic anisometropic | |||||||
| SA1 | 45 | F | R | +0.25 − 0.50 × 090 | 20/12.5−2 | 70″ | |
| L | +3.75 − 1.00 × 030 | 20/50+2 (20/50+2) | |||||
| SA2 | 30 | M | R | −1.25 − 0.25 × 160 | 20/16+1 | 200″ | |
| L | +0.75 − 0.75 × 170 | 20/63+1 (20/50+2) | |||||
2.2. Motion capture
A routine by two dancers (recruited from the UC Berkeley Ballroom Dancers) performing the Rumba was filmed using a camera device (Logitech QuickCam) that generated digital AVI movies at 10 Hz and 640 × 480 pixel resolution (Fig. 1). Each dancer was outfitted with clothing that carried battery-powered wire light markers (ClubThings, Los Angeles, CA) positioned at 13 points on the body: one at the head, and one at each shoulder, elbow, wrist, hip, knee, and ankle. We created customized Matlab software to aid in the movie processing and to provide computer-assisted motion capture. The software used basic clustering analysis to detect regions of high luminance on the body of each dancer to determine the positions of the light markers. The trajectories of these markers were then tracked through each frame of the movie. A graphic user interface included in this software allowed the user to view the automated tracking frame-by-frame and make corrections when needed to correct the numerous errors made in the automated process. This user interface allowed for the tracking of the full trajectories of the 13 major joints on each dancer in x–y–t space (the sequence was interpolated to obtain 30 Hz sampling), and allowed for the marking of joint disappearances due to occlusion. The final tracked dancing routine was 24 s in length.
Fig. 1.

Motion capture. One frame of the digital AVI movie generated by filming a Rumba routine performed by two dancers recruited from the UC Berkeley Ballroom Dancers. Each dancer was outfitted in clothing carrying battery-powered wire light markers positioned at 13 points on the body: one at the head, and one at each shoulder, elbow, wrist, hip, knee, and ankle.
2.3. Stimuli
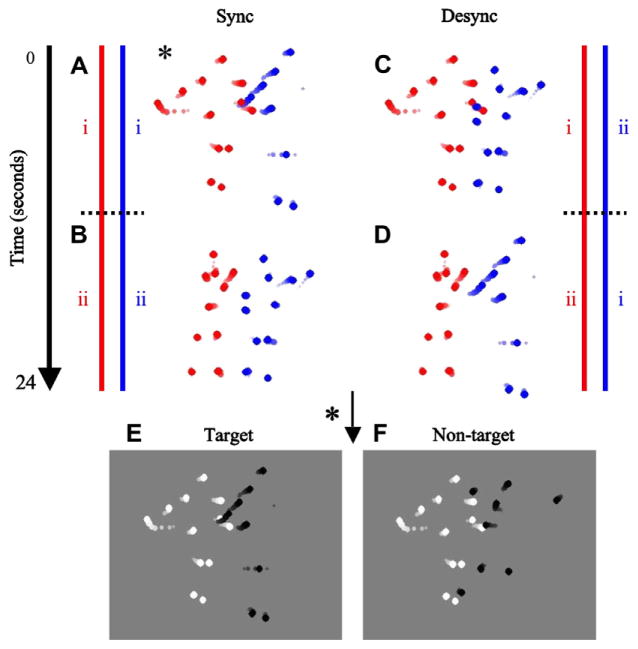
The trials in this experiment were of either ‘Sync’ or ‘Desync’ type. Sync and Desync trials were randomly presented within a block, and in each trial both intervals were either Sync or Desync. Sync trials consisted of a short segment randomly selected from the two sequences that result from the first and second halves of the original tracked movie (Fig. 2A and B). Desync trials consisted of a short segment randomly selected from the two sequences that result from cross-pairing the trajectories of one agent (red) in one half of the original tracked movie (i) with the trajectories of the other agent (blue) in the other half of the original tracked movie (ii), and vice versa (Fig. 2C and D). The duration of each segment was 3 s. In each segment, the dot (6 arcmin diameter) trajectories of one agent were randomly selected to be bright (157.5 cd m−2) while the trajectories of the other agent were dark (0.75 cd m−2) on a gray background (80.73 cd m−2) (Fig. 2E and F). The trajectories were sized so that their overall center of mass was centered on a Sony Trinitron Multiscan G400 monitor driven by a VSG 2/5 graphics card (Cambridge Research Systems, Rochester, UK), and did not extend outside a 13.0° × 13.4° region. Observers sat a distance of 114 cm from the monitor and fixated on a marker located at the center of the screen.
Fig. 2.
