Abstract
Background
In adults, primary sclerosing cholangitis (PSC), a cholestatic liver disease characterized by inflammation/fibrosis of intra/extra-hepatic bile ducts, associates with a milder form of inflammatory bowel disease (IBD), particularly ulcerative colitis (UC). The pediatric PSC-IBD phenotype is less well characterized.
Methods
We performed a retrospective, single-center study examining PSC-IBD patients at Texas Children’s Hospital (TCH) between 2000–2015. IBD-phenotype (Modified Montreal Classification), medications, lab values, endoscopic records, and IBD-based hospital admissions were collected. PSC-UC phenotype was compared to UC, non-PSC patients (n=95) from TCH. Elevated gamma-glutamyl transpeptidase (GGT) levels were compared to calcitonin levels and IBD-flare activity, i.e. GI symptoms resulting in office/emergency room visits or hospital admission.
Results
Of thirty-nine PSC-IBD patients, thirty-four (87.2%) had UC (PSC-UC) and five (12.8%) had Crohn’s Disease. Pancolitis was more common in PSC-UC than UC, non-PSC (96.3%, 64%, p=0.0009). PSC-UC patients required less treatment with steroids (76.5%, 91.6%, p=0.0326) or infliximab (8.8%, 37.9%, p=0.0011), and fewer had at least one IBD-related hospital admission (32.4%, 63.2%, p=0.0025) than UC, non-PSC. Progression to colectomy was significantly less (5.8%, 24.2%, p=0.0223) in PSC-UC. Median diagnosis-to-colectomy time tended to be longer in PSC-UC (6.37, 2.5 years, p=0.0792). In two smaller subsets, GGT did not correlate with calprotectin in PSC-UC (n=11, p=0.7922) and less strongly associated with IBD-flares in PSC-UC than UC, non-PSC (n=33, n=67; 15.2%, 41.8%, p=0.0120).
Conclusion
Pediatric PSC appears to associate with milder pancolitic-UC. PSC and IBD activity do not appear to correlate. Our findings may provide useful information towards etiology and management of pediatric PSC-IBD.
Introduction
Primary sclerosing cholangitis (PSC) is a rare progressive cholestatic liver disease characterized by inflammation and fibrosis of intra- and/or extra-hepatic bile ducts. Clinical manifestations of PSC are typically secondary to cholestasis and progressive fibrosis, including jaundice, hepatomegaly, splenomegaly, recurrent cholangitis and intractable pruritus1–3. Little is known about the etiology and treatment for PSC, with no current therapies conclusively proven to alter the natural history of the disease2.
PSC is strongly associated with inflammatory bowel diseases (IBD)2. In adult patients, studies indicate that the most common PSC-IBD phenotype is a clinically milder, but more extensive (larger portion of the colonic mucosa affected) form of ulcerative colitis2, 4. Adult-based population studies found 46–98% co-diagnoses of IBD in patients with PSC, with UC as the most common form (48%–90% of PSC-IBD)2, 4, 5. Based on various adult reports, it appears as though the majority of the PSC-IBD population is white males. It should be noted, however, that these reviews or reports were predominantly based in North America and Northern Europe5, 6. Rectal sparing in PSC-IBD has wide variance in adult population studies, with incidence ranging from 5.6% to 66.4% in one large systematic review5. Additionally, PSC-IBD patients have an increased risk of cholangiocarcinoma (CCA), with one study indicating a 14% risk of developing CCA within ten years in patients co-diagnosed with IBD (versus 2% in patients without IBD, p=0.008)7. Colorectal cancer (CRC) has also been associated with PSC-IBD, as a large-scale meta-analysis concluded higher risk of colorectal cancer at a younger age when compared to non-IBD PSC patients (OR 4.79, 95% CI 3.58–6.41; 19 vs. 29 years, p=0.04)8.
Few studies have focused on the pediatric PSC population, with even less examining the PSC-IBD phenotype. One retrospective review reported a pediatric PSC incidence of 0.23 per 100,000 patients, significant for shorter life expectancy than the age and sex-matched general population1. A recent study by Lascurain et al.9 compared a multi-center cohort of pediatric PSC-IBD patients (n=37) to pediatric IBD patients without PSC (n=137). Though prevalence of pancolitis was higher in their PSC-IBD population (89.7% vs. 72.4%, p=0.051), other clinical characteristics—rectal sparing, disease severity scores, IBD-related admissions, and colonic surgery rates—showed no statistical difference when the groups were compared. The goal of our single center based work was to provide added information about the pediatric IBD phenotype associated with PSC. Previous studies have shown that interobserver variation of IBD care exists between different tertiary adult and pediatric centers. This includes differences in diagnostic pathological accuracy and variations in therapeutic treatment plans (i.e. significant variation in use of antibiotics, immunomodulators, steroids, aminosalycilates and infliximab)11–13. Our single center approach attempted to provide means to overcome center-based practice bias in regards to IBD care.
