Abstract
Background
Our institution has published damage control laparotomy (DCL) rates of 30% and documented the substantial morbidity associated with the open abdomen. The purpose of this quality improvement (QI) project was to decrease the rate of DCL at a busy, Level 1 trauma center in the United States.
Study Design
A prospective cohort of all emergent trauma laparotomies from 11/2013–10/2015 (QI group) were followed. The QI intervention was multi-faceted and included audit and feedback for every DCL case. Morbidity and mortality of the QI patients were compared to a published control (control group – emergent laparotomy from 01/2011–10/2013).
Results
A significant decrease was observed immediately upon beginning the QI project, from a 39% DCL rate in the control period to 23% in the QI group (p<0.001). This decrease was sustained over the two year study period. There were no differences in demographics, Injury Severity Score, or transfusions between the groups. No differences organ/space infection (control 16% vs QI 12%, p=0.15), fascial dehiscence (6% vs 8%, p=0.20), unplanned re-laparotomy (11% vs 10%, p=0.58), or mortality (9% vs 10%, p=0.69) was observed. The reduction in utilization resulted in a decrease of 68 DCLs over the two year period. There was a further reduction in the rate of DCL to 17% following completion of the QI project.
Conclusion
A QI initiative rapidly changed the utilization of DCL and improved quality of care by decreasing resource utilization without an increase morbidity or mortality. This decrease was sustained during the QI period and further improved upon following its completion.
Keywords: trauma, damage control laparotomy, morbidity, mortality, quality improvement
INTRODUCTION
Damage control laparotomy (DCL) in severely injured and physiologically deranged patients has revolutionized trauma care.1,2 While initially described in coagulopathic patients requiring packing and massively transfused patients with a major abdominal vascular injury, the indications for DCL have slowly increased without evidence to support this expansion.3 Our institution has previously reported DCL rates of 30% and the substantial morbidities associated with the open abdomen necessitated by DCL, including organ/space surgical site infection and fascial dehiscence.4,5,6
In January 2009, damage control resuscitation (high ratios of red blood cells, fresh frozen plasma, and platelets with minimal crystalloid and colloid) became usual practice at our institution. Significant improvements compared to large volume crystalloid resuscitation were observed, namely decreased overall transfusion volumes, lower rates of clinically significant coagulopathy, less bowel edema, and more frequent fascial closure at the first take back.7,8,9,10,11 Although the rate of DCL was expected to decrease with the implementation of damage control resuscitation, the yearly rate of DCL at our institution after January 2009 actually increased.
The rationale behind this quality improvement (QI) project was that these improvements in resuscitation should allow surgeons to stay longer in the operating room in order to address anatomic problems by preventing or reversing the abnormal physiology which previously may have driven the need to perform DCL. A decrease in the rate of DCL should decrease hospital resource utilization, decrease the rate of associated morbidities, and improve the quality of patient care. While the QI process was driven by a physician champion, the entire division agreed to participate because we felt this to be a gap in quality care.
In November 2013, this QI project was begun with the following objectives: 1) to decrease the rate of DCL, 2) to determine if decreasing the rate of DCL decreased morbidity, 3) to determine the ideal rate of DCL at our institution, and 4) to develop a process for addressing perceived gaps in the quality of care for trauma patients undergoing emergency procedures.
METHODS
The described study was a pre-post intervention QI project.12 This manuscript was written using the guidelines provided by the Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) framework.13 The Committee for the Protection of Human Subjects approved this project as QI.14
Study Setting
The Red Duke Trauma Institute at Memorial Hermann Hospital-Texas Medical Center is an American College of Surgeons verified Level 1 trauma center that is the primary teaching hospital for the UT Houston McGovern Medical School (UT Health). The Red Duke Trauma Institute is one of the only two adult Level 1 trauma centers in Houston, Texas, the fourth largest city in the United States.
Patient Population
The QI study group included all adult trauma patients (>15 years of age) undergoing emergent laparotomy from November 1, 2013 through October 31, 2015. Emergent laparotomy was defined as admission directly to the operating room from the emergency department. The control group included all adult trauma patients who underwent emergent trauma laparotomy from January 1, 2011 through October 31, 2013.
