Abstract
Background and Objectives
Gastric ischemic preconditioning has been proposed to improve blood flow and reduce the incidence of anastomotic complications following esophagectomy with gastric pull-up. This study aimed to evaluate the effect of prolonged ischemic preconditioning on the degree of neovascularization in the distal gastric conduit at the time of esophagectomy.
Methods
A retrospective review of a prospectively maintained database identified 30 patients who underwent esophagectomy. The patients were divided into three groups: control (no preconditioning, n=9), partial (short gastric vessel ligation only, n=8), and complete ischemic preconditioning (left and short gastric vessel ligation, n=13). Microvessel counts were assessed, using immunohistologic analysis to determine the degree of neovascularization at the distal gastric margin.
Results
The groups did not differ in age, gender, BMI, pathologic stage, or cancer subtype. Ischemic preconditioning durations were 163±156 days for partial ischemic preconditioning, compared to 95±50 days for complete ischemic preconditioning (p=0.2). Immunohistologic analysis demonstrated an increase in microvessel counts of 29% following partial ischemic preconditioning (p=0.3) and 67% after complete ischemic preconditioning (p < 0.0001), compared to controls.
Conclusions
Our study indicates that prolonged ischemic preconditioning is safe and does not interfere with subsequent esophagectomy. Complete ischemic preconditioning increased neovascularization in the distal gastric conduit.
Keywords: Esophagectomy, Esophageal cancer, Preconditioning, Anastomosis
Introduction
Esophageal cancer is a deadly disease with an overall five-year survival of less than 20%.[1] An important method of treatment involves esophagectomy with esophageal reconstruction by means of a gastric conduit. Over the last decade, the mortality rate associated with esophagectomy has decreased from 8.3% to 4.2%.[2] Analysis of prospective nationwide data, however, demonstrates an associated morbidity rate as high as 50%.[3] Anastomotic leak and stricture constitute common and dreaded sources of surgical morbidity following esophagectomy. Anastomotic complication rates as high as 50% have been reported in some studies with significant associated mortality.[4–6] Ischemia of the gastric conduit, due to the division of the left gastric and short gastric vessels at the time of conduit creation, contributes to poor anastomotic healing and subsequent complications. Laparoscopic ischemic conditioning with ligation of these vessels prior to esophagectomy has been proposed to allow neovascularization of the proximal stomach to improve blood flow and decrease the incidence of anastomotic complications.
Preclinical studies have examined the effect of ischemic conditioning on gastric blood flow and esophagogastric anastomotic healing. Ureschel et al. showed that partial ligation of the gastric vessels led to a reduction of anastomotic dehiscence and increase in wound bursting pressures in a rat model.[7] Another study demonstrated that ligation of the left gastric vessels in a rodent model led to an immediate decrease in gastric perfusion that significantly increased after 28 days and leveled off by 56 days after left gastric vessel ligation.[8] Preclinical studies of gastroesophageal anastomotic healing have demonstrated that an ischemic duration of 30 days or greater resulted in increased anastomotic bursting pressure and tensile strength, but that these benefits were not observed with an ischemic conditioning time of seven days.[9, 10] Taken together, these results suggest that prolonged gastric ischemic conditioning may be of benefit in clinical practice.
The length of time required to provide an adequate increase in gastric perfusion to significantly improve anastomotic healing in clinical practice is unknown. Clinical trials utilizing short ischemic conditioning intervals of four to seven days have failed to demonstrate significant improvements in anastomotic outcomes. One single institution randomized trial with ischemic conditioning performed two weeks prior to esophagectomy demonstrated a trend toward lower anastomotic leak rates, compared to patients undergoing immediate reconstruction, but this did not reach statistical significance.[11] Others have not seen any significant changes in vascular endothelial growth factor (VEGF) expression within the gastric conduit of patients who had ischemic conditioning performed up to five days prior to esophagectomy.[12] These studies suggest that the duration of ischemic conditioning may be critical to optimizing its effect on anastomotic healing, and no studies to date have examined the effect of ischemic conditioning beyond two weeks before esophagectomy.
Consequently, the aim of this study was to assess the effect of prolonged ischemic conditioning prior to esophagectomy. Our primary endpoint was the degree of neovascularization at the tip of the gastric conduit. Secondary outcomes included operative and postoperative outcomes after esophagectomy.