Materials and Methods
We performed a retrospective, single-center study to examine PSC-IBD in patients seen at Texas Children’s Hospital (TCH) between January 2000 and June 2015. Located in the diverse metropolitan area of Houston, TX, TCH offered a geographic and ethnically unique populace of pediatric patients diagnosed with this rare condition from a single tertiary center. PSC was defined by a combination of clinical, biochemical, and cholangiographic standards. This included a cholestatic biochemical profile (i.e. elevated transaminase levels, elevated gamma-glutamyl transpeptidase [GGT]) with at least one radiological or histological finding. Cutoff levels for elevated AST, ALT, and GGT were based on standardized lab reference values, corrected for age and gender. Radiologic findings were based on cholangiography (i.e. magnetic resonance cholangiography, endoscopic retrograde cholangiography, percutaneous transhepatic cholangiography) consistent with characteristic bile duct changes (i.e. multifocal strictures, segmental dilatations)4. Histological records of liver biopsies were reviewed for each patient, noting findings by expert pathologists compatible with PSC (i.e. bile duct damage, “onion-skin” fibrosis). These findings were important for defining the small-duct PSC variant, where typical cholestatic and histologic features of PSC are present without bile duct findings on cholangiogram4. Patient records were reviewed for other etiologies of liver disease and were excluded if pathology reports were consistent with alternative cholestatic diagnoses (i.e. biliary atresia). None of the patients included in our study had history of viral hepatitis, although only 50% (n=17) had records of serologic screening. Those with incomplete or poorly documented records were also removed. Thirteen (n=13) patients in our cohort had co-diagnosis of autoimmune hepatitis and were not excluded from the study.
All patients with inflammatory bowel disease had clinical diagnoses based on established records of clinical features (i.e. hematochezia, chronic watery diarrhea, severe abdominal pain) in combination with characteristic endoscopic, (i.e. inflammation, ulceration, friability, fissures, fat wrapping, etc.), imaging and histopathologic findings (i.e. inflammation, crypt architecture irregularity, crypt abscesses, granulomas, etc.).
Patients were either diagnosed prior to evaluation, or were newly diagnosed with PSC at TCH. The data extracted from patient charts included age at diagnosis of PSC and IBD, IBD phenotype/severity (Modified Montreal Classification)10, medication history, IBD-based hospital admissions (admission secondary to abdominal pain, diarrhea, weight loss, and/or fevers), disease focused laboratory values (i.e. aspartate transaminase [AST], alanine transaminase [ALT], GGT, calprotectin), presence or absence of rectal sparing upon IBD diagnosis and colectomy status. PSC-IBD (with particular focus of PSC patients with ulcerative colitis) was compared to a cohort of UC patients without PSC (UC, non-PSC) from the same center. The two groups were matched by gender, ethnic distribution, and by median age of UC diagnosis (11.72 years vs. 12.0 years, p=0.65). Full breakdown of age, gender and ethnicity can be found in Table 1 for the groups studied. IBD flares were based on patient chart review, defined as clinical symptoms of IBD (i.e. loose stools, hematochezia, weight loss, abdominal pain, tenesmus, stool occult blood) leading to an office visit, emergency room visit or hospital admission. Patients with IBD flares were reviewed for concomitant Clostridium difficile infection. One patient (n=1) in the control group had documented positive Clostridium difficile toxin during their UC flare. This patient was not removed from the UC flare subset and was treated for both the UC symptoms and C. difficile colitis during their hospital admission. Of the remaining subset of patients with documented IBD flares, none were positive for C. difficile toxin.
Table 1.