The QI Intervention
Year One
During the first year of the QI project, an audit and feedback strategy was implemented for all surgeons on the trauma service. On all patients undergoing DCL, surgeons filled out a 5”x8” index card detailing emergency department, operating room, and immediate post-operative intensive care unit data. Also, the primary indication for DCL was reported. Every other month, a report listing the overall DCL rate, blinded surgeon-specific DCL rates, indications, and overall complication rates during the QI period was sent to faculty and posted on a wall in the administrative offices of the division.15 This mechanism allowed the surgeons to receive immediate feedback, view blinded surgeon-specific DCL rates, and follow prospective quality metrics. Surgeon-specific DCL rates were only un-blinded when surgeons asked to know their individual rate. All aspects of care, such as choice of temporary abdominal closure and wound management, were left to the discretion of the surgeon.
Year Two
Starting in year two of the QI project, all DCLs cases were presented for review by the group every other month at our regularly scheduled faculty meeting. The regularly scheduled faculty meeting was chosen as the site of review as it is the most highly attended meeting in the division, with >90% normal attendance. This review included date of operation, surgeon, procedures, indications, resuscitation volumes, and outcomes. Every DCL was discussed to determine if the patient could have safely undergone definitive laparotomy. Adjudication of whether the patients could have safely undergone definitive laparotomy was by majority vote during the weekly faculty meeting.
Using themes from the Knowledge-to-Action framework and concepts from a Learning Healthcare System, the theory behind this staged method of QI was to first generate and disseminate knowledge and then to identify, in real-time, barriers to implementation of this information.16,17 The transparent publication of blinded surgeon-specific rates identified variability in practice.
Measures and Analysis
Baseline Characteristics
Patient demographics, injury severity, emergency department/operating room/intensive care unit vital signs, laboratory values, and resuscitation, operating room procedures, and DCL-specific data (indications, surgeon) were recorded to assess the two groups for significant baseline differences. Over the course of the study period, the measurement of coagulopathy transitioned from conventional coagulation tests to thromboelastography. To account for this, a composite coagulopathy variable was created and reported. Coagulopathy was defined as: International Normalized Ratio >1.5, partial thromboplastin time>53.7 (>1.5 normal at our institution), activated clotting time >128, alpha angle <60, maximum amplitude <50, and percent lysis at 30 minutes >3%.18,19
Primary Outcome
The primary outcome for the project was the overall DCL rate for the trauma service.
Secondary Outcomes
Secondary outcomes included surgeon-specific DCL rates, morbidities, disposition, and mortality. A binary, composite “major abdominal complication” variable was also analyzed and consisted of any one of the following: reopening of fascia following closure, enteric suture line failure, enterocutaneous fistula, fascial dehiscence, and organ/space surgical site infection.
Variables were analyzed using Wilcoxon rank sum, Chi Square, and Fisher’s Exact test for continuous, binary, and sparse binary variables, respectively. All calculations were performed using STATA statistical software (version 13.1; Stata Corporation, College Station, TX).
RESULTS
Emergent Laparotomies and Utilization of DCL
Over the control time period (34 months), there were 581 emergent laparotomies (5% of all admissions) with a DCL rate of 39%. Over the QI time period (24 months), there were 448 emergent laparotomies (5% of all admissions), with a 23% DCL rate.
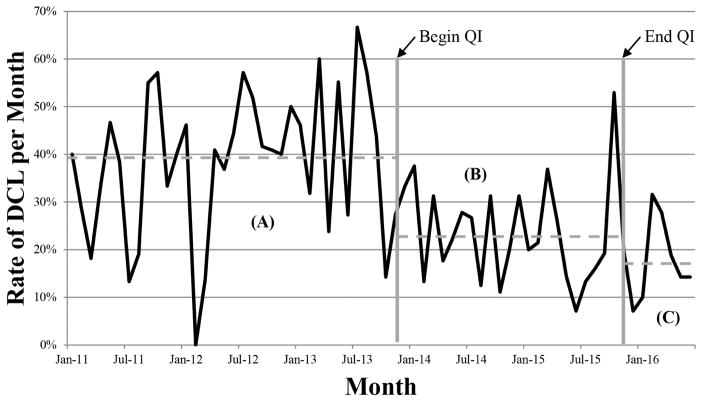
The line graph of monthly rates of DCL from 2011 through October 2015 is shown in Figure 1. A statistically significant decrease (p<0.001) in the utilization of DCL was observed almost immediately following introduction of the QI project. At 23%, this DCL rate persisted for the entirety of the QI period. This decrease in the rate of DCL was actually improved upon following completion of the QI project, with a rate of 17% over the subsequent seven months.