Stimulus and psychophysical procedure. (A–D) Red and blue lines depict the database consisting of a 24 s sequence of tracked trajectories of a dancing routine between two agents obtained through motion capture. Static depictions representing movie segments are represented by dot streaks in which the contrast and size of the individual dots increase with time. The sequence is divided into two halves, i and ii, as depicted by the dotted black lines. Sync trials consist of a short segment randomly selected from the two synchronized sequences (A) i–i and (B) ii–ii. Desync trials consist of a short segment randomly selected from the two desynchronized sequences obtained by cross-pairing i and ii for the two agents, (C) i–ii and (D) ii–i. Each trial could be of either Sync or Desync type, and were randomly presented within a block. Each trial consisted of two intervals. The Sync trial depicted with the * marker in (A) was chosen to illustrate these two intervals (E and F). (E) The target interval showed a randomly selected 3 s segment with the dot trajectories of one agent randomly selected to be dark while the dot trajectories of the second agent were bright on a gray background. (F) The non-target interval showed another segment, but with one of the agents randomly selected to have its 13 dot trajectories scrambled so that the structure of the agent was destroyed while the local motion information of the individual dots was preserved. The observers’ task was to determine the target interval. Static depictions representing the moving stimuli are represented by dot streaks in which the contrast and size of the individual dots increase with time (these manipulations were not present in the actual stimuli). In order to vary the strength of biological motion, we randomly varied the number of dot trajectories selected to be displayed in each trial (6, 11, 16, 21, or 26, out of a total of 26 dot trajectories). The stimulus level represented in this figure has 26 dot trajectories. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Psychophysical procedure
We used the method of constant stimuli to present the biological motion stimulus at five different stimulus levels, varying the strength of biological motion by varying the number of dot trajectories. For a given trial, the number of randomly selected dot trajectories to be displayed was 6, 11, 16, 21, or 26, out of a total of 26 dot trajectories. Within a single block, each stimulus level was tested 10 times for both Sync and Desync trials, resulting in 100 trials total for a single block. Each trial consisted of two 3 s stimuli shown consecutively with a 1 s ISI. Each trial consisted of two intervals, one containing the ‘target’ stimulus (Fig. 2E) and the other containing the ‘non-target’ stimulus (Fig. 2F), with the order of presentation randomly selected. In the non-target interval one of the agents was randomly selected to have its 13 dot trajectories scrambled by selecting different segments from the original sample for each dot trajectory (Neri, Luu, & Levi, 2006). Since each individual joint is sampled uniformly for both scrambled and nonscrambled agents, this procedure ensures that there is no difference in the raw motion content averaged across trials. The temporal window in which the scrambled joints could sample the original sequence matched the duration of the entire sequence, allowing for maximum scrambling. The target interval was described to the observers as the interval in which there could be seen two humans dancing. Before testing, observers performed practice runs with fully sampled versions of the sequence. The observers were able to immediately recognize the dancing action and were able to perform the detection task without difficulty. We used a temporal two-alternative forced choice paradigm with feedback, in which the observer indicated the target interval by pressing one of two buttons on a keyboard. Observers performed the experiment monocularly, with the untested eye covered with a black eye patch. Observers with normal vision tested their dominant eye only. Observers with amblyopic vision tested both eyes separately, alternating between their non-amblyopic eye and their amblyopic eye between blocks.
2.5. Threshold determination
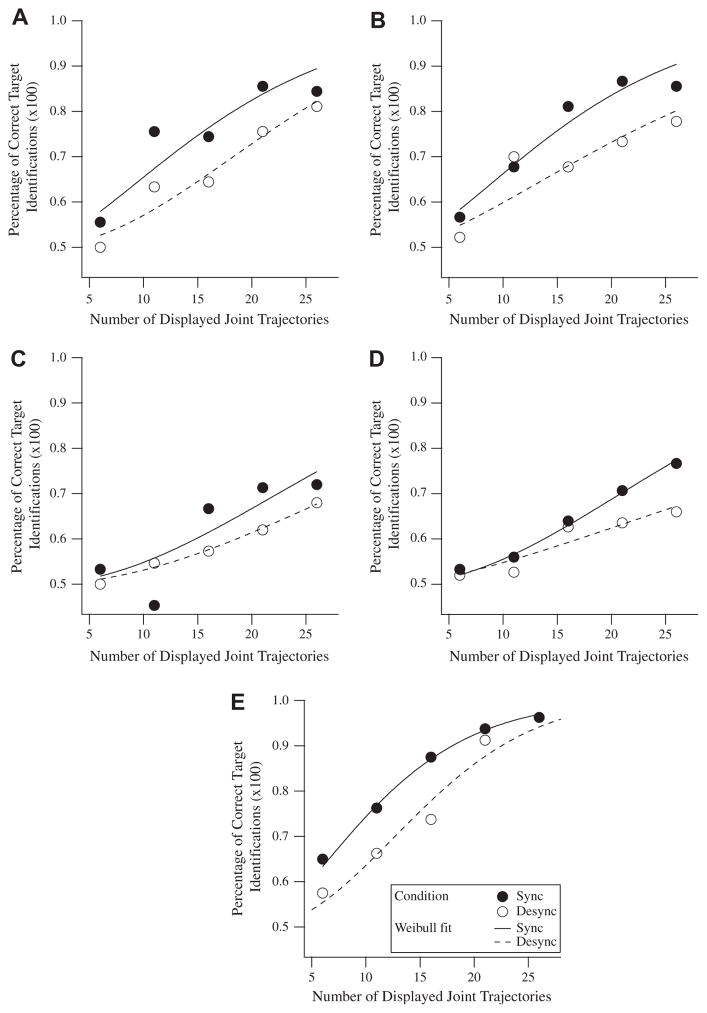
The probability of correct target identifications (P) was plotted as a function of the number of dot trajectories displayed (D) for both Sync and Desync conditions, which were carried out in interleaved trials. A Weibull function, P = 1 − 0.5 exp(−(D/α)β), was fitted to each psychometric curve to determine the threshold (α parameter), with the shape (β parameter) fixed at a value of either 1.5 or 2 depending on which value of β minimized the value of Chi-square. For example, the psychometric curve for the amblyopic eye of amblyopic observer SS1 was fitted with the Weibull function P = 1 − 0.5 exp(−(D/19)1.5) for Sync conditions, and P = 1 − 0.5 exp(−(D/26)2) for Desync conditions (Fig. 3A). The psychometric curve for the non-amblyopic eye of amblyopic observer SS1 was fitted with the Weibull function P = 1 − 0.5 exp(−(D/19)1.5) for Sync conditions, and P = 1 − 0.5exp(−(D/27)1.5) for Desync conditions (Fig. 3B). And the psychometric curve for one normal control observer (inverted triangle in Fig. 4C) was fitted with the Weibull function P = 1 − 0.5 exp(−(D/13)1.5) for Sync conditions, and P = 1 − 0.5 exp(−(D/18)2) for Desync conditions (Fig. 3E). The Weibull function is commonly used to parameterize psychometric functions because of its ability to provide a good model for actual data (Klein, 2001; Wichmann & Hill, 2001). Note that in some cases thresholds are extrapolated beyond the range of measurements. Data determined through larger extrapolations resulted in correspondingly larger standard errors. The Weibull functions fitted to amblyopic observer SB1, an example of a large extrapolation of data, is depicted for the amblyopic eye (Fig. 3C) and the non-amblyopic eye (Fig. 3D).