Patient Demographics and Outcomes
| Variable | PSC-UC | UC, non-PSC | p-value | Statistical Test |
|---|---|---|---|---|
| # of Patients | 34 | 95 | ||
| Age of UC Diagnosis (years) [Median] | 11.72 | 12 | p=0.65 | Mann-Whitney U Test |
| Age of PSC Diagnosis (years) [Median] | 12.83 | |||
| Gender - Males | 58.8% | 49% | p=0.32 | Fisher’s Exact Test |
| Race - White | 58.8% | 46.3% | p=0.23 | |
| Race - Black | 17.6% | 9.5% | p=0.22 | |
| Race - Hispanic | 14.7% | 17.9% | p=0.79 | |
| Race - Asian | 0% | 5.2% | p=0.32 | |
| Pancolitis* | 96.3% | 64% | p=<0.001 | |
| History of Steroid Use | 76.5% | 91.6% | P=0.03 | |
| History of Infliximab Use | 8.8% | 37.9% | p=0.001 | |
| IBD-related Hospital Admission (minimum of 1 since Dx of IBD) | 32.4% | 63.2% | p=0.002 | |
| Rectal Sparing* | 0.7% | 2.2% | p=0.08 | |
| Colectomy | 5.8% | 24.2% | p=0.02 | |
| Mean Time from UC Diagnosis to Colectomy (years) | 6.37 | 2.5 | p=0.07 | Unpaired T-Test |
UC= ulcerative colitis, PSC-UC = ulcerative colitis with primary sclerosing cholangitis, UC, non-PSC = ulcerative colitis without primary sclerosing cholangitis, IBD = inflammatory bowel disease, Dx = diagnosis,
Corrected for patients without full colonoscopic evaluation
PSC-IBD subgroups were defined by histologic and endoscopic results, in combination with review of clinical records. Modified Montreal classification10 was used in determination of colitis in the UC patients, ranked from E1 (ulcerative proctitis) to E4 (pancolitis; a category delineated in the pediatric population). Modified Montreal Classification was also used for our five Crohn’s disease (CD) patients, which ranked from L1 (distal 1/3 ileum +/− limited cecal disease) to L4b (upper disease distal to ligament of Treitz and proximal to distal 1/3 ileum)10. Rectal sparing was based on endoscopic findings (i.e. lack of gross inflammatory findings of the rectum). It should be noted that 45 of the 95 control patients had either incomplete or missing colonoscopic records and were not included in this subset analysis of the degree of colitis based on Modified Montreal Classification. Otherwise, all 95 patients in the control were analyzed for treatment history and frequency of hospital admissions.
For our study, we associated elevated GGT levels as a marker of PSC disease activity and elevated calprotectin as a marker of UC disease activity. All calprotectin levels were extracted from the PSC-UC cohort. GGT levels drawn within 14 days of these calprotectin labs were paired and analyzed for correlation. Furthermore, the highest recorded GGT value (GGTmax) was extracted from each of the PSC-UC patients (n=33, one patient was removed from this specific sub-analysis due to lack of GGT recorded). Within 14 days of GGTmax, medical records were investigated for evidence of IBD flare activity. Positive flare activity was defined as active GI symptoms and physical exam findings (i.e. loose stools, hematochezia, abdominal pain, tenesmus, stool occult blood) leading to urgent office visit, emergency room visit or hospital admission.
Qualitative variables were reported numerically and as percentages; quantitative variables were calculated as averages. Fisher’s Exact Test was used to analyze patient demographics (i.e. gender, sex), clinical outcome data (i.e. pancolitis, IBD-based hospital admissions, medication history, colectomy) and presence of IBD flares during periods of elevated GGT. Two-tailed Pearson’s test was used to analyze correlation between calprotectin and GGT levels. Mann-Whitney U Test was used to analyze the median age of UC diagnosis. Unpaired T Test was used to analyze mean time from UC diagnosis to colectomy and mean time between diagnosis of UC and PSC. All tests were two-tailed, and statistical significance was set at p < 0.05. Colectomy data were analyzed using uncensored Kaplan – Meier probability estimators with subsequent log rank analysis. Kaplan-Meier analysis was performed with RStudio version 0.99.491 with the aid of ‘survival’ version 2.38-1.
Results
Patient population
Our initial search of TCH electronic medical records resulted sixty-two (62) patients with possible diagnosis of PSC. After removal of patients for insufficient records (i.e. lack of and/or poor documentation, misdiagnosis), a total of fifty-two (52) patients identified with PSC were included. Thirty-nine (75%) had a co-diagnosis of IBD. Thirty-four (87.2%) of the IBD patients had UC (PSC-UC) and five (12.8%) had Crohn’s Disease.
PSC-UC
Secondary to the overwhelming majority of UC amongst the PSC-IBD patients, we further focused on PSC-UC and compared those to a cohort of UC patients without PSC (UC, non-PSC, n=95). PSC was diagnosed before (i.e. more than 6 months before), simultaneously (i.e. within 6 months), or after the diagnosis (i.e. more than 6 months after) of UC in 5.9%, 64.7%, and 29.4% of patients, respectively. Average time between diagnoses was 44.8 months when UC was diagnosed first and 15.0 months when PSC was diagnosed first (p=0.14). All 34 patients with PSC-UC had history of ursodiol therapy aimed at treatment of PSC.
