Abstract
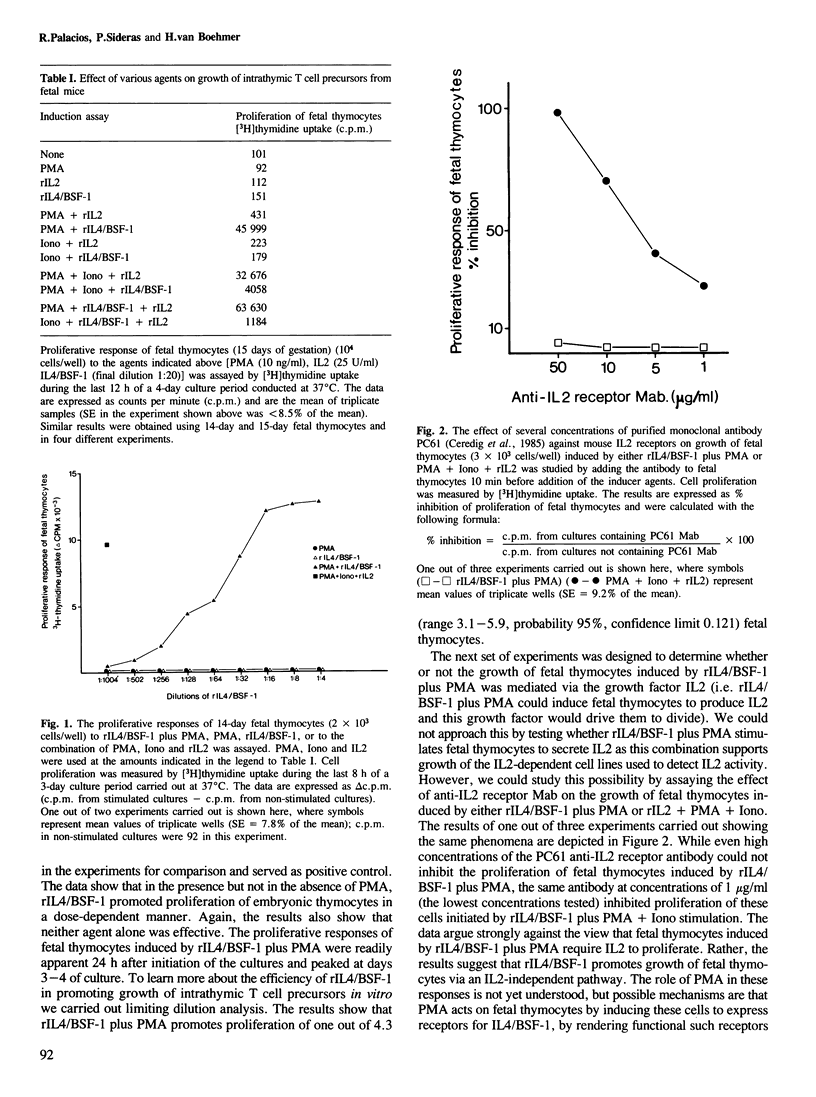
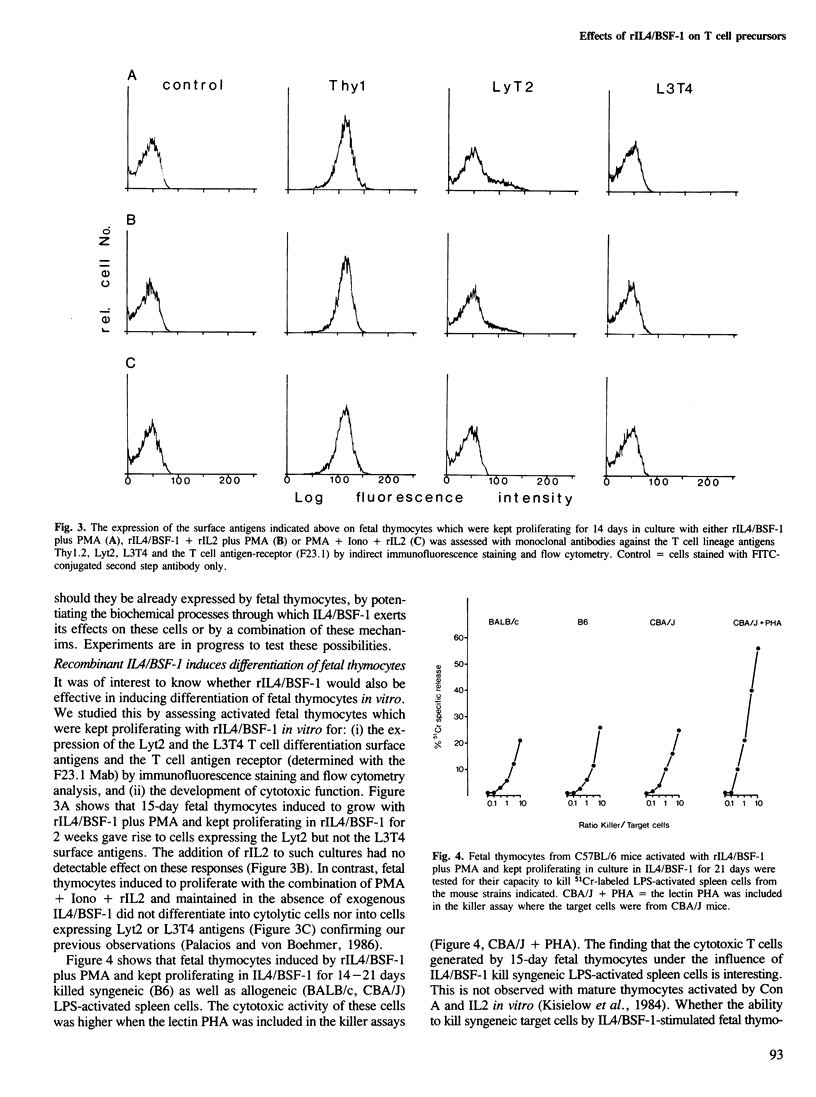
Recombinant mouse interleukin 4/BSF-1 (rIL4/BSF-1) together with phorbol myristate acetate (PMA) promotes growth of one out of approximately four intrathymic T cell precursors from fetal mice (14-15 days gestation). This response is not inhibited by even high concentrations of monoclonal antibody against the receptor for interleukin 2. Fetal thymocytes activated by rIL4/BSF-1 plus PMA give rise to cytolytic T cells after 7-21 days of culture. All the proliferating cells are Thy1+, some of them express Lyt2 but none has detectable L3T4 T cell differentiation antigens nor T cell antigen receptor (F23.1) on the cell membrane as assessed by immunofluorescence staining and flow fluorocytometry analysis. It is concluded that rIL4/BSF-1 exerts both growth and differentiation activities on normal intrathymic T cell precursors. The results provide evidence for an alternative growth factor to interleukin 2 involved in proliferation of T cell precursors. These findings open new and direct ways of studying cellular and molecular events during the differentiation of normal intrathymic T cell precursors in vitro and extend the spectrum of target cells for IL4/BSF-1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M. Molecular genetics of the T cell-receptor beta chain. Annu Rev Immunol. 1985;3:537–560. doi: 10.1146/annurev.iy.03.040185.002541. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K., Eisenman J., Mochizuki D., Shanebeck K., Conlon P., Hopp T., March C., Gillis S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J Exp Med. 1986 Jun 1;163(6):1405–1414. doi: 10.1084/jem.163.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S., Okumura K., Diamantstein T., Shevach E. M. Expression of interleukin 2 receptor on murine fetal thymocytes. Eur J Immunol. 