Fig. 3.
Weibull functions fitted to psychometric curves. Scatter plots show the number of displayed joint trajectories versus the probability of correct target identifications for Sync (closed circles) and Desync (open circles) conditions. The Weibull functions fitted to the psychometric curves are also shown. (A) Scatter plot for amblyopic eye of amblyopic observer SS1. (B) Scatter plot for non-amblyopic eye of amblyopic observer SS1. (C) Scatter plot for amblyopic eye of amblyopic observer SB1. (D) Scatter plot for non-amblyopic eye of amblyopic observer SB1. (E) Scatter plot for dominant eye of one normal observer (inverted triangle in Fig. 4C).
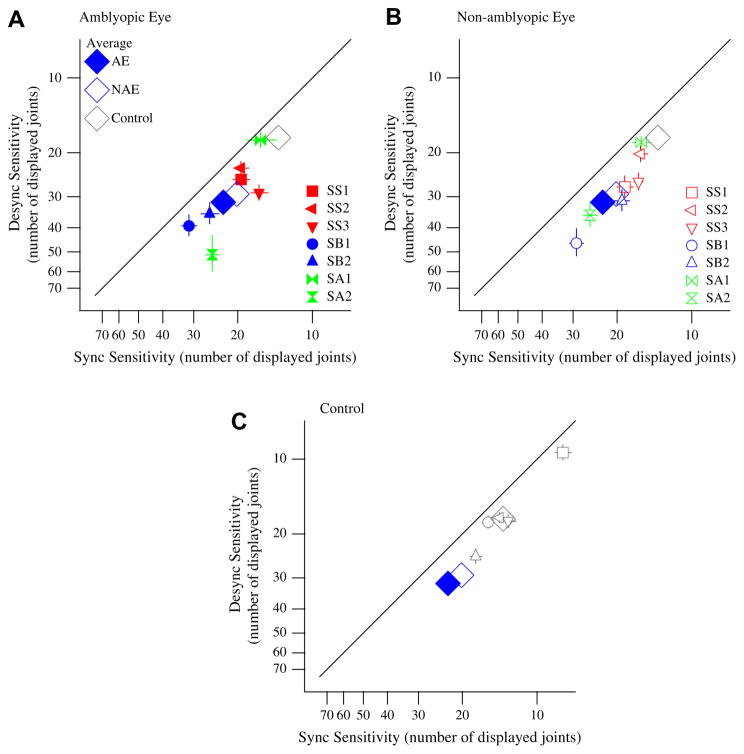
Fig. 4.
Sync sensitivity is greater than Desync sensitivity. Scatter plots show sensitivity (measured by threshold number of displayed joints) for Sync versus Desync trials in strabismic (red), strabismic and anisometropic (blue), non-strabismic anisometropic (green), and normal control (open gray). Smaller threshold values of number of displayed joints correspond to better sensitivity (axes have been reversed to represent this). (A) Solid colored symbols represent data from the amblyopic eye (AE). (B) Open colored symbols represent data from the non-amblyopic eye (NAE). (C) Open gray symbols represent data from normal observers (Control). Different symbols refer to different observers. All data points fall below the unity line, representing a greater sensitivity for Sync trials as compared to Desync trials. For all scatter plots, the average Sync and Desync thresholds for AE, NAE, and Control data are indicated by the large symbols labeled in the caption in Panel A. One author (gray square) participated in the experiment; all other observers were naïve. Error bars indicate ±1 SEM. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Results
3.1. Sensitivity is better for synchronized than desynchronized dancing
Amblyopic and normal control observers were asked to discriminate between sequences of point-light dancers to determine the target interval which contained two agents performing a dancing routine. These sequences of point-light dancers were generated as either Sync or Desync trials, with no difference between the trials in regard to the visual information provided to the observer. We ensured there was no difference in the raw motion content averaged across trials by sampling each individual joint uniformly. In fact, whether a trial was of Sync or Desync type had no bearing on the task. The ability to discriminate between target and nontarget intervals in Sync and Desync trials was analyzed to determine if the difference resulted in differing abilities to use the actions of one agent to predict the expected actions of a second agent. Any difference that exists could then be attributed to human processing of the interaction between two agents.
Sync and Desync sensitivities were measured by varying the number of displayed point-lights to determine the threshold number of displayed joints needed for discrimination between target and non-target intervals. Desync (ordinate) versus Sync (abscissa) thresholds are plotted for each observer in Fig. 4, with each point representing either the amblyopic eye (solid colored symbols) (Fig. 4A) or non-amblyopic eye (open colored symbols) (Fig. 4B) of an amblyopic observer, or the dominant eye (open gray symbols) (Fig. 4C) of an observer with normal vision. Note that the axes in Fig. 4 are reversed, so that points below the 1:1 line indicate lower sensitivity (higher thresholds) for Desync trials as compared to Sync trials.
The data for all observers fall below the unity line showing higher sensitivity for Sync trials as compared to Desync trials. Paired t-tests (one-tailed) across observers for Sync > Desync sensitivity show that Sync sensitivity was significantly higher than Desync sensitivity for both the amblyopic and non-amblyopic eyes of amblyopic observers, and for the dominant eye of normal observers (p = 0.01 (AE), p = 0.001 (NAE), and p = 0.01 (Control)). This finding is in agreement with, and extends upon, earlier findings showing that significantly higher noise tolerance is possible for Sync trials as compared to Desync trials in observers with normal vision (Neri, Luu, & Levi, 2006). Note however, that the present study does not use noise (as did the previous study), rather we show that observers need less information (number of displayed joints) in order to detect agents whose actions are synchronized than when they are desynchronized.