Clinical Outcomes in PSC-UC
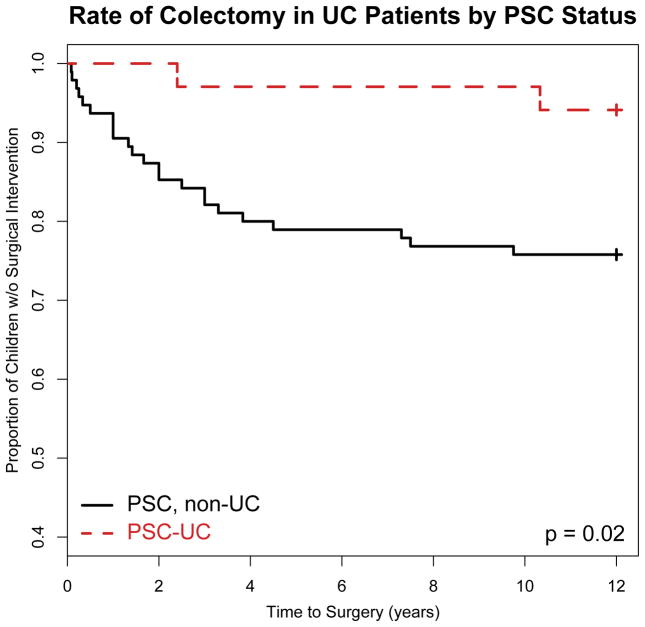
Among the patients who had a full colonoscopic evaluation (27 PSC-UC, 50 UC, non-PSC), pancolitis was more common in PSC-UC than in UC, non-PSC (96.3% vs. 64%, p=0.0009). There was a trend for more rectal sparing at diagnosis in PSC-UC, but this difference was not statistically significant (10.7% vs. 2.2%, p=0.0861). PSC-UC patients were significantly less commonly treated with steroids (i.e. prednisone, prednisolone) than UC, non-PSC patients (76.5% vs. 91.6%, p=0.0326). PSC-UC patients were also significantly less commonly treated with infliximab than UC, non-PSC patients (8.8% vs. 37.9%, p=0.0011). Significantly fewer PSC-UC patients had at least one IBD-related hospital admission than UC, non-PSC patients (32.4% vs. 63.2%; p=0.0025). Disease progression to colectomy was also significantly less common, with only 2 of 34 (5.8%) PSC-UC patients eventually requiring surgery. In comparison, nearly a quarter (24.2%) of the 95 UC, non-PSC patients followed at TCH had eventual colectomy within 10 years from diagnosis (Table 1, Figure 1). Median time from diagnosis of UC to colectomy tended to be longer in PSC-UC (6.37 years vs. 2.5 years, p=0.0739) as well.
Figure 1.
Primary sclerosing cholangitis (PSC) in children with Ulcerative Colitis (PSC-UC) is associated with a reduced rate of progression to surgery as compared to UC children without PSC (UC, non-PSC). In total, two PSC-UC patients (n=2) required colectomy (n @ 4 years =1; n @ 12 years =2). In total, twenty-three UC, non-PSC patients (n=23) required colectomy (n @ 4 years =19; n @ 8 years = 22; n @ 12 years = 23).
PSC-CD
Of the five Crohn’s patients, one patient was classified as L2 (colonic) and two patients were classified as L3 (ileocolonic). The two remaining patients did not have complete colonoscopies adequate for classification. Therefore, all CD patients with complete endoscopic evaluation had colonic involvement. Within the CD sub-cohort (n=5), PSC was diagnosed either concomitantly (i.e. within 6 months) or after the diagnosis (i.e. more than 6 months after) of CD in 40% and 60% of patients, respectively.
Disease Activity Correlation: calprotectin and GGT
Given the strong historical evidence for association between PSC and UC, we extracted clinical markers of both diseases for analysis of correlation. Of the PSC-UC cohort (n=34), 11 patients had at least one calprotectin level collected, with a total of 15 calprotectin levels overall. In these patients, 14 individual GGT levels collected within two weeks of these calprotectin labs were extracted for paired comparison (excluding one patient without history of GGT). Average levels of calprotectin and GGT were 433.00 ug/g and 157.64 U/L, respectively. Based on analysis of calprotectin/GGT pairs in this smaller PSC-UC subset by two-tailed Pearson’s test (r=0.0775, p=0.7922), PSC activity (GGT) did not correlate with or predict UC activity (calprotectin).