Material and Methods
This study involved two tertiary care medical centers. Informed consent was obtained and procedures followed were in accordance with the ethical standards, as approved by institutional review boards at both institutions. A retrospective review of a prospectively maintained esophageal disease registry was performed. We identified all patients undergoing esophagectomy with reconstruction between July 2008 and January 2014 at both institutions. Patients who had adequate tissue available for histopathologic analysis were included in the study. Only patients with tissue blocks from the distal gastric conduit were included. Twenty-one patients were identified with esophageal cancer who underwent partial or complete laparoscopic ischemic conditioning prior to esophagectomy. These patients were compared to nine control patients who underwent esophagectomy for cancer without laparoscopic ischemic conditioning. Of the 21 patients who underwent laparoscopic ischemic conditioning, ligation of the left gastric and short gastric vessels was performed in 13 patients (complete IC group), whereas eight patients had short gastric vessel ligation only (partial IC group). All patients subsequently underwent minimally invasive esophagectomy without operative mortality. The primary endpoint was the degree of neovascularization at the distal tip of the resected specimen, measured by microvessel counts per high powered field by an expert pathologist. The charts of all 30 patients were reviewed for patient demographics, including age, gender, body mass index (BMI), American Society of Anesthesiologist (ASA) class, clinical staging, patient co-morbidities, and cancer type. Perioperative data, and 30-day morbidity and mortality were also collected. Morbidities included any infections (wounds, pulmonary, urinary), respiratory distress requiring re-intubation, myocardial infarction, deep venous thrombosis, anastomotic leaks, anastomotic strictures, and supraventricular tachycardias.
Ischemic Conditioning
Ischemic conditioning was performed at the time of staging laparoscopy or pre-treatment jejunostomy placement. Staging laparoscopy was performed on patients in line with the current National Comprehensive Cancer Network (NCCN) guidelines, and jejunostomy was performed on patients with dysphagia, significant weight loss, or evidence of malnutrition. Additionally, a preconditioning procedure was also performed on patients determined to be high-risk for anastomotic ischemia based on clinician judgement. Factors considered high-risk for ischemia included current smokers, diabetics, and patients with a history of vascular disease. Most commonly the preconditioning procedure was performed prior to the initiation of neoadjuvant chemoradiotherapy, with the exception of three patients who received the gastric preconditioning procedure after neoadjuvant therapy. Patients who were treated with neoadjuvant therapy received ischemic conditioning two to three weeks prior to induction therapy. Laparoscopy was performed, using five trocars. Starting in the right upper quadrant, all the peritoneal surfaces, liver surfaces, omentum, and intra-peritoneal organs were systematically examined, and any suspicious deposits were biopsied. Patients in the complete IC group had the left gastric vessel ligated just prior to its branching along the lesser curvature of the stomach, using 10mm laparoscopic clip-appliers. These patients also had their short gastric vessels divided, using laparoscopic ultrasonic shears. The patients in the partial IC group had only the short gastric vessels divided, using ultrasonic shears. At the completion of the staging laparoscopy, a feeding jejunostomy tube was placed in anticipation of esophagectomy.
Assessment of Neovascularization
Esophagectomy specimens from patients who underwent ischemic conditioning prior to esophagectomy were compared to patients who did not undergo ischemic conditioning. Three serial sections from the distal gastric margin of each surgical specimen were examined. This area on the conduit was representative of the location where the esophagogastric anastomosis was created. All specimens were routinely submitted for pathology review to assure that the distal margins were free of malignancy. Evaluation of the microvascular density was performed as previously described by a single gastrointestinal pathologist who was blinded to all clinical information.[13] Briefly, gross descriptions and cassette keys from the surgical pathology reports of all subjects were reviewed to identify all blocks containing distal margin(s) from the resection specimens. CD34 immunohistochemical stains were performed on all these blocks. Three “hotspots” were identified in the submucosa on each block, and microvessel counts were performed in each at 200X magnification.[14] Vessels with muscular walls were not counted. The average microvascular density was calculated from counts of the three hotspots on each tissue block.
Esophagectomy
All patients underwent esophagectomy in one of the following manners: 1) minimally invasive three-field (thoracoscopic esophageal mobilization, laparoscopic gastric mobilization with conduit creation and neck dissection with the anastomosis in the neck), 2) minimally invasive antegrade transhiatal esophagectomy, or 3) minimally invasive Ivor-Lewis esophagectomy.[15–17]
Statistical Analysis
We compared patient characteristics, procedure details, and postoperative and oncologic outcomes among patients undergoing esophagectomy without ischemic conditioning and those undergoing esophagectomy following ischemic conditioning. Given the small sample size, continuous variables were evaluated for normality, using normal quantile plots. Where normality assumption was reasonable, Student’s t-test was used; otherwise, a permutation test was performed. Fischer’s exact test was used to compare categorical variables. A two-sided p-value of less than 0.05 was considered significant. The statistical analysis was carried out, using STATA version 14 (StataCorp, College Station, TX).