Upon completion of the experiment, naïve observers were asked if they had noticed that in some of the trials the two agents had moved in a desynchronized manner. The naïve observers re-ported that they had noticed that in some trials the two agents were synchronized and in others they were desynchronized. However, they were unaware that this had any bearing on the task since the task had been stated to them as determining the target interval that contained two agents performing a dancing routine.
3.2. Sync and Desync sensitivities in amblyopic observers relative to normal controls
To compare the Sync sensitivities of each eye of amblyopic observers (amblyopic eye and non-amblyopic eye) relative to the dominant eye of normal control observers we performed a oneway ANOVA showing a significant difference between the groups (F(2,16) = 5.29, p = 0.02). Subsequent t-tests (one-tailed) with Bonferroni correction for multiple comparisons at β = 0.017 confirmed a drop in Sync sensitivities in both the amblyopic (p = 0.002) and non-amblyopic (p = 0.016) eyes relative to normal control observers; however, no significant difference was found between amblyopic and non-amblyopic eyes (p = 0.02).
To compare the Desync sensitivities of each eye of amblyopic observers (amblyopic eye and non-amblyopic eye) relative to the dominant eye of normal control observers we performed a oneway ANOVA showing no significant difference between the groups (F(2,16) = 3.62, p = 0.05).
Furthermore, t-tests (two-tailed) found no difference between strabismic and non-strabismic amblyopic observers for both Sync and Desync sensitivities in the amblyopic and non-amblyopic eyes (p = 0.64 (AE, Sync), p = 0.88 (NAE, Sync), p = 0.85 (AE, Desync), p = 0.77 (NAE, Desync)).
3.3. Synchronicity sensitivity in amblyopic observers relative to normal controls
To determine quantitatively whether amblyopic observers have an impaired ability to detect synchronized interactions relative to desynchronized interactions, referred to here as synchronicity sensitivity, we compared the ratio of their Desync and Sync sensitivities. Specifically, we compared the ratio of the number of displayed joints needed for discrimination between target and non-target intervals in Desync and Sync trials. We performed a one-way ANOVA comparing synchronicity sensitivities in each eye of amblyopic observers (amblyopic eye and non-amblyopic eye) and the dominant eye of normal control observers and found that there was no significant difference between the groups (F(2,16) = 0.91, p = 0.42). This suggests that there is no significant difference between the synchronicity sensitivities of the non-amblyopic and amblyopic eyes of amblyopic observers and the dominant eye of normal observers.
In addition, t-tests (two-tailed) found no difference between strabismic and non-strabismic amblopic observers for synchronicity sensitivity in the amblyopic and non-amblyopic eyes (p = 0.66 (AE), p = 0.25 (NAE)).
3.4. Effect of visual acuity on amblyopic sensitivity to synchronicity
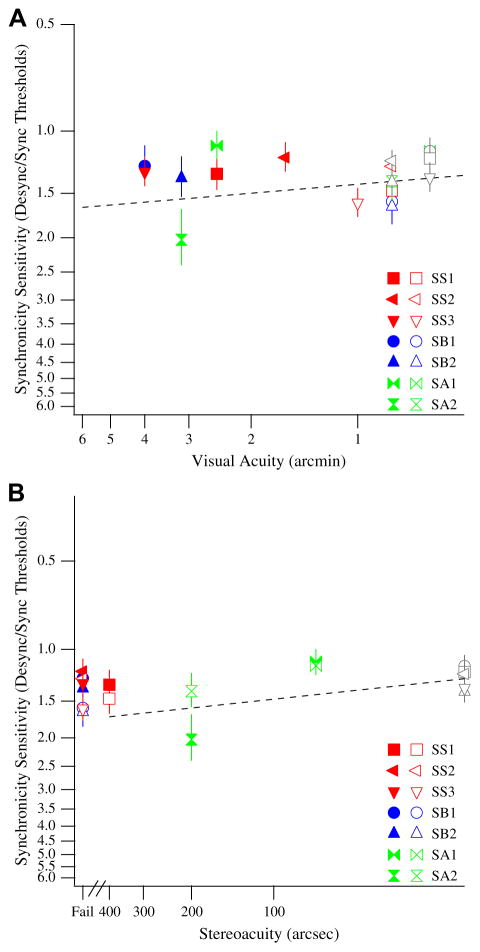
To determine if visual acuity has an effect on synchronicity sensitivity in the amblyopic visual system, we plotted the visual acuities of our amblyopic observers (by taking the inverse of their line letter visual acuities in Table 1) against the synchronicity sensitivities of their amblyopic (solid symbols) and non-amblyopic (open symbols) eyes (Fig. 5A). Included in Fig. 5A are the synchronicity sensitivities for our control (open gray symbols) observers. To look at the relationship between synchronicity sensitivity and stereoacuity, we also plotted the synchronicity sensitivities of our amblyopic and control observers against their stereoacuities (Fig. 5B). Our results suggest no clear relationship between visual acuity and sensitivity to synchronicity (Fig. 5A). The slope of the best fitting line is 0.08 ± 0.59, consistent with a slope of zero.
Fig. 5.