Figure 1.
Rate of damage control laparotomy (DCL) from January 2011 to June 2016. (A) DCL rate during historical control period was 38%. (B) After initiation of quality improvement (QI) project, there was an immediate and sustained decrease in the DCL rate to 23% (p<0.001). (C) After completion of the QI project, the DCL rate continued to decrease, averaging 18% in the subsequent 8 months.
Impact of Decreasing the Utilization of DCL on Morbidity and Mortality
No difference in patient demographics was seen between the two groups (Table 1). Additionally, no difference in the indications used to determine DCL was seen. There was a significantly higher percentage of patients undergoing emergent laparotomy for penetrating trauma in the QI group.
Table 1.
Admissions, Demographics, and Injury Severity
| Variable | Control (01/2011– 10/2013) | QI (11/2013– 11/2015) | p Value |
|---|---|---|---|
| Total trauma admissions, n | 12,922 | 9,695 | |
| Emergent laparotomies, n | 581 | 448 | 0.250 |
| Definitive laparotomies, n (%) | 338 (58) | 326 (73) | |
| Damage control laparotomies, n (%) | 221 (38) | 101 (23) | |
| Operative deaths, n (%) | 22 (4) | 21 (5) | |
| Indication for damage control, n (%) | |||
| Packing | 134 (61) | 60 (59) | 0.343 |
| Second look | 29 (13) | 16 (16) | |
| Hemodynamic instability | 30 (14) | 15 (15) | |
| Expedite CT/ICU | 14 (6) | 3 (3) | |
| ACS prophylaxis | 3 (1) | 5 (5) | |
| Contamination | 7 (3) | 1 (1) | |
| Expedite IR | 1 (0) | 1 (1) | |
| Unclear | 2 (1) | 0 (0) | |
| Sex, n (%) | |||
| Male | 423 (76) | 330 (77) | 0.555 |
| Female | 136 (24) | 97 (23) | |
| Mechanism, n (%) | |||
| Blunt | 349 (62) | 220 (52) | 0.001 |
| Penetrating | 210 (38) | 207 (48) | |
| BMI, kg/m2, median (IQR) | 26 (24, 30) | 27 (24, 31) | 0.175 |
| Head AIS, median (IQR) | 0 (0, 2) | 0 (0, 0) | 0.222 |
| Face AIS, median (IQR) | 0 (0, 0) | 0 (0, 0) | 0.095 |
| Chest AIS, median (IQR) | 2 (0, 3) | 2 (0, 3) | 0.753 |
| Abdomen AIS, median (IQR) | 3 (2, 4) | 3 (2, 4) | 0.142 |
| Extremity AIS, median (IQR) | 1 (0, 3) | 0 (0, 3) | 0.098 |
| External AIS, median (IQR) | 1 (0, 1) | 1 (0, 1) | 0.126 |
| Injury Severity Score, median (IQR) | 19 (11, 34) | 19 (10, 29) | 0.165 |
QI, quality improvement; CT, computed tomography; ICU, intensive care unit; ACS, abdominal compartment syndrome; IR, interventional radiology; AIS, abbreviated injury score
No difference in emergency department temperature, systolic blood pressure, acidosis, or coagulopathy was observed (Table 2). Intraoperatively, no significant differences in transfusions were observed (Table 3). There was a shift towards significantly longer operative durations during the QI period. Despite longer operating room times, the QI group actually had higher operative temperatures, arterial pH values, and base excess values at the end of the cases.
Table 2.