Results
A total of 30 esophagectomy specimens were reviewed for the study, with 13 specimens from patients who had complete IC, eight from patients with partial IC, and nine patients who did not have IC (control group). Table 1 shows the demographic data amongst the groups. There were no observed differences between groups with respect to age, gender, body mass index (BMI), cancer subtypes, or pre-operative co-morbidities. Five of the patients from the complete IC group received the procedure because they were determined to be high ischemic risk, and the remaining 16 patients received IC at the time of planned laparoscopy for feeding tube or staging purposes. The majority of the tumors were esophageal adenocarcinoma, with four squamous cell tumors. Twenty-eight of the tumors were located in the distal esophagus, with two mid esophageal squamous cell lesions. Twenty-five of the 30 patients were treated with neoadjuvant chemoradiotherapy prior to esophagectomy, and IC patients tended to receive neoadjuvant therapy more commonly than controls (p=0.02). ASA class was lower in patients undergoing complete IC than those of the control and partial IC (p=0.01, Table 1). Patients in the control group also had a lower clinical stage, compared to those in the partial IC and complete IC groups (p=0.02), but there were no significant differences in the final pathologic staging (p=0.51).Table 2 shows the operative and postoperative data for patients in the partial and complete IC groups. Operative time for the complete IC procedure tended to be longer compared to partial IC, but this did not reach significance (p=0.06). Length of hospital stay, 30-day morbidity, and 30-day mortality were not significantly different between the partial and complete IC groups. There was no procedural morbidity or mortality.
Table 1.
Patient Demographics for all 30 patients in the study,
| Control | Partial IC | Complete IC | P value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Age, Yrs (mean ± SD) | 62±6.8 | 65±4.8 | 64±9.7 | 0.801 | ||
|
| ||||||
| Gender (M:F) | 9:0 | 8:0 | 10:03 | 0.172 | ||
|
| ||||||
| BMI, (mean ± SD) | 31±5.8 | 27±4.8 | 26±4.0 | 0.091 | ||
|
| ||||||
| ASA class, No.(%) | 0.012 | |||||
| 1–2 | 0 | 0 | 6(46) | |||
| 3–4 | 9(100) | 8(100) | 7(54) | |||
|
| ||||||
| Indications for IC, No. (%) | ||||||
| Planned Laparoscopy | - | 8(100) | 8(62) | 0.112 | ||
| High-Risk Ischemia | 0 | 5(38) | ||||
|
| ||||||
| Clinical Stage, No. (%) | 0.022 | |||||
| I | 4(44) | 1(13) | 0 | |||
| II | 1(11) | 0 | 5(38) | |||
| III | 4(44) | 7(88) | 8(62) | |||
|
| ||||||
| Pathologic Stage, No. (%) | 0.512 | |||||
| (Pathologic Complete Response) 0 | 2(22) | 2(25) | 4(31) | |||
| I | 2(22) | 0 | 1(8) | |||
| II | 2(22) | 3(38) | 3(23) | |||
| III | 3(33) | 3(38) | 5(38) | |||
|
| ||||||
| Neoadjuvant Therapy, No. (%) | 0.022 | |||||
| Yes | 5(56) | 7(88) | 13(100) | |||
| No | 4(44) | 1(8) | 0 | |||
|
| ||||||
| Cancer Histology, No.(%) | 0.962 | |||||
| Adenocarcinoma | 8(89) | 7(88) | 11(85) | |||
| Squamous Cell | 1(9) | 1(13) | 2(15) | |||
|
| ||||||
| Comorbidities, No.(%) | ||||||
|
| ||||||
| Current Tobacco Use | 0 | 3(38) | 4(31) | 0.142 | ||
|
| ||||||
| Former Tobacco Use | 8(89) | 5(63) | 7(54) | 0.232 | ||
|
| ||||||
| Cardiac Arrhythmia | 0 | 0 | 2(15) | 0.502 | ||
|
| ||||||
| DM | 3(33) | 1(13) | 2(15) | 0.262 | ||
|
| ||||||
| CAD | 2(22) | 1(13) | 3(23) | 0.652 | ||
|
| ||||||
| CHF | 1(11) | 0 | 1(8) | 0.832 | ||
|
| ||||||
| PVD | 1(11) | 1(13) | 4(31) | 0.452 | ||
|
| ||||||
| CKD | 0 | 0 | 1(8) | 0.522 | ||
|
| ||||||
| Pulmonary Disease | 2(22) | 0 | 3(23) | 0.352 | ||
|
| ||||||
| Liver Disease | 0 | 1(13) | 0 | 0.252 | ||
IC, Ischemic Conditioning; BMI, body mass index; ASA, American Society of Anesthesiologists; DM, Diabetes Mellitus; CAD, Coronary Artery Disease; CHF, Congestive Heart Failure; PVD, Peripheral Vascular Disease; CKD, Chronic Kidney Disease
One way Analysis of Variance,
Fisher’s Exact Test
Table 2.
