Abstract
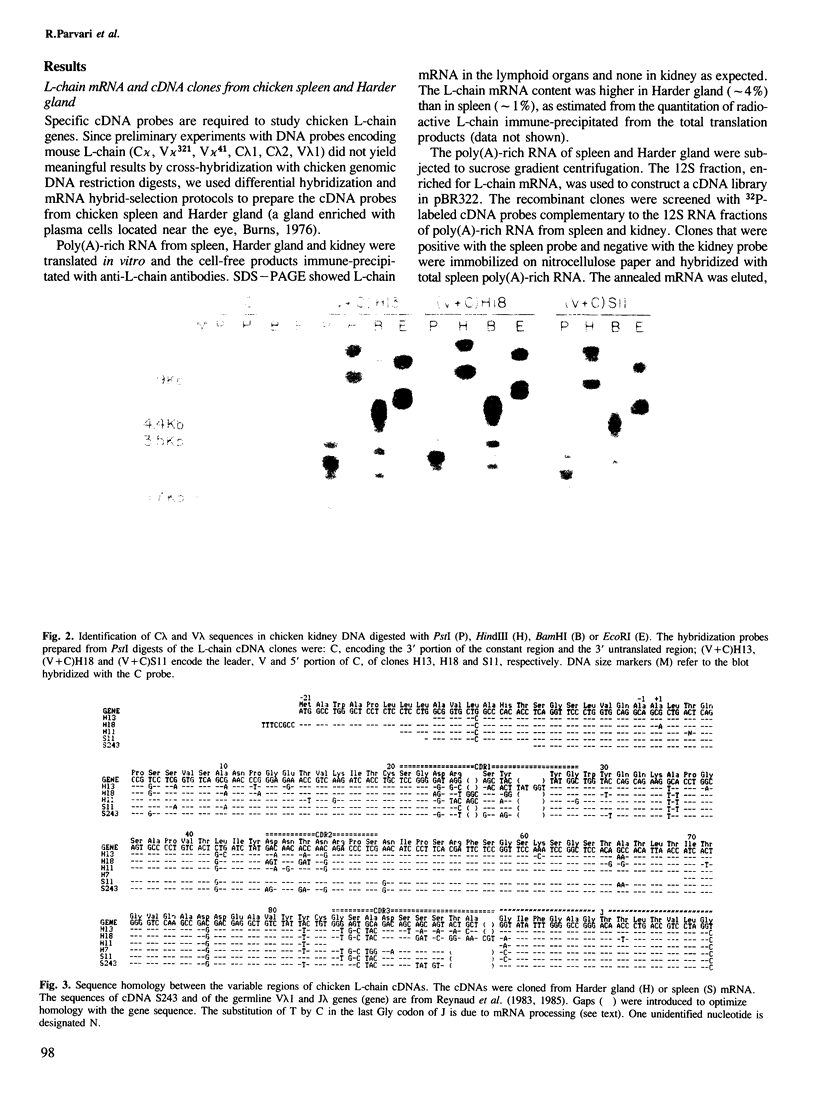
cDNA libraries of chicken spleen and Harder gland (a gland enriched with immunocytes) constructed in pBR322 were screened by differential hybridization and by mRNA hybrid-selected translation. Eleven L-chain cDNA clones were identified from which VL probes were prepared and each was annealed with kidney DNA restriction digests. All VL probes revealed the same set of bands, corresponding to about 15 germline VL genes of one subgroup. The nucleotide sequences of six VL clones showed greater than or equal to 85% homology, and the predicted amino acid sequences were identical or nearly identical to the major N-terminal sequence of L-chains in chicken serum. These findings, and the fact that the VL clones were randomly selected from normal lymphoid tissues, strongly indicate that the bulk of chicken L-chains is encoded by a few germline VL genes, probably much less than 15 since many of the VL genes are known to be pseudogenes. Therefore, it is likely that somatic mechanisms operating prior to specific triggering by antigen play a major role in the generation of antibody diversity in chicken. Analysis of the constant region locus (sequencing of CL gene and cDNAs) demonstrate a single CL isotype and suggest the presence of CL allotypes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Bentley D. L. Most kappa immunoglobulin mRNA in human lymphocytes is homologous to a small family of germ-line V genes. Nature. 1984 Jan 5;307(5946):77–80. doi: 10.1038/307077a0. [DOI] [PubMed] [Google Scholar]
- Breiner A. V., Brandt C. R., Milcarek C., Sweet R. W., Ziv E., Burstein Y., Schechter I. Somatic DNA rearrangement generates functional rat immunoglobulin kappa chain genes: the J kappa gene cluster is longer in rat than in mouse. Gene. 1982 May;18(2):165–174. doi: 10.1016/0378-1119(82)90114-7. [DOI] [PubMed] [Google Scholar]
- Burns R. B. Specific antibody production against a soluble antigen in the Harderian gland of the domestic fowl. Clin Exp Immunol. 1976 Nov;26(2):371–374. [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Glutamine as a precursor to N-terminal pyrrolid-2-one-5-carboxylic acid in mouse immunoglobulin lambda-type light chains. Amino acid-sequence variability at the N-terminal extra piece of lambda-type light-chain precursors. Biochem J. 1977 Aug 1;165(2):347–354. doi: 10.1042/bj1650347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Cory S., Tyler B. M., Adams J. M. Sets of immunoglobulin V kappa genes homologous to ten cloned V kappa sequences: implications for the number of germline V kappa genes. J Mol Appl Genet. 1981;1(2):103–116. [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. A., Sanders B., Hood L. Partial amino acid sequences of chicken and turkey immunoglobulin light chains. Homology with mammalian lambda chains. Biochemistry. 1971 Aug 3;10(16):3123–3132. doi: 10.1021/bi00792a022. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Granfors K., Toivanen P. Defect in the generation of light-chain diversity in bursectomized chickens. Nature. 1984 Sep 6;311(5981):69–71. doi: 10.1038/311069a0. [DOI] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Solomon A., Zachau H. G. Contribution of human V kappa II germ-line genes to light-chain diversity. Nature. 1984 May 3;309(5963):73–76. doi: 10.1038/309073a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Weill J. C. Complete sequence of a chicken lambda light chain immunoglobulin derived from the nucleotide sequence of its mRNA. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4099–4103. doi: 10.1073/pnas.80.13.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schechter I. Region of immunoglobulin light-chain mRNA transcribed into complementary DNA by RNA-dependent DNA polymerase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2511–2514. doi: 10.1073/pnas.72.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Ziv E. Binding of 2,4-dinitrophenyl derivatives by the light chain dimer obtained from immunoglobulin A produced by MOPC-315 mouse myeloma. Biochemistry. 1976 Jun 29;15(13):2785–2790. doi: 10.1021/bi00658a013. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Valbuena O., Marcu K. B., Weigert M., Perry R. P. Multiplicity of germline genes specifying a group of related mouse kappa chains with implications for the generation of immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):780–784. doi: 10.1038/276780a0. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeelon E. P., Bothwell A. L., Kantor F., Schechter I. An experimental approach to enumerate the genes coding for immunoglobulin variable-regions. Nucleic Acids Res. 1981 Aug 11;9(15):3809–3820. doi: 10.1093/nar/9.15.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]