Emergency Department Vital Signs, Labs, and Resuscitation
| Variable | Control (n=559) | QI (n=427) | p Value |
|---|---|---|---|
| Vital sign | |||
| Temperature, °F, median (IQR) | 97.6 (96.7, 98.3) | 97.7 (97.0, 98.4) | 0.158 |
| Systolic blood pressure, mmHg, median (IQR) | 111 (90, 130) | 115 (92, 130) | 0.165 |
| Heart rate, bpm, median (IQR) | 99 (82, 115) | 100 (83, 117) | 0.625 |
| Glasgow Coma Scale, median (IQR) | 15 (6, 15) | 15 (13, 15) | 0.003 |
| Laboratory value | |||
| Lactic acid, mmol/L, median (IQR) | 3.5 (2.2, 5.5) | 3.3 (2.0, 5.0) | 0.185 |
| Base excess, mmol/L, median (IQR) | −3 (−7, −1) | −4 (−7, −1) | 0.085 |
| Hemoglobin, g/dL, median (IQR) | 13.2 (11.9, 14.4) | 13.2 (11.8, 14.4) | 0.556 |
| Platelet level, k/cm2, median (IQR) | 224 (190, 266) | 233 (194, 280) | 0.115 |
| Abnormal coagulation profile, n (%) | 172 (35) | 146 (39) | 0.181 |
| Resuscitation, median (IQR) | |||
| Crystalloid, mL | 0 (0, 0) | 0 (0, 0) | 0.013 |
| Red blood cells, units | 0 (0, 2) | 0 (0, 1) | <0.001 |
| Fresh frozen plasma, units | 0 (0, 2) | 0 (0, 1) | <0.001 |
| Other | |||
| Positive FAST, n (%) | 263 (49) | 206 (50) | 0.781 |
| Time in ED, min, median (IQR) | 64 (27, 132) | 47 (23, 103) | 0.005 |
QI, quality improvement; F, Fahrenheit; FAST, focused abdominal sonography for trauma; ED, emergency department
Table 3.
Operating Room Vital Signs, Labs, and Resuscitation
| Variable | Control (n=559) | QI (n=427) | p Value |
|---|---|---|---|
| OR duration, min, median (IQR) | 109 (73, 162) | 135 (94, 192) | <0.001 |
| First documented vital sign and lab, median (IQR) | |||
| Temperature, °F | 96.6 (95.4, 97.7) | 97.0 (96.1, 98.1) | <0.001 |
| Systolic blood pressure, mmHg | 120 (100, 140) | 118 (97, 139) | 0.279 |
| Heart rate, bpm | 98 (85, 110) | 96 (82, 112) | 0.369 |
| pH | 7.30 (7.22, 7.36) | 7.31 (7.24, 7.38) | 0.044 |
| Lactic acid, mmol/L | 2.8 (1.8, 4.3) | 2.8 (1.7, 4.6) | 0.983 |
| Base excess, mmol/L | −5 (−8, −2) | −4 (−7, −1) | 0.079 |
| Resuscitation , median (IQR) | |||
| Crystalloid, mL | 1500 (1000, 2000) | 1200 (800, 1700) | <0.001 |
| Colloid, mL | 500 (0, 1000) | 500 (0, 1000) | 0.068 |
| Red blood cells, units | 1 (0, 4) | 1 (0, 5) | 0.853 |
| Fresh frozen plasma, units | 0 (0, 4) | 0 (0, 4) | 0.561 |
| Platelets, units | 0 (0, 6) | 0 (0, 6) | 0.197 |
| Estimated blood loss, mL | 350 (100, 1000) | 400 (150, 1000) | 0.430 |
| Last documented vital signs and labs, median (IQR) | |||
| Temperature, °F | 96.8 (95.5, 97.9) | 97.2, (96.1, 98.2) | <0.001 |
| Systolic blood pressure, mmHg | 125 (115, 140) | 128 (115, 142) | 0.105 |
| Heart rate, bpm | 91 (80, 100) | 90 (79, 105) | 0.534 |
| pH | 7.33 (7.29, 7.37) | 7.34 (7.30, 7.39) | 0.003 |
| Lactic acid, mmol/L | 3.2 (2.0, 4.9) | 3.3 (1.8, 5.0) | 0.823 |
| Base excess, mmol/L | −4 (−6, −2) | −4 (−6, −2) | 0.036 |
QI, quality improvement; OR, operating room; F, Fahrenheit; TXA, tranexamic acid
There were no significant differences in morbidities or mortality between the two groups (Table 4). There was also not a significant difference in hospital-, ventilator-, or intensive care unit-free days.