Operative and postoperative outcomes following laparoscopic ischemic conditioning.
| Partial IC | Complete IC | P value | |
|---|---|---|---|
| Mean IC Time, Days (mean ± SD) | 163±156 | 95±50 | 0.331 |
| IC OR Time, Minutes (mean ± SD) | 92±37 | 157±54 | 0.061 |
| IVF (mL, mean ± SD) | 1057±360 | 1333±356 | 0.241 |
| Length of Stay, Days (mean ± SD) | 3±3 | 2±1 | 0.481 |
| 30 Day Morbidity Rate (%) | 0 | 0 | 1.002 |
| 30 Day Mortality (%) | 0 | 0 | 1.002 |
Mean IC Time, Mean Ischemic Conditioning Time; IC OR Time, Ischemic Conditioning Operating Room Time; IVF, operative IV fluid administration
Students T-test,
Fisher’s Exact Test
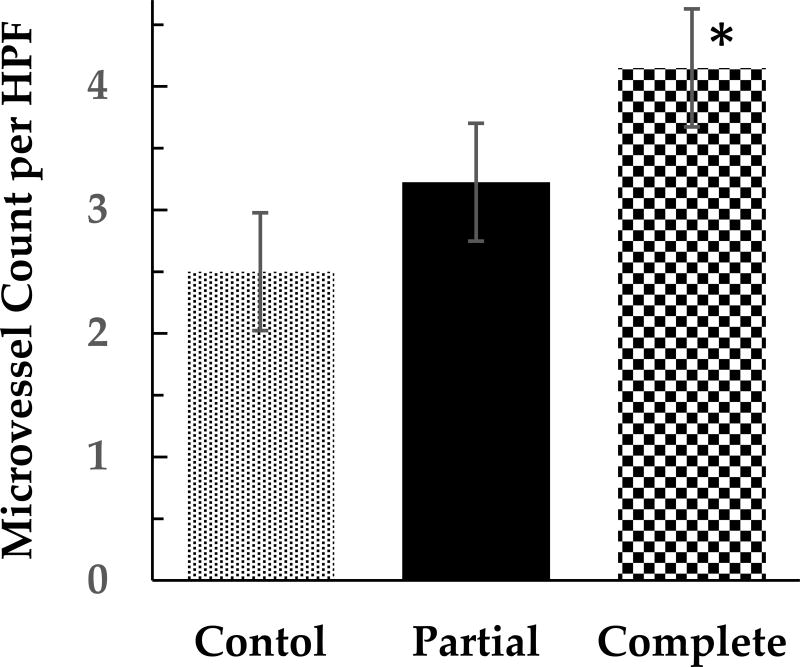
The average ischemic conditioning time prior to esophagectomy was 121 (±105) days for both groups, combined with the shortest duration being eight days and the longest 533 days. The partial IC group averaged 163 (±156) days and the complete IC group averaged 95 (±50) days (p=0.33, Table 2). CD34 immunostaining showed a 29% increase in microvessel counts in patients undergoing partial IC, as compared to controls (p=0.3, Figure 1). Complete IC produced a 67% increase in microvessel counts, compared to controls (p < 0.0001), and a 29% increase in microvessel counts over the partial IC group (p=0.05, Figure 1).
Figure 1.