Disease Activity Correlation: UC flares and GGT
We further explored possible correlation between UC and PSC disease activities by analyzing presence of IBD flares during episodes of elevated GGT (GGTmax). Thirty-three of thirty-four PSC-UC patients and sixty-seven of ninety-five UC, non-PSC patients had a history of at least one GGT lab collected. Average levels of GGTmax were 481.23 U/L and 43.88 U/L in PSC-UC and UC, non-PSC patients, respectively. Average levels of GGTmax in patients with associated IBD flare were 353.00 U/L and 58.60 U/L in PSC-UC and UC, non-PSC patients, respectively. Average levels of GGTmax in patients without associated IBD flare were 520.86 U/L and 33.30 U/L in PSC-UC and UC, non-PSC patients, respectively. Within 14 days of GGTmax collection, 5 of 33 (15.2%) PSC-UC patients had a recorded IBD flare. In comparison, within 14 days of GGTmax collection, 28 of 67 (41.8%) UC, non-PSC patients had a recorded IBD flare. Therefore, PSC-UC patients in our cohort were less likely to have an IBD flare in the setting of high GGT levels (GGTmax) when compared to the smaller subset of UC, non-PSC patients with history of collected GGT (15.2% vs. 41.8%, p=0.0120). (Table 2).
Table 2.
Disease Activity Correlation: GGT and IBD Flares
| Variable | PSC-UC | UC, non-PSC | p-value | Statistical Test |
|---|---|---|---|---|
| # of patients with flare during highest GGT recorded* | 5 (33%) | 28 (41.8%) | p=0.01 | Fisher’s Exact Test |
| Average highest GGT (U/L) | 481.23 | 43.88 | ||
| Average highest GGT (U/L) in patients with associated flare** | 353.00 | 58.60 | ||
| Average highest GGT (U/L) in patients with no associated flare** | 520.86 | 33.30 |
UC= ulcerative colitis, PSC-UC = ulcerative colitis with primary sclerosing cholangitis, UC, non-PSC = ulcerative colitis without primary sclerosing cholangitis, IBD = inflammatory bowel disease, GGT = gamma-glutamyl transpeptidase,
Corrected to remove patients from analysis without history of GGT,
Flare within 14 days of highest GGT value collected
Caucasian vs. non-Caucasian PSC-IBD patients
Upon review of recent literature, we were unable to find specific comparison of clinical outcomes in pediatric PSC-IBD between ethnic groups. Among the thirty-four (34) PSC-UC patients, twenty (20) were self-reported in medical records as Caucasian. Of the remaining fourteen, six (6) and five (5) were reported as Black and Hispanic, respectively. Three (3) patients without ethnic classification were removed from this specific subgroup comparison. Each group had high rates of pancolitis, with E4 Modified Montreal Classification found in 92.8% and 100% of Caucasian and non-Caucasian patients, respectively (p=1.000). Both patients (n=2) requiring eventual colectomy were Caucasian, but this finding was not statistically significant (p=0.527). No significant differences were found between therapeutic treatments, including history of use of Infliximab, Azathioprine, or 6-MP (p=0.2814, p=0.2814, p=1.000), respectively. However, there was significantly less steroid use (i.e. prednisone, prednisolone) amongst Caucasian PSC-UC patients when compared with non-Caucasian PSC-UC patients (35% vs 90.9%, p=0.0068). There was no significant difference between rates of having at least one IBD-related hospital admission between Caucasian and non-Caucasian PSC-UC patients (20% vs. 27.2%, p=0.675).