Table 4.
Outcomes
| Variable | Control (n=559) | QI (n=427) | p Value |
|---|---|---|---|
| Morbidity,n (%) | |||
| Major abdominal complication | 128 (23) | 106 (25) | 0.481 |
| Organ/space SSI | 69 (12) | 66 (16) | 0.148 |
| Fascial dehiscence | 32 (6) | 33 (8) | 0.195 |
| Enteric suture line failure | 15 (3) | 17 (4) | 0.243 |
| Enterocutaneous fistula | 12 (2) | 10 (2) | 0.815 |
| Reopened | 56 (11) | 41 (10) | 0.575 |
| Superficial SSI, n (%) | 44 (8) | 29 (7) | 0.544 |
| Ileus, n (%) | 107 (19) | 98 (23) | 0.124 |
| Pulmonary embolus, n (%) | 39 (7) | 34 (8) | 0.530 |
| Deep vein thrombosis, n (%) | 18 (3) | 12 (3) | 0.795 |
| Sepsis, n (%) | 98 (18) | 92 (22) | 0.098 |
| Acute renal failure, n (%) | 73 (13) | 57 (13) | 0.849 |
| Length of stay, d, median (IQR) | |||
| ICU-free | 27 (16, 30) | 27 (17, 30) | 0.096 |
| Ventilator-free | 29 (22, 30) | 29 (24, 30) | 0.074 |
| Hospital-free | 17 (3, 24) | 17 (1, 24) | 0.684 |
| Disposition, n (%) | |||
| Death | 52 (9) | 43 (10) | 0.686 |
| Home | 392 (70) | 392 (70) | 0.058 |
| Skilled nursing facility | 24 (4) | 29 (7) | |
| Long term acute care | 35 (6) | 14 (3) | |
| Rehabilitation hospital | 48 (9) | 30 (7) | |
| Other | 8 (1) | 8 (2) |
QI, quality improvement; SSI, surgical site infection; ICU, intensive care unit
DCL Patients who May Have Safely Undergone Definitive Laparotomy
During adjudication of individual cases, discussion focused on the indication-specific outcome of each patient and the perceived definitions of relatively vague indications. As an example, patients who underwent DCL for a planned second look at bowel viability and had a negative re-exploration were felt to be those who could have safely undergone definitive laparotomy. As another example, patients who underwent DCL to expedite imaging when there was concern for a traumatic brain injury and then were found to have not have a traumatic brain injury (or a traumatic brain injury where minimal therapy was needed to maintain normal intracranial pressures) were felt to be those who could have safely undergone definitive laparotomy.
The indications for DCL in this group of patients who could have safely undergone definitive laparotomy are listed in Table 5. Indications consistently determined by group adjudication to be those safe for definitive laparotomy were: contamination, expedition of postoperative imaging or intensive care, and second look.
Table 5.
Indications for Damage Control Laparotomy in Patients Who May Have Safely Undergone Definitive Laparotomy
| Indication | Voted safe for definitive laparotomy | |
|---|---|---|
| n | % | |
| Contamination | 1 | 100 |
| Expedite CT/ICU | 3 | 100 |
| Second look | 13 | 81 |
| Hemodynamic instability | 5 | 33 |
| Packing | 5 | 8 |
| Abdominal compartment prophylaxis | 0 | 0 |
| Expedite transport to IR | 0 | 0 |
| Heterogeneity in definition of hemodynamic instability | ||
| Ongoing transfusions at end of laparotomy | 0 | 0 |
| Continuous vasopressor use at end of laparotomy | 0 | 0 |
| Isolated, persistent acidosis at end of laparotomy | 5 | 100 |
CT, computed tomography; ICU, intensive care unit; IR, interventional radiology
Hemodynamic instability was a vague indication for DCL and heterogeneity existed in its definitions. Hemodynamic instability was not defined a priori and left up to the determination of the operating surgeon. Patients who underwent DCL for hemodynamic instability were divided into three definitions: 1) ongoing transfusions at end of laparotomy, 2) continuous vasopressor use at end of laparotomy, or 3) isolated, persistent acidosis without continuous vasopressor use or ongoing transfusions. During the adjudication meetings, only continuous vasopressor use and ongoing transfusion requirement were considered to be appropriate definitions for hemodynamic instability. All patients with a persistent acidosis without continuous vasopressor use or an ongoing transfusion requirement were felt to be patients who could have safely undergone definitive laparotomy.