Microvessel counts per high power field after immunohistochemical staining for CD 34, in patients undergoing no (Control), partial (Partial) or complete (Complete) ischemic conditioning. HPF, high-power field. Error bars represent standard error of the mean. *(Students t-test, p < 0.0001)
All patients who underwent ischemic conditioning went on to have minimally invasive esophagectomy. Table 3 summarizes the operative and perioperative outcomes for patients undergoing esophagectomy. In all, 19 (63%) patients had minimally invasive three-field esophagectomy, two (7%) had minimally invasive Ivor-Lewis esophagectomy, and nine (30%) had minimally invasive transhiatal esophagectomy. All patients who had complete IC went on to have minimally invasive three-field esophagectomy. In contrast, only two (22%) of the controls and four (50%) of the partial IC patients went on to have minimally invasive three-field esophagectomy (p < 0.001). This may account for the significantly longer OR time for the complete IC group (Table 3). There were no conversions to an open procedure, and no observed differences in blood loss, operative IV fluid administration, length of stay, R0 resection margin status, or lymph node harvest among the patients undergoing esophagectomy following ischemic conditioning versus controls (Table 3). Thirty-day mortality rates were not statistically different amongst the groups. There was no difference in overall thirty-day morbidity that included infectious complications, respiratory distress requiring re-intubation, myocardial infarction, deep venous thrombosis, anastomotic complications, and supraventricular tachycardia. Specifically, looking at anastomotic complications, there was no difference between leak (p=0.58) and stricture rates (p=0.61) among the three groups (Table 3).
Table 3.
Operative and postoperative outcomes after esophagectomy.
| Control | Partial IC | Complete IC | P value | ||
|---|---|---|---|---|---|
| OR Time, Minutes (mean ± SD) | 454±67 | 526±95 | 551±44 | 0.011 | |
| EBL, No. (%) | 0.892 | ||||
| 0–200 mL | 5(56) | 5(63) | 9(69) | ||
| >200 mL | 4(44) | 3(38) | 4(31) | ||
| IVF, mL (mean ± SD) | 5950±2213 | 5901±1876 | 3826±2705 | 0.181 | |
| Length of Stay, Days (mean ± SD) | 14±9 | 11±4 | 11±5 | 0.541 | |
| R0 Margin, No. (%) | 9(100) | 7(88) | 13(100) | 0.502 | |
| Lymph node Count, No. | 12±6 | 13±8 | 17±4 | 0.131 | |
| 30 Day Morbidity, No. (%) | 4(44) | 4(50) | 2(15) | 0.182 | |
| Anastomotic Leak, No. (%) | 0(0) | 1(13) | 1(8) | 0.582 | |
| Anastomotic Stricture, No. (%) | 1(11) | 1(13) | 1(8) | 0.612 | |
| 30 Day Mortality, No. (%) | 1(11) | 0(0) | 1(8) | 0.642 | |
Partial IC, Partial Ischemic Conditioning; Complete IC, Complete Ischemic Conditioning; EBL, estimated blood loss; IVF, operative IV fluid administration
One way Analysis of Variance,
Fisher’s Exact Test
Discussion
This is the first clinical study to objectively demonstrate increased neovascularization in the proximal stomach of patients after ischemic conditioning of the stomach. It demonstrates that complete laparoscopic ischemic conditioning of the stomach, including division of both the left gastric and short gastric vessels at an average of four months prior to esophagectomy leads to increased neovascularization in the fundus of the stomach. In addition, prolonged laparoscopic gastric ischemic conditioning was safe and did not affect the operative or perioperative outcomes of subsequent minimally invasive esophagectomy.