Discussion
This is the largest single center pediatric study of PSC-IBD to date. Our results indicate that a clinically less severe UC phenotype is the most common form of IBD associated with PSC in pediatric patients. Similar to our findings in children, PSC-IBD in adults has been repeatedly observed to be a clinically less severe form of UC2, 4–6. Of the recent reports on pediatric specific PSC-IBD, few were found to include specific comparisons between PSC-IBD and non-PSC-IBD. The previously mentioned study by Lascurain et al. demonstrated a close to significant difference in pancolitis rates (89.7%, 72.4%, p=0.051) between PSC-IBD and non-PSC-IBD patients. Other outcomes in this study, including incidence of IBD-related hospital admissions (0.19, 0.25, p=0.111), follow-up Mayo/PGA severity scores (1.7, 1.7, p=0.708; 1.0, 1.3, p=0.141), and 5-year colectomy rates (16.4%, 24.7%, p=0.27), were not significantly different between PSC-IBD and non-PSC-IBD9. This latter study was multicenter, which may have introduced bias by different center based practice methods in regards to IBD care, as significant variation in the diagnostic accuracy between clinical care practices for IBD has been well described in the literature11–13. Though the single-center approach of our study does not remove diagnostic or therapeutic variation between our own institution’s pathologists and gastroenterologists, it does allow for elimination of variable practice standards that have been demonstrated to exist between separate institutions11–13. We argue that in respect to IBD, the relatively consistent clinical practice within a single academic center (secondary to formal IBD case conferences encouraging similar diagnostic and therapeutic approaches) can provide advantage over multi-center based studies, especially if those significantly depend on IBD subcategorization (i.e. differential diagnosis between UC and CD, and subclassification of UC) as is the case in our study.
In a pediatric single center study by Ordonez et al.14, pancolitis (Modified Montreal Classification)10 was found in 18 of 24 patients with UC and concomitant PSC, versus 8 of 27 patients with only UC (p=0.0006). The authors also observed that evolution of the colitis was less aggressive in colitis associated with autoimmune disease (CAI) than in classic UC (CUC). Another smaller retrospective study, which found 100% pancolitis among 13 PSC-IBD patients, made no comparisons to a non-PSC-IBD group as control15. On the contrary, a recent single center pediatric paper described a clinically similar course of colitis in 22 CAI patients compared to 45 CUC16. As comparison, our work examined 50% more patients: 34 “CAI” (more precisely: PSC-UC) and 95 “CUC” (more precisely: UC, non-PSC). These numbers are comparable to prior multicenter pediatric cohorts and carry the potential benefit to overcome center-based bias. Consistent with the adult studies, our findings revealed a relatively milder form of pancolitic UC in pediatric patients with co-diagnosis of PSC when compared to UC, non-PSC patients. Of note, all completely evaluated PSC-CD patients within our cohort had colonic involvement indicating a possible unique association between the large intestine and PSC.
A majority of our cohort had under a 6-month span between diagnoses of UC and PSC (n=22). Of the ten patients diagnosed initially with UC (i.e. >6 months between diagnoses), there was 44.8-month average lag period before diagnosis of PSC. These patients were found to have elevated liver enzymes (i.e. elevated AST, ALT, alkaline phosphatase, etc.) leading to subsequent investigation and diagnosis of PSC. On the contrary, there was only a 15-month lag period in the two patients initially diagnosed of PSC with subsequent UC diagnosis (i.e. >6 months between diagnoses). These findings are consistent with higher prevalence of UC in patients with a primary diagnosis of PSC (70–80%) versus lower prevalence of PSC in patients with a primary diagnosis of UC (2–7.5%) based on a study by Loftus et. al. These findings, including the prevalence of UC within our study’s PSC population (34 of 52 PSC patients) suggest need for IBD surveillance in all children diagnosed with PSC.
Within our cohort, there was a trend towards rectal sparing in PSC-UC when compared to our UC, non-PSC group. This finding is consistent with both adult and pediatric literature, as the characteristic of rectal sparing in PSC-UC is suggestive of distinct disease in contrast to “classic” standalone UC17, 18. Notably, there were no findings of adenocarcinoma or CCA in our pediatric cohort.
Historically, oral glucocorticoids have been a mainstay in treatment of UC with severe exacerbations. In our study, rates of steroid use (i.e. prednisone, prednisolone) in PSC-UC patients were lower than UC, non-PSC patients, which is consistent with the already emphasized, more quiescent form of PSC-UC. It should also be noted that Caucasian patients in our study had a lower rate of steroid use when compared to non-Caucasian PSC-UC patients. While this finding was statistically significant, it is hard to decipher specific reasons for such a medication trend, though one may consider ethnicity related differences in therapeutic responses. Infliximab, an anti-TNF agent, has been used in treatment against refractory IBD since its approval for use in the US in 199819, though approval of use of infliximab in pediatric CD and UC was not until 2006 and 2011, respectively. In a meta-analysis of the efficacy of biological therapies in IBD, results reported superior remission induction rates in infliximab versus placebo in moderate-to-severely active UC20. In our study, rates of infliximab use in PSC-UC patients was significantly lower than UC, non-PSC. Again, this likely reflects the clinically milder phenotype of PSC-UC, as infliximab has typically been reserved for steroid and/or thiopurine refractory cases of UC in our practice, similarly to other groups20, 21.