(A) Visual acuity versus synchronicity sensitivity. The synchronicity sensitivities for the amblyopic eye (solid colored symbols) and the non-amblyopic eye (open colored symbols) of strabismic (red), strabismic and anisometropic (blue), and non-strabismic anisometropic (green) observers are plotted against their visual acuities. The synchronicity sensitivities for control (open gray symbols) observers are also plotted against their visual acuities. Different symbols refer to different observers. Smaller synchronicity sensitivities and smaller visual acuities correspond to better sensitivity (axes have been reversed to represent this). The regression line for control, amblyopic, and non-amblyopic data is plotted. (B) Stereoacuity versus synchronicity sensitivity. The synchronicity sensitivities for the amblyopic eye (solid colored symbols) and the non-amblyopic eye (open colored symbols) of strabismic (red), strabismic and anisometropic (blue), and non-strabismic anisometropic (green) observers are plotted against their stereoacuities. The synchronicity sensitivities for control observers (open gray symbols) are also plotted against their stereoacuities. Observers who did not pass the stereoacuity test are denoted as ‘Fail’ on the abscissa. Different symbols refer to different observers. Smaller synchronicity sensitivities and smaller stereoacuities correspond to better sensitivity (axes have been reversed to represent this). The regression line for control, amblyopic, and non-amblyopic data is plotted (regression line does not include data from observers who failed the stereoacuity test). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Our results also suggest no clear relationship between stereoacuity and sensitivity to synchronicity (Fig. 5B). The slope of the best fitting line is 0.10 ± 0.47, consistent with a slope of zero. Across a large range of visual acuities and stereoacuities, the sensitivity to synchronicity is more or less invariant (ratio ≈ 1.4).
4. Discussion
4.1. Controls for low-level deficits
The aim of this experiment was to determine whether the higher-level processing stages of the human visual system responsible for the ability to extract visual information from the actions of others are impaired in amblyopic observers. To do this we evaluated whether the synchronized interaction of two agents dancing would result in an increased ability of amblyopic observers to use the actions of one agent to serve as a predictor of the expected actions of a second agent. In order to isolate higher-level processing as the possible cause of impairment for biological motion perception in the amblyopic visual system, we needed to rule out lower-level processing of motion information as the limiting factor in the processing of motion information. To do this, we ensured that our stimuli were easily visible to our amblyopic observers. We chose dots of large size and high contrast so that the motions of the individual point-lights were easily detectable to our amblyopic observers. Before running the experiment, we piloted our stimuli on each of our observers to ensure that the details of the point-light display were visible. Before each testing session, observers performed practice runs with fully sampled versions of the sequence. In addition, amblyopic observers performed these practice runs monocularly with their amblyopic eye, in order to familiarize the observers with our stimuli and to ensure that the stimuli were easily visible to them. Our observers were able to immediately recognize the dancing action, and they were able to perform the detection task without difficulty.
To further control for low-level cues, in an earlier study we performed several simulations with full statistical knowledge of the low-level position and motion content of our Sync and Desync sequences to determine if any underlying differences existed in the low-level content of the stimuli (Neri, Luu, & Levi, 2006). Our findings indicated that the Sync and Desync sequences could not be differentiated by low-level spatio-temporal properties. For additional confirmation, in this previous study we performed an inversion control where the non-scrambled agent in each interval was inverted (Neri, Luu, & Levi, 2006). Inverting a point-light display has been found to make it more difficult to perceive biological motion (Pavlova & Sokolov, 2000; Sumi, 1984), while at the same time retaining low-level structure. If low-level cues were the reason for the difference that resulted between Sync and Desync sequences, than they should still be present after inversion. However, there was no significant difference between Sync and Desync thresholds after inversion, confirming that low-level structures were not the reason for the differential effect (Neri, Luu, & Levi, 2006).
4.2. Higher- and lower-level motion processing
The first reporting of a brain area, the superior temporal sulcus (STS), containing cells responsive to faces was performed using single-cell recording studies on the macaque temporal cortex (Perrett, Rolls, & Caan, 1982). Further research determined that neurons in the STS were selectively responsive to biological motion stimuli, or whole body motion (Oram & Perrett, 1994). Subsequent studies have established that biological motion perception activates neural areas of the STS in both human and non-human primates (Puce & Perrett, 2003). Neurons of the STS largely respond to visual stimuli; however, studies have reported that these neurons can be affected by projections from the motor system (Hietanen & Perrett, 1996) and from the amygdala (Aggleton, Burton, & Passingham, 1980). Biological motion perception has also been reported to activate mirror neurons of area F5 in monkey premotor cortex (Rizzolatti & Craighero, 2004). Mirror neurons are a type of visuomotor neuron that respond both when the monkey performs a particular action and when the monkey observes another individual (monkey or human) performing a similar action (di Pellegrino et al., 1992; Rizzolatti & Craighero, 2004; Rizzolatti et al., 1996). The visual processing of biological motion is thought to be composed of two streams, a dorsal pathway specialized for the processing of motion information and a ventral pathway specialized for the processing of form information (Giese & Poggio, 2003). The STS receives neural input from both the dorsal and the ventral streams, allowing for the integration of both form and motion information arising from the same visual stimulus (Giese & Poggio, 2003; Oram & Perrett, 1996). Importantly, amblyopes show abnormalities in both the ventral and dorsal streams (Simmers et al., 2006).
The results of our experiment reflect upon the later stages in the motion processing hierarchy that follows after the extraction of optic flow (Neri, Luu, & Levi, 2006). We refer to these later stages as higher-level motion processing. The successful extraction of optic flow from visual motion establishes global translation and rotation features, as well as local average flow velocities and variations (Koenderink, 1986). In the human visual system, higher-level motion processing is based on the retrieval of structure from motion. In regards to this experiment, higher-level motion processing stages of the human visual system are involved in the grouping of moving point-light stimuli to form coherent percepts of human agents (Aggarwal & Cai, 1999; Hoffman & Flinchbaugh, 1982).
The results of our experiment do not relate to the earlier stage of the motion processing hierarchy preceding the extraction of optic flow, which we refer to as lower-level motion processing. Therefore, our results do not address the question of whether there exists lower-level motion processing deficits in amblyopic observers. The results of previous studies on lower-level motion processing in amblyopic observers have been mixed. Some studies have found little or no deficits in lower-level motion processing in amblyopic observers (Hess et al., 2006; Kubova et al., 1995; Levi & Tripathy, 2006). In contrast, other studies have found lower-level motion processing to be impaired in amblyopic humans (Ho et al., 2006; Rislove et al., 2010; Simmers et al., 2003) and monkeys (El-Shamayleh et al., 2010; Kiorpes, Tang, & Movshon, 2006). This area remains an open and active topic of research.