It is well established that gastric conduit ischemia is a major contributor to anastomotic complications.[18] As a result, a number of techniques have been devised to improve blood flow to the gastric conduit. These methods include anastomosing donor vessels to the gastric circulation, use of a pedicled omental flap around the anastomosis, phlebotomy through the short gastric veins to decrease vascular congestion, and delayed anastomosis with gastric ischemic conditioning.[19] The basis of ischemic conditioning is that perfusion to conduit tip can be enhanced by ligation of select vessels to the stomach prior to esophagogastrostomy, allowing new microcirculation to develop to the gastric fundus at the site of the anastomosis. A recent review on the topic of microcirculation following ischemic conditioning found ten studies that demonstrated improved circulation at the gastric conduit tip following ischemic conditioning.[19] There were a number of different methods used to assess the microvasculature of the gastric conduit, including contrast enhanced ultrasound, laser Doppler flowmetry, reflectance spectrophotometry, and clearance techniques of various tracers. [19] For our study, we elected to perform microvessel counts of postoperative biopsy specimens at the conduit tip. This method was well suited for our purposes, because it provided a quantitative measurement, did not add an additional step to the operative procedure, and allowed us to take advantage of our database of available surgical specimens. Furthermore, this method is an established protocol to evaluate microvasculature and angiogenesis within a biopsy specimen.[14
The creation of the neoesophagus from the stomach during esophagectomy can lead to malperfusion and ischemia of the gastric conduit. Schilling et al showed a 69% reduction in gastric tissue perfusion immediately following gastric devascularization with further reduction in perfusion after gastric conduit creation, using laser Doppler.[24] Using white light fiber optic spectroscopy probe, Pham et al have shown that oxygen saturation at the most proximal tip of gastric conduit decreased by an average of 29.4% shortly after conduit creation.[25] Additionally, patients who subsequently developed anastomotic complications had a significantly greater drop in oxygen saturation at the time of gastric conduit creation than those that did not develop anastomotic complications.[25] Finally, Briel et al demonstrated that ischemia was the most significant risk factor for the development of anastomotic complications. Conduit ischemia increased the odds of leak or stricture by nearly fivefold.[18] Ischemic conditioning of the stomach prior to esophagectomy provides a means to obviate this reduction in perfusion; the improvements, however, are not immediate. In an opossum animal model of gastric ischemic conditioning, Reavis et al showed that it took 28 days before gastric perfusion returned to baseline levels after gastric devascularization.[10] Mittermair et al, using a rodent model, showed significant decrease in perfusion of the stomach after ligation of the left and short gastric vessels. They demonstrated that the perfusion of the stomach did not return to pre-vessel ligation levels for average of 56 days.[8] In a preclinical study of anastomotic healing, Perry et al demonstrated significantly improved esophagogastric anastomotic healing, compared to controls in animals with at least a 30-day ischemic conditioning time, but no improvement in anastomotic healing with an ischemic conditioning interval of seven days. [9] The data from these pre-clinical animal models suggest that longer duration of ischemic conditioning may be necessary in order for neovascularization to return perfusion back to baseline levels and to have a clinically relevant impact on anastomotic healing.
Multiple studies have demonstrated the safety of laparoscopic ischemic conditioning. In 2007, Holscher et al described laparoscopic ischemic conditioning in 83 patients, with a mean duration of 4.3 days. Although there was a 3.6% conversion to open rate with 2.4% reoperation rate, there was no mortality associated with the ischemic conditioning procedure, and all patients went on to have esophagectomy. This study, however, did not show any differences in anastomotic complication rates.[26] Similar findings regarding the safety of laparoscopic ischemic conditioning were reported by Berrisford et al in 2009. They reported no morbidity associated with laparoscopic gastric ischemic conditioning with a mean duration of 15.5 days. There were no differences in operative time or blood loss during the subsequent esophagectomies. Despite a trend towards lower anastomotic complication rates in the ischemic conditioned group, it did not reach statistical significance.[22] In a small, single institution randomized control trial of laparoscopic ischemic conditioning with a two-week conditioning interval, there were no complications attributed to the ischemic conditioning procedures. In the 16 patients randomized to ischemic conditioning group, however, the perfusion of the gastric conduit had not returned to baseline, and there were no differences in anastomotic complications noted.[11] The most common reason cited for the short duration of ischemic conditioning in these studies was the risk of scaring that would interfere with the ability to perform esophagectomy safely. In contrast, the present study indicates that longer ischemic conditioning periods (average 121 days) do not interfere with subsequent esophagectomy.
Such clinical studies involving ischemic conditioning of the stomach have failed to consistently demonstrate significant reductions in anastomotic complication rates. One recent meta-analysis attempted to identify technical factors that are associated with anastomotic integrity following esophagectomy. This review identified 12 studies, compromising over 1,200 patients.[20] In patients undergoing ischemic preconditioning, anastomotic leak rates were 8.8% versus 14.1% in controls, although this failed to meet significance (p=0.1).[20] Based on these findings, the authors could not recommend ischemic conditioning for all patients, but did acknowledge that an individualized approach to each patient’s physiology and esophageal cancer stage is the most important factor to achieve the best anastomotic integrity after esophagectomy. Another recent systematic review examined clinical studies of ischemic conditioning, and concluded that there was no evidence that gastric ischemic conditioning decreased anastomotic complications.[21] The authors, however, noted that the study with the longest mean ischemic conditioning time was only 15.5 days.[22] Finally, a review of 11 clinical studies identified only six studies that demonstrated decreased anastomotic leak rate with a laparoscopic ischemic preconditioning procedure, and one study that actually demonstrated increased leak rates with the preconditioning procedure.[23] Again, the mean duration from preconditioning to esophagectomy was short, ranging from four days to two weeks.[23] It is possible that these trials failed to demonstrate improved anastomotic outcomes due to insufficient duration of ischemic conditioning to allow for neovascularization to develop and for gastric perfusion to improve.