While there was no significant difference in treatment rates of patients in either PSC-UC or UC, non-PSC with thiopurines, there was a predilection of using azathioprine for PSC-UC, while UC, non-PSC was treated more often with 6-Mercaptopurine (6-MP). Based on our own observations, this pattern may represent clinical preference amongst our group’s gastroenterologists (i.e. hepatologists favoring azathioprine over 6-MP).
In cases of severe refractory ulcerative colitis poorly responsive to medical therapies, eventual surgical colectomy is usually pursued. In our study, there was a significantly lower rate of colectomy in PSC-UC compared to UC, non-PSC. This finding further emphasizes that a clinically less severe phenotype of UC is associated with PSC in pediatric patients.
Given the historical association between PSC and UC, our study made attempts to find markers of correlation between both diseases. Fecal calprotectin, a stable protein produced within neutrophils, has commonly been used as a simple non-invasive marker of IBD activity in both adults and children. Over the past 15 years, multiple studies, including two large meta-analyses by Henderson et al. and Holtman et al., have demonstrated the high sensitivity of fecal calprotectin in the setting of pediatric IBD activity22, 23. Gamma-glutamyl transpeptidase (GGT) is an enzyme found within multiple organs, but is frequently utilized as a marker of disease activity in the biliary system24. A recent pediatric study concluded that abnormal liver enzymes, including GGT, were commonly found in pediatric IBD with associated PSC and that elevated GGT levels (>252 U/L) were highly specific for PSC in pediatric IBD patients (specificity 99%, sensitivity 71%, AUC= 0.96)25. In our study, we used GGT and calprotectin as non-invasive biomarkers of PSC and UC activity, respectively. When comparing calprotectin levels in our PSC-UC population to paired GGT levels drawn within 14 days of each another, there was no significant correlation between these disease activity markers. Furthermore, we also compared the highest GGT value from each patient to documentation of IBD flare activity within two weeks of the elevated lab value. There was no significant correlation between elevated GGT in prediction of IBD flares leading to office visits, emergency room visits or hospital admissions. It should be noted, however, that the population analyzed is smaller than the total cohort, with only 67 of 95 patients in the control group with history of GGT lab values. While previous studies have concluded specificity of elevated liver enzymes for diagnosis of PSC in the setting of IBD, our study is the first to examine whether IBD activity may correlate with PSC activity or if the two disorders follow an independent course within PSC-UC patients. Based on non-invasive biomarkers (i.e. GGT, calprotectin), our findings appear to support the latter result, but with the inherent limitation of our calculations secondary to small sample size for this analysis.
Future directions include prospective studies following clinical management and outcomes of pediatric PSC-UC patients as they transition to adulthood. Other considerations include implementation of screening protocols for pediatric patients in our institution with UC for evaluation of PSC. This is especially important as nearly 30% of our PSC-UC population had more than a 6-month gap between the initial diagnosis of UC and the diagnosis of PSC.
Limitations of our data include a small sample size compared to adult cohorts, smaller cohort size in sub-analysis of GGT/calprotectin/IBD-based hospital admissions, incomplete or missing colonoscopy records among nearly half of the control patient group, and the retrospective nature of the analysis. The sample size in our single center, however, is comparable to other high impact observations from multicenter retrospective analyses in the pediatric PSC population.
In summary, our work suggests that pediatric PSC has a significant association with a milder form of pancolitic UC when compared with UC, non-PSC. This observation implicates the importance of right-sided (i.e. cecum and ascending colitis) UC related colon pathology in the etiology of PSC. Furthermore, PSC and UC activity appears to run an independent course amongst PSC-UC pediatric patients, with the limitation of a small cohort of patients studied. These findings warrant further larger scale and prospective studies, and are likely to provide useful information towards the etiology, management, and possible future treatment of pediatric PSC and related IBD.
SUMMARY BOX TEXT.
What is known about this subject? (3–4 bullet points)
Primary sclerosing cholangitis (PSC) is a rare cholestatic disease with inflammation/fibrosis of intra-and/or extra-hepatic bile ducts
In adults, PSC strongly associates with a clinically extensive but milder inflammatory bowel disease (IBD) phenotype
There is little evidence of etiology/treatment of PSC in either adults or children
What are the new findings and/or what is the impact on clinical practice?(3–4 bullet points)
Similar to adults, pediatric PSC appears to associate with a milder pancolitic-UC phenotype
Pediatric PSC-UC patients appear to require less medical therapy and have lower colectomy rates for IBD control than pediatric UC patients
In a smaller cohort subset with history of GGT lab collection, elevated GGT levels do not correlate with elevated calprotectin or UC-flare activity in pediatric PSC-UC
Acknowledgments
RK was supported by the Gutsy Kids Fund including philanthropic donation from the Karen and Brock Wagner family. FDI was supported by grant NIH T32 DK007664-24S1.