4.3. Feature integration is impaired due to undersampling in the amblyopic visual system
Undersampling in the visual system of observers with strabismic amblyopia may result from widespread loss of cortical neurons, scrambling of functional neural connections leading to neural disarray, or both (Asper, Crewther, & Crewther, 2000; Levi & Klein, 1986; Levi, Klein, & Sharma, 1999; Levi, Klein, & Yap, 1987). Undersampling is one hypothesis for the visual deficits found in amblyopic observers. Support for this hypothesis comes from previous studies reporting impaired sampling in the central vision of strabismic observers relative to the central vision of normal observers (Levi & Klein, 1986; Levi, Klein, & Sharma, 1999; Levi, Klein, & Yap, 1987). Consequently, the strabismic fovea requires more sampling information, and has an increased “sample threshold”, compared to the normal fovea. This increased sample threshold suggests that as a result of undersampling, the amblyopic visual system under represents visual stimuli at the stage of feature integration (Levi, Klein, & Sharma, 1999; Levi, Sharma, & Klein, 1997). Undersampling is especially detrimental to amblyopic vision relative to normal vision because of a lack in redundancy; therefore, the removal of samples results in impaired amblyopic visual performance (Levi, Klein, & Sharma, 1999).
In this study, we found the ability to detect biological motion requires more samples (displayed dot trajectories) in both eyes of amblyopes than in the eyes of normal control observers. More specifically, there is a drop in both the Sync and Desync sensitivities of both amblyopic and non-amblyopic eyes relative to normal control eyes. The increase in sample thresholds is not the result of low-level losses (dot trajectories were highly visible), but may instead reflect losses in feature integration due to undersampling in the amblyopic visual system (Levi & Klein, 1986; Levi, Klein, & Sharma, 1999; Levi, Klein, & Yap, 1987). Particularly noteworthy is the fact that the non-amblyopic eye showed a similar impairment, despite normal visual acuity. This implies a central locus for undersampling in the amblyopic visual system.
4.4. Higher-level processes involved in biological motion detection is unimpaired by amblyopia
Our results show that in the dominant eye of normal observers Sync sensitivity is significantly greater than Desync sensitivity (Fig. 4C). This finding is consistent with our previous finding that Sync sensitivity is significantly greater than Desync sensitivity in normal observers under binocular viewing conditions (Neri, Luu, & Levi, 2006). Similarly, in amblyopic observers Sync sensitivity was found to be significantly greater than Desync sensitivity in both amblyopic (Fig. 4A) and non-amblyopic (Fig. 4B) eyes. Our findings reveal that like normal observers, amblyopes are more sensitive to synchronized versus desynchronized interactions, indicating that the higher-level processing of biological motion remains intact, as previously reported (Neri, Luu, & Levi, 2007; Thompson et al., 2008).
In this study, we quantitatively measured Sync and Desync sensitivities by establishing the threshold number of displayed joints required for discrimination of target and non-target intervals. Different performance metrics have been used to measure biological motion detection in amblyopic observers. Using fully sampled stimuli while varying the amount of scrambling (Thompson et al., 2008) and measuring the inversion effect with the addition of masking noise dots (Neri, Luu, & Levi, 2007) have proven to be effective methods of measurement. This study extends upon these findings and establishes varying the number of displayed joints to determine the threshold number of dot trajectories as another performance metric that can be used in the quantitative analysis of biological motion detection in amblyopic observers.
4.5. Disruption due to desynchronization impairs amblyopic biological motion detection
The disruption due to desynchronization results in a markedly impaired ability to detect biological motion in both normal and amblyopic vision. For both the amblyopic and non-amblyopic eyes, desynchronization requires that the threshold number of displayed joints for Desync sensitivity be extrapolated beyond the 26 dot trajectories available in the point-light display. A previous study found that in normal observers the difference in synchronized and desynchronized biological motion detection resulted from disruption by desynchronization rather than enhancement by synchronization (Neri, Luu, & Levi, 2006). Our results extend upon this finding by showing that desynchronization also disrupts the ability to detect biological motion in the amblyopic visual system.
4.6. Synchronicity sensitivity is not impaired in the amblyopic visual system
Synchronicity sensitivity is the ability to detect synchronized versus desynchronized interactions. To determine whether synchronicity sensitivity is impaired in the amblyopic and non-amblyopic eyes of amblyopic observers relative to the eyes of normal control observers, we calculated the ratio of Desync and Sync sensitivities. We found that there is no significant difference between the synchronicity sensitivities of the amblyopic and non-amblyopic eyes of amblyopic observers and the dominant eye of normal control observers. Our results suggest that the higher order processing of biological motion is intact.
In considering the visual performance of the amblyopic eye relative to the non-amblyopic eye, it is reasonable to expect that the performance in the amblyopic eye will be worse than in the clinically unaffected non-amblyopic eye. In fact, previous studies have reported motion perception deficits in the non-amblyopic eye of amblyopic observers (Ho et al., 2006; Simmers & Bex, 2004; Simmers et al., 2003). However, this assumption proves to be naïve when considering the finding that there is no difference in the synchronicity sensitivities of amblyopic and non-amblyopic eyes. Furthermore, across a large range of visual acuities and stereoacuities, the sensitivity to synchronicity is more or less invariant across amblyopic and normal observers (Fig. 5A and B). These findings support our conclusion that there is no significant difference in the synchronicity sensitivities of amblyopic and normal observers.