In our study, the mean interval between ischemic conditioning and esophagectomy was 121 days, but did have a broad range (8–533 days). The vast majority of patients received preconditioning prior to neoadjuvant chemoradiotherapy. Three patients, however, received ischemic conditioning after neoadjuvant therapy, and therefore had shorter intervals between preconditioning and esophagectomy. One patient who received the ischemic conditioning procedure prior to neoadjuvant therapy had significant delay, due to complications associated with systemic therapy, thus a prolonged ischemic conditioning period. Nonetheless, in our study, the interval between ischemic conditioning and surgery was significantly longer on average than seen in prior analyses. Going forward, randomized trials of prolonged ischemic conditioning may be the best option to demonstrate reduced anastomotic complications, compared to short interval ischemic conditioning.
Although our findings provide objective evidence that prolonged laparoscopic ischemic conditioning is safe and induces neovascularization of the gastric conduit, there are important weaknesses that must be addressed. The most important outcome that cannot be adequately addressed in this study is the subsequent rate of anastomotic complications. The reason for this is that our study cohort was too small, and only two patients developed anastomotic leaks, both of which lead to anastomotic strictures that improved with dilation. In addition, it would have been prudent to demonstrate that the observed neovascularization was accompanied by a return of perfusion to near baseline levels. No direct measurement of perfusion was taken in our study, but it has been previously demonstrated that increased histologic microvascular density of gastric mucosa is directly correlated with in vivo measures of perfusion.[27] Despite these weaknesses, the data presented can be used as a foundation for the development of larger prospective multi-center studies of laparoscopic prolonged ischemic conditioning, where anastomotic outcomes can be correlated to neovascularization and perfusion of the gastric conduit.
Conclusions
Prolonged laparoscopic gastric ischemic conditioning induces neovascularization in the gastric conduit. The greatest increase in neovascularization is seen with the ligation of both the short and left gastric vessels. In addition, prolonged laparoscopic gastric ischemic conditioning is feasible, safe, and did not interfere with subsequent minimally invasive esophagectomy.
Acknowledgments
Funding: The funding for this research was provided by the Dallas Veteran’s Affairs Research Corporation (Pham), the Oregon Clinical and Translational Research Institute (OCTRI), and a grant (no. UL1TR000128) from the National Center for Advancing Translational Sciences (NACATS) of the National Institutes of Health (Dolan).
This research was presented at the Annual Meeting of the Association of Veteran Affairs Surgeons on May 3rd, 2015, in Miami, FL. The funding for this research was provided by the Dallas Veteran’s Affairs Research Corporation (Pham), the Oregon Clinical and Translational Research Institute (OCTRI), and a grant (no. UL1TR000128) from the National Center for Advancing Translational Sciences (NACATS) of the National Institutes of Health (Dolan). The corresponding author, Dr. Thai Pham, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosure: No author has any financial conflict of interest to disclose
References
- 1. [Access Date: Dec 7, 2016];National Cancer Institute: Surveillance, Epidemiology and End Results Program. http://seer.cancer.gov/csr/1975_2012/browse_csr.php?sectionSEL=8&pageSEL=sect_08_table.08.html.