1985 May;15(5):456–460. doi: 10.1002/eji.1830150508. [DOI] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Leiserson W., Von Boehmer H. Differentiation of thymocytes in fetal organ culture: analysis of phenotypic changes accompanying the appearance of cytolytic and interleukin 2-producing cells. J Immunol. 1984 Sep;133(3):1117–1123. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Henry Kunkel. 1916-1983. Immunol Rev. 1984 Apr;78:5–6. [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Leu T. CC11: a monoclonal antibody specific for interleukin 3-sensitive mouse cells defines two major populations of B cell precursors in the bone marrow. Immunol Rev. 1986 Oct;93:125–146. doi: 10.1111/j.1600-065x.1986.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Von Boehmer H. Requirements for growth of immature thymocytes from fetal and adult mice in vitro. Eur J Immunol. 1986 Jan;16(1):12–19. doi: 10.1002/eji.1830160104. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Expression and function of interleukin-2 receptors on immature thymocytes. Nature. 1985 Mar 7;314(6006):101–103. doi: 10.1038/314101a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Bergstedt-Lindqvist S., Severinson E. Partial biochemical characterization of IgG1-inducing factor. Eur J Immunol. 1985 Jun;15(6):593–598. doi: 10.1002/eji.1830150612. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Van Ewijk W., Jenkinson E. J., Owen J. J. Detection of Thy-1, T-200, Lyt-1 and Lyt-2-bearing cells in the developing lymphoid organs of the mouse embryo in vivo and in vitro. Eur J Immunol. 1982 Apr;12(4):262–271. doi: 10.1002/eji.1830120403. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Crisanti A., Kisielow P., Haas W. Absence of growth by most receptor-expressing fetal thymocytes in the presence of interleukin-2. Nature. 1985 Apr 11;314(6011):539–540. doi: 10.1038/314539a0. [DOI] [PubMed] [Google Scholar]