Indications for DCL that were consistently adjudicated to be appropriate were packing abdominal compartment syndrome prophylaxis, and expedition of patient transport to Interventional Radiology for hemorrhage control.
Surgeon-Specific Rates of DCL
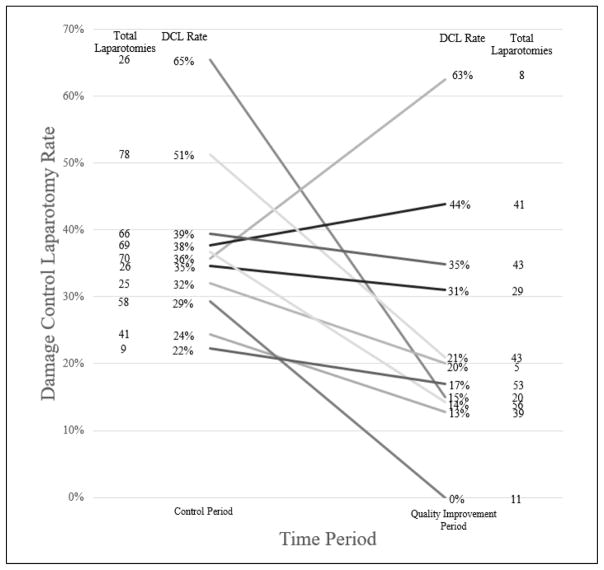
During the QI period, surgeon-specific rates of DCL varied significantly, ranging from 0% – 100% (p=0.005). DCL rates did not vary according to surgeon-specific laparotomy volume. Ten of the 21 surgeons included from 2011 through 2015 had at least one laparotomy in both the control and QI periods. For those 10 surgeons, pre-QI and post-QI rates of DCL and the total number of laparotomies performed during the respective time periods are shown in Figure 2. Most surgeons had a decrease in the rate of DCL between the two periods with percent decreases ranging from to 4 to 50%. Two surgeons had an increase in the rate of DCL, one of which was more likely reflective of few laparotomies in the QI period.
Figure 2.
Surgeon-specific rates of damage control laparotomy (DCL) during the control period (01/2011–10/2013) and the quality improvement period (11/2013–10/2015). Most surgeons had a decrease in the rate of DCL between the 2 periods with varying degrees of change. Two surgeons had an increase in the rate of DCL between the 2 periods.
The ideal rate of DCL over the QI project was 17% (appropriate DCL [74] divided by total laparotomies [448]). Using 17% as an ideal DCL rate, the six surgeons with rates below this during the QI period did not significantly differ from the twelve surgeons with DCL rates above in terms of age (median 46 years, range 36–60 versus 43, range 34–68, p=0.512), years since the completion of fellowship (median 12 years, range 3–27 versus 8, range 0–33, p=0.615), the number of institutions at which they have trained or worked (median 3, range 1–6 versus 1, range 1–4, p=0.091), the percent who did residency at UT Health (33% versus 33%, p=1.000), or the percent who completed fellowship training at UT Health (33% versus 67%, p=0.321).
DISCUSSION
The implementation of a QI process successfully resulted in an immediate, significant, and sustained decrease in the rate of DCL at our institution. The temporal relationship between the intervention and the drop the rate of DCL suggested a causal relationship between the intervention and the observed reduction in DCL. No associated increase in the need for unplanned reoperation was observed. With an absolute DCL rate reduction of 16% in a time period in which 427 patients underwent emergent laparotomy, 68 DCLs and 68 subsequent planned re-laparotomies were avoided during this 2 year time period.