- 2.Jafari MD, et al. A decade analysis of trends and outcomes of partial versus total esophagectomy in the United States. Ann Surg. 2013;258:450–8. doi: 10.1097/SLA.0b013e3182a1b11d. [DOI] [PubMed] [Google Scholar]
- 3.Dhungel B, et al. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005–2008. J Gastrointest Surg. 2010;14:1492–501. doi: 10.1007/s11605-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 4.Lam TC, et al. Anastomotic complications after esophagectomy for cancer. A comparison of neck and chest anastomoses. J Thorac Cardiovasc Surg. 1992;104:395–400. [PubMed] [Google Scholar]
- 5.Martin LW, et al. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg. 2005;242:392–9. doi: 10.1097/01.sla.0000179645.17384.12. discussion 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junemann-Ramirez M, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg. 2005;27:3–7. doi: 10.1016/j.ejcts.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Urschel JD, Antkowiak JG. The effect of ischemic conditioning on gastric wound healing in the rat: implications for esophageal replacement with stomach. J Cardiovasc Surg (Torino) 1997;5:4. [PubMed] [Google Scholar]
- 8.Mittermair C, et al. Functional capillary density in ischemic conditioning: implications for esophageal resection with the gastric conduit. Am J Surg. 2008;196:88–92. doi: 10.1016/j.amjsurg.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Perry KA, et al. Gastric ischemic conditioning increases neovascularization and reduces inflammation and fibrosis during gastroesophageal anastomotic healing. Surg Endosc. 2013;27:753–60. doi: 10.1007/s00464-012-2535-6. [DOI] [PubMed] [Google Scholar]
- 10.Reavis KM, et al. Utilization of the delay phenomenon improves blood flow and reduces collagen deposition in esophagogastric anastomoses. Ann Surg. 2005;241:736–45. doi: 10.1097/01.sla.0000160704.50657.32. discussion 745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veeramootoo D, Shore AC, Wajed SA. Randomized controlled trial of laparoscopic gastric ischemic conditioning prior to minimally invasive esophagectomy, the LOGIC trial. Surg Endosc. 2012;26:1822–9. doi: 10.1007/s00464-011-2123-1. [DOI] [PubMed] [Google Scholar]
- 12.Bludau M, et al. Vascular endothelial growth factor expression following ischemic conditioning of the gastric conduit. Dis Esophagus. 2013;26:847–52. doi: 10.1111/j.1442-2050.2012.01391.x. [DOI] [PubMed] [Google Scholar]
- 13.Nico B, et al. Evaluation of microvascular density in tumors: pro and contra. Histol Histopathol. 2008;23:601–7. doi: 10.14670/HH-23.601. [DOI] [PubMed] [Google Scholar]
- 14.Weidner N, et al. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 15.Luketich JD, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–94. doi: 10.1097/01.sla.0000089858.40725.68. discussion 494–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry KA, et al. Comparison of laparoscopic inversion esophagectomy and open transhiatal esophagectomy for high-grade dysplasia and stage I esophageal adenocarcinoma. Arch Surg. 2009;144:679–84. doi: 10.1001/archsurg.2009.113. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen NT, et al. Minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2001;72:593–6. doi: 10.1016/s0003-4975(00)02261-x. [DOI] [PubMed] [Google Scholar]
- 18.Briel JW, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198:536–41. doi: 10.1016/j.jamcollsurg.2003.11.026. discussion 541–2. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, et al. Vascular conditioning of the stomach before esophageal reconstruction by gastric interposition. Dis Esophagus. 2012;25:740–9. doi: 10.1111/j.1442-2050.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- 20.Markar SR, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol. 2013;20:4274–81. doi: 10.1245/s10434-013-3189-x. [DOI] [PubMed] [Google Scholar]
- 21.Ney A, Kumar R. Does preoperative ischaemic conditioning with gastric vessel ligation reduce anastomotic leaks in oesophagectomy? Interact Cardiovasc Thorac Surg. 2014;19:121–4. doi: 10.1093/icvts/ivu070. [DOI] [PubMed] [Google Scholar]
- 22.Berrisford RG, et al. Laparoscopic ischaemic conditioning of the stomach may reduce gastric-conduit morbidity following total minimally invasive oesophagectomy. Eur J Cardiothorac Surg. 2009;36:888–93. doi: 10.1016/j.ejcts.2009.01.055. discussion 893. [DOI] [PubMed] [Google Scholar]
- 23.Kechagias A, et al. Ischemic Conditioning of the Stomach in the Prevention of Esophagogastric Anastomotic Leakage After Esophagectomy. Ann Thorac Surg. 2016;101:1614–23. doi: 10.1016/j.athoracsur.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Schilling MK, et al. Gastric microcirculatory changes during gastric tube formation: assessment with laser Doppler flowmetry. J Surg Res. 1996;62:125–9. doi: 10.1006/jsre.1996.0184. [DOI] [PubMed] [Google Scholar]
- 25.Pham TH, et al. Decreased conduit perfusion measured by spectroscopy is associated with anastomotic complications. Ann Thorac Surg. 2011;91:380–5. doi: 10.1016/j.athoracsur.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Holscher AH, et al. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg. 2007;245:241–6. doi: 10.1097/01.sla.0000245847.40779.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamura M, et al. Analysis of microvascular density in early gastric carcinoma using magnifying endoscopy with narrow-band imaging. Endosc Int Open. 2016;4:E832–7. doi: 10.1055/s-0042-110095. [DOI] [PMC free article] [PubMed] [Google Scholar]