Acknowledgments
We thank Dr. Peter Neri for his many contributions and insightful comments. We also thank Dr. Shuang Song and Dr. Karl Saldanha for their help and useful suggestions. This project was supported by the grant R01EY01728 from the National Eye Institute, National Institutes of Health, Bethesda, MD.
References
- Aaen-Stockdale C, Ledgeway T, Hess RF. Second-order optic flow deficits in amblyopia. Investigative Ophthalmology & Visual Science. 2007;48(12):5532–5538. doi: 10.1167/iovs.07-0447. [DOI] [PubMed] [Google Scholar]
- Aggarwal JK, Cai Q. Human motion analysis: A review. Computer Vision and Image Understanding. 1999;73(3):428–440. [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Research. 1980;190(2):347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Asper L, Crewther D, Crewther SG. Strabismic amblyopia. Part 2. Neural processing. Clinical and Experimental Ophthalmology. 2000;83(4):200–211. doi: 10.1111/j.1444-0938.2000.tb05003.x. [DOI] [PubMed] [Google Scholar]
- Barclay CD, Cutting JE, Kozlowski LT. Temporal and spatial factors in gait perception that influence gender recognition. Perception & Psychophysics. 1978;23(2):145–152. doi: 10.3758/bf03208295. [DOI] [PubMed] [Google Scholar]
- Bingham GP. Kinematic form and scaling: Further investigations on the visual perception of lifted weight. Journal of Experimental Psychology: Human Perception and Performance. 1987;13(2):155–177. doi: 10.1037//0096-1523.13.2.155. [DOI] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 1981;21(3):467–476. [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and clinical aspects. Boston: Butterworth-Heinemann; 1991. [Google Scholar]
- Cutting JE, Kozlowski LT. Recognizing friends by their walk: Gait perception without familiarity cues. Bulletin of the Psychonomic Society. 1977;9(5):353–356. [Google Scholar]
- Cutting JE, Proffitt DR, Kozlowski LT. A biomechanical invariant for gait perception. Journal of Experimental Psychology: Human Perception and Performance. 1978;4(3):357–372. doi: 10.1037//0096-1523.4.3.357. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dittrich WH. Action categories and the perception of biological motion. Perception. 1993;22(1):15–22. doi: 10.1068/p220015. [DOI] [PubMed] [Google Scholar]
- Dittrich WH, Troscianko T, Lea SE, Morgan D. Perception of emotion from dynamic point-light displays represented in dance. Perception. 1996;25(6):727–738. doi: 10.1068/p250727. [DOI] [PubMed] [Google Scholar]
- El-Shamayleh Y, Kiorpes L, Kohn A, Movshon JA. Visual motion processing by neurons in area MT of macaque monkeys with experimental amblyopia. Journal of Neuroscience. 2010;30(36):12198–12209. doi: 10.1523/JNEUROSCI.3055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Konig P, Singer W. Direct physiological evidence for scene segmentation by temporal coding. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(20):9136–9140. doi: 10.1073/pnas.88.20.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese MA, Poggio T. Neural mechanisms for the recognition of biological movements. Nature Reviews Neuroscience. 2003;4(3):179–192. doi: 10.1038/nrn1057. [DOI] [PubMed] [Google Scholar]
- Hess RF, Holliday IE. The spatial localization deficit in amblyopia. Vision Research. 1992;32(7):1319–1339. doi: 10.1016/0042-6989(92)90225-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: Evidence for a two type classification. Vision Research. 1977;17(9):1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Dakin SC, Allen HA. Integration of local motion is normal in amblyopia. Journal of the Optical Society of America A – Optics Image Science an Vision. 2006;23(5):986–992. doi: 10.1364/josaa.23.000986. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Perrett DI. Motion sensitive cells in the macaque superior temporal polysensory area: Response discrimination between self-generated and externally generated pattern motion. Behavioural Brain Research. 1996;76(1–2):155–167. doi: 10.1016/0166-4328(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Ho CS, Paul PS, Asirvatham A, Cavanagh P, Cline R, Giaschi DE. Abnormal spatial selection and tracking in children with amblyopia. Vision Research. 2006;46(19):3274–3283. doi: 10.1016/j.visres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Hoffman DD, Flinchbaugh BE. The interpretation of biological motion. Biological Cybernetics. 1982;42(3):195–204. doi: 10.1007/BF00340076. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–211. [Google Scholar]
- Johansson G. Spatio-temporal differentiation and integration in visual motion perception. An experimental and theoretical analysis of calculus-like functions in visual data processing. Psychological Research. 1976;38:379–393. doi: 10.1007/BF00309043. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: Animal studies. Strabismus. 2006;14(1):3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Current Opinion in Neurobiology. 1999;9(4):480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Tang C, Movshon JA. Sensitivity to visual motion in amblyopic macaque monkeys. Visual Neuroscience. 2006;23(2):247–256. doi: 10.1017/S0952523806232097. [DOI] [PubMed] [Google Scholar]
- Klein SA. Measuring, estimating, and understanding the psychometric function: A commentary. Perception & Psychophysics. 2001;63(8):1421–1455. doi: 10.3758/bf03194552. [DOI] [PubMed] [Google Scholar]
- Koenderink JJ. Optic flow. Vision Research. 1986;26(1):161–179. doi: 10.1016/0042-6989(86)90078-7. [DOI] [PubMed] [Google Scholar]
- Kubova Z, Kuba M, Juran J, Blakemore C. Is the motion system relatively spared in amblyopia? Evidence from cortical evoked responses. Vision Research. 1995;36(1):181–190. doi: 10.1016/0042-6989(95)00055-5. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Malach R, Harel M, Leiba H, Stolovitch C, et al. Selective fovea-related deprived activation in retinotopic and high-order visual cortex of human amblyopes. Neuroimage. 2006;33(1):169–179. doi: 10.1016/j.neuroimage.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Pianka P, Azmon B, Leiba H, Stolovitch C, Loewenstein A, et al. Area-specific amblyopic effects in human occipitotemporal object representations. Neuron. 2003;40(5):1023–1029. doi: 10.1016/s0896-6273(03)00720-7. [DOI] [PubMed] [Google Scholar]