References
- 1.Miloh T, Arnon R, Shneider B, et al. A retrospective single-center review of primary sclerosing cholangitis in children. Clin Gastroenterol Hepatol. 2009;7:239–45. doi: 10.1016/j.cgh.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Rossi RE, Conte D, Massironi S. Primary sclerosing cholangitis associated with inflammatory bowel disease: an update. Eur J Gastroenterol Hepatol. 2016;28:123–31. doi: 10.1097/MEG.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 3.Deneau M, Jensen MK, Holmen J, et al. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013;58:1392–400. doi: 10.1002/hep.26454. [DOI] [PubMed] [Google Scholar]
- 4.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 5.de Vries AB, Janse M, Blokzijl H, et al. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956–71. doi: 10.3748/wjg.v21.i6.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar S, Bowlus CL. Primary Sclerosing Cholangitis: Multiple Phenotypes, Multiple Approaches. Clin Liver Dis. 2016;20:67–77. doi: 10.1016/j.cld.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claessen MM, Vleggaar FP, Tytgat KM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–64. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 9.Lascurain L, Jensen MK, Guthery SL, et al. Inflammatory Bowel Disease Phenotype in Pediatric Primary Sclerosing Cholangitis. Inflamm Bowel Dis. 2016;22:146–50. doi: 10.1097/MIB.0000000000000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 11.Farmer M, Petras RE, Hunt LE, et al. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol. 2000;95:3184–8. doi: 10.1111/j.1572-0241.2000.03199.x. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN, Kwon J, Raffals L, et al. Variation in treatment of patients with inflammatory bowel diseases at major referral centers in the United States. Clin Gastroenterol Hepatol. 2015;13:1197–200. doi: 10.1016/j.cgh.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronman MP, Gerber JS, Prasad PA, et al. Variation in Antibiotic Use for Children Hospitalized With Inflammatory Bowel Disease Exacerbation: A Multicenter Validation Study. J Pediatric Infect Dis Soc. 2012;1:306–13. doi: 10.1093/jpids/pis053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordonez F, Lacaille F, Canioni D, et al. Pediatric ulcerative colitis associated with autoimmune diseases: a distinct form of inflammatory bowel disease? Inflamm Bowel Dis. 2012;18:1809–17. doi: 10.1002/ibd.22864. [DOI] [PubMed] [Google Scholar]
- 15.Yoon J, Oh SH, Kim HJ, et al. Primary Sclerosing Cholangitis with Inflammatory Bowel Disease in Korean Children. Pediatr Gastroenterol Hepatol Nutr. 2015;18:268–75. doi: 10.5223/pghn.2015.18.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinet-Charpentier C, Champigneulle J, Williet N, et al. The association of autoimmune diseases with pediatric ulcerative colitis does not influence its disease course. Scand J Gastroenterol. 2016;51:33–40. doi: 10.3109/00365521.2015.1058415. [DOI] [PubMed] [Google Scholar]
- 17.Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–51. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Bjarnason I, Hayee B, Pavlidis P, et al. Contrasting Pattern of Chronic Inflammatory Bowel Disease in Primary and Autoimmune Sclerosing Cholangitis. EBioMedicine. 2015;2:1523–7. doi: 10.1016/j.ebiom.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malaty HM, Abraham BP, Mehta S, et al. The natural history of ulcerative colitis in a pediatric population: a follow-up population-based cohort study. Clin Exp Gastroenterol. 2013;6:77–83. doi: 10.2147/CEG.S40259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–59. doi: 10.1038/ajg.2011.73. quiz 660. [DOI] [PubMed] [Google Scholar]
- 21.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 22.Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–7. doi: 10.1097/00005176-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:637–45. doi: 10.1038/ajg.2013.131. [DOI] [PubMed] [Google Scholar]
- 24.Lum G, Gambino SR. Serum gamma-glutamyl transpeptidase activity as an indicator of disease of liver, pancreas, or bone. Clin Chem. 1972;18:358–62. [PubMed] [Google Scholar]
- 25.Valentino PL, Feldman BM, Walters TD, et al