This QI process was emblematic of how a learning trauma care system would address a perceived gap in quality care.20 First, a problem in the quality of care was identified. Next, a QI process using evidence-based implementation strategies, including audit and feedback, stakeholder involvement, a physician champion, local consensus discussions, and ongoing stakeholder consultation, was designed and executed.21 To monitor the QI process, data was collected, analyzed, and repeatedly published for the clinicians to review. Prospectively collected evidence was used to influence practice and practice influenced our prospectively collected evidence. This process will be one model of QI at our institution moving forward as we aim to create a learning trauma care system. While this process could be repeated for another perceived gap in quality care, the presence of a physician champion was absolutely necessary as the multi-faceted process was time consuming.
The process would be significantly easier with the use automatic capture of data. This project identified four indications for DCL that our group of surgeons generally accepted as appropriate: 1) therapeutic packing to control hemorrhage, 2) expedition to Interventional Radiology for hemorrhage control, 3) hemodynamic instability defined as continuous vasopressor and/or ongoing transfusion requirement, and 4) abdominal compartment syndrome treatment or prophylaxis. Our group of surgeons also identified four indications for DCL in which appropriateness in unclear at this time: 1) second look, 2) expedition to imaging or intensive care, 3) hemodynamic instability defined as persistent acidosis with a continuous vasopressor or ongoing transfusion requirement, and 4) contamination. It is important to note that the group did not feel these indications were inappropriate, simply the group began to question if the indications were necessary and acknowledged that high quality data supporting the indications was both lacking and needed.
Contrary to our hypothesis and the current available literature, our study did not show a decrease in morbidity associated with the reduction in the rate of DCL. As the majority of peer-reviewed publications evaluating morbidity following DCL have been retrospective and non-randomized, the identified association between DCL and morbidity may be confounded by the increased severity of patient injury in those studies undergoing DCL compared to definitive laparotomy. As this is a pre-post intervention QI project, it is also possible that temporal changes in patient injury patterns or changes in clinical management over the course of the study may have affected the incidence of post-operative complications.
The initial reduction in the DCL rate at the beginning of the QI project is suggestive of a Hawthorne, or observer, effect – surgeons’ behavior changed as a result of their knowledge of being observed. Though present, this is not a bias, but rather the mechanism by which an audit and feedback QI strategy works. The sustained decrease in utilization of DCL over the two year time period suggests a change in practice and culture. This change is further supported by the fact that the rate of DCL continued to decrease in the months following the completion of the project, averaging 17% in the subsequent seven months.
There are several limitations to this study. First, though prospective in nature, this study compares outcomes to those in a historical control group. The historical control, however, was the immediate time period prior to the QI period and was collected in detail for other peer reviewed studies. Second, given that audit and feedback alone has been demonstrated to have variable effectiveness, we chose to use a multi-faceted strategy to effect change.22,23 The multi-faceted process described was complex and time consuming. While there is no clear evidence that multi-faceted interventions are routinely more effective than single-component interventions, it can be difficult to identify a priori the single intervention that would be most effective given the specific context of the project.24 Lastly, long-term observation of the DCL rate to determine if the change was in fact institutionalized was not possible as the QI study led to a randomized clinical trial that began enrolling in July 2016.
As a follow up to this study, a pilot randomized controlled trial comparing morbidity between DCL and definitive laparotomy has been started to obtain the least-biased estimates of outcomes and to define objective inclusion and exclusion criteria for a potential multicenter randomized controlled trial. Also, a multicenter QI project is underway to assess the replicability of this QI process and to determine if additional indications for which there are clinical equipoise to perform DCL or definitive laparotomy. And, finally, an economic analysis of the impact of decreasing the rate of DCL will be performed.
CONCLUSIONS
A QI project to transparently share surgeon-specific rates of, the indications for, and appropriateness of DCL resulted in a significant decrease in the rate of DCL, from 39% to 23%. This decrease was not associated with increased morbidity or mortality. This QI project changed the culture and clinical practice for an emergent intervention at our institution using prospectively collected data and will be one model of QI at our institution as we build a learning trauma care system.
Acknowledgments
The authors would like to thank Dr Jeff Tomasek for his invaluable work with the trauma registry.
Support: This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award KL2 TR000370 from the National Center for Advancing Translational Sciences.
Footnotes
Presented at the 75th Meeting of the American Association for the Surgery of Trauma, Baltimore, MD, September 2016.