- Levi DM. Spatial vision in amblyopia. In: Regan D, editor. Spatial vision. Vol. 10. London: Macmillan Press; 1991. pp. 212–238. [Google Scholar]
- Levi DM. Visual processing in amblyopia: Human studies. Strabismus. 2006;14(1):11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- Levi DM, Carkeet A. Amblyopia: A consequence of abnormal visual development. In: Simons K, editor. Early visual development, normal and abnormal. Berlin: Oxford University Press; 1993. pp. 391–408. [Google Scholar]
- Levi DM, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1977;16(1):90–95. [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25(7):979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Sampling in spatial vision. Nature. 1986;320(6060):360–362. doi: 10.1038/320360a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Sharma V. Position jitter and undersampling in pattern perception. Vision Research. 1999;39(3):445–465. doi: 10.1016/s0042-6989(98)00125-4. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Yap YL. Positional uncertainty in peripheral and amblyopic vision. Vision Research. 1987;27(4):581–597. doi: 10.1016/0042-6989(87)90044-7. [DOI] [PubMed] [Google Scholar]
- Levi DM, Sharma V, Klein SA. Feature integration in pattern perception. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(21):11742–11746. doi: 10.1073/pnas.94.21.11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Tripathy SP. Is the ability to identify deviations in multiple trajectories compromised by amblyopia? Journal of Vision. 2006;6(12):1367–1379. doi: 10.1167/6.12.3. [DOI] [PubMed] [Google Scholar]
- Li X, Mullen KT, Thompson B, Hess RF. Effective connectivity anomalies in human amblyopia. Neuroimage. 2011;54(1):505–516. doi: 10.1016/j.neuroimage.2010.07.053. [DOI] [PubMed] [Google Scholar]
- Neri P, Luu JY, Levi DM. Meaningful interactions can enhance visual discrimination of human agents. Nature Neuroscience. 2006;9(9):1186–1192. doi: 10.1038/nn1759. [DOI] [PubMed] [Google Scholar]
- Neri P, Luu JY, Levi DM. Sensitivity to biological motion drops by approximately 1/2 log-unit with inversion, and is unaffected by amblyopia. Vision Research. 2007;47(9):1209–1214. doi: 10.1016/j.visres.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram MW, Perrett D. Reponses of anterior superior temporal polysensory (STPa) neurons to “biological motion” stimuli. Journal of Cognitive Science. 1994;6:99–116. doi: 10.1162/jocn.1994.6.2.99. [DOI] [PubMed] [Google Scholar]
- Oram MW, Perrett DI. Integration of form and motion in the anterior superior temporal polysensory area (STPa) of the macaque monkey. Journal of Neurophysiology. 1996;76(1):109–129. doi: 10.1152/jn.1996.76.1.109. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Sokolov A. Orientation specificity in biological motion perception. Perception & Psychophysics. 2000;62(5):889–899. doi: 10.3758/bf03212075. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Experimental Brain Research. 1982;47(3):329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Popple AV, Levi DM. The attentional blink in amblyopia. Journal of Vision. 2008;8(13):1–9. doi: 10.1167/8.13.12. [DOI] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 2003;358(1431):435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rislove EM, Hall EC, Stavros KA, Kiorpes L. Scale-dependent loss of global form perception in strabismic amblyopia. Journal of Vision. 2010;10(12):25. doi: 10.1167/10.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Research Cognitive Brain Research. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Konig P, Engel AK, Sireteanu R, Singer W. Reduced synchronization in the visual cortex of cats with strabismic amblyopia. European Journal of Neuroscience. 1994;6(11):1645–1655. doi: 10.1111/j.1460-9568.1994.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Runeson S, Frykholm G. Kinematic specification of dynamics as an informational basis for person-and-action perception: Expectation, gender recognition, and deceptive intention. Journal of Experimental Psychology: General. 1983;112(4):585–615. [Google Scholar]
- Shadlen MN, Movshon JA. Synchrony unbound: A critical evaluation of the temporal binding hypothesis. Neuron. 1999;24(1):67–77. 111–125. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- Sharma V, Levi DM, Klein SA. Undercounting features and missing features: Evidence for a high-level deficit in strabismic amblyopia. Nature Neuroscience. 2000;3(5):496–501. doi: 10.1038/74872. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Bex PJ. The representation of global spatial structure in amblyopia. Vision Research. 2004;44(5):523–533. doi: 10.1016/j.visres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Research. 2003;43(6):729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Mansouri B, Hutchinson CV, Hess RF. The extent of the dorsal extra-striate deficit in amblyopia. Vision Research. 2006;46(16):2571–2580. doi: 10.1016/j.visres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Sumi S. Upside-down presentation of the Johansson moving light-spot pattern. Perception. 1984;13(3):283–286. doi: 10.1068/p130283. [DOI] [PubMed] [Google Scholar]
- Thompson B, Troje NF, Hansen BC, Hess RF. Amblyopic perception of biological motion. Journal of Vision. 2008;8(4):21–24. doi: 10.1167/8.4.22. article no. 22. [DOI] [PubMed] [Google Scholar]
- Troje NF, Westhoff C, Lavrov M. Person identification from biological motion: Effects of structural and kinematic cues. Perception & Psychophysics. 2005;67(4):667–675. doi: 10.3758/bf03193523. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception & Psychophysics. 2001;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wong EH, Levi DM, McGraw PV. Is second-order spatial loss in amblyopia explained by the loss of first-order spatial input? Vision Research. 2001;41(23):2951–2960. doi: 10.1016/s0042-6989(01)00189-4. [DOI] [PubMed] [Google Scholar]