Disclosure Information: Nothing to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532–535. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal trauma. J Trauma. 1993;35:375–382. [PubMed] [Google Scholar]
- 3.Roberts DJ, Zygun DA, Faris PD, et al. Indications for Trauma Damage Control Surgery International Study Group. Opinions of practicing surgeons on the appropriateness of published indications for use of damage control surgery in trauma patients: an international cross-sectional survey. J Am Coll Surg. 2016;223:515–529. doi: 10.1016/j.jamcollsurg.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Hatch QM, Osterhout LM, Podbielski J, et al. Impact of closure at the first take back: complication burden and potential overutilization of damage control laparotomy. J Trauma. 2011;71:1503–1511. doi: 10.1097/TA.0b013e31823cd78d. [DOI] [PubMed] [Google Scholar]
- 5.Hatch QM, Osterhout LM, Ashraf A, et al. Current use of damage-control laparotomy, closure rates, and predictors of early fascial closure at the first take-back. J Trauma. 2011:1429–1436. doi: 10.1097/TA.0b013e31821b245a. [DOI] [PubMed] [Google Scholar]
- 6.Harvin JA, Wray CJ, Steward J, et al. Control the damage: morbidity and mortality after emergent trauma laparotomy. Am J Surg. 2016;212:34–39. doi: 10.1016/j.amjsurg.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 9.Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha B, Holcomb JB, Camp EA, et al. Damage control resuscitation increases successful non-operative management rates and survival after severe blunt liver injury. J Trauma Acute Care Surg. 2015;78:336–341. doi: 10.1097/TA.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 11.Joseph B, Zangbar B, Pandit V, et al. The conjoint effect of reduced crystalloid administration and decreased damage control laparotomy use in the development of abdominal compartment syndrome. J Trauma Acute Care Surg. 2014;76:457–461. doi: 10.1097/TA.0b013e3182a9ea44. [DOI] [PubMed] [Google Scholar]
- 12.fan e, laupacis a, pronovost pj, et al. how to use an Article About Quality Improvement. JAMA. 2010;304:2279–2287. doi: 10.1001/jama.2010.1692. [DOI] [PubMed] [Google Scholar]
- 13.Revised Standards for Quality Improvement Reporting Excellence – SQUIRE 2.0. [Accessed August 1, 2016];Standards for Quality Improvement Reporting Excellence web site. Available at: http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&PageID=471.
- 14.Guidance for Determining Whether a QI/QA Activity Needs CPHS Review and Approval. [Accessed August 1, 2016];UT Health Committee for the Protection of Human Subjects web site. Available at: https://www.uth.edu/dotAsset/71bc373b-0ef3-4974-bd89-a368e13985d5.pdf.
- 15.Lau BD, Amaoutakis GJ, Streiff MB, et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study. Ann Surg. 2016;264:1181–1187. doi: 10.1097/SLA.0000000000001512. [DOI] [PubMed] [Google Scholar]
- 16.Knowledge-to-Action Framework. [Accessed August 25, 2016];World Health Organization web site. Available at: http://www.who.int/reproductivehealth/topics/best_practices/greatproject_KTAframework/en/
- 17.Grumbach K, Lucey CR, Johnston SC. Transforming from centers of learning to learning health systems. JAMA. 2014;311:1109–1110. doi: 10.1001/jama.2014.705. [DOI] [PubMed] [Google Scholar]
- 18.Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 19.Chapman MP, Moore EE, Ramos CR, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75:961–967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A National Trauma Care System. Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury. Washington, DC: the National Academies Press; 2016. [Accessed December 7, 2016]. http://www.nationalacademies.org/hmd/Reports/2016/A-National-Trauma-Care-System-Integrating-Military-and-Civilian-Trauma-Systems.aspx. [PubMed] [Google Scholar]
- 21.Waltz TJ, Powell BJ, Matthieu MM, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10:109. doi: 10.1186/s13012-015-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012 Jun; doi: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey G, Kitson A. Translating evidence into healthcare policy and practice: single versus multi-faceted implementation strategies – is there a simple answer to a complex question? Int J Health Policy Manag. 2015;4:123–126. doi: 10.15171/ijhpm.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squires JE, Sullivan K, Eccles MP, et al. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals’ behaviours? An overview of systematic reviews. Implement Sci. 2014;9:152. doi: 10.1186/s13012-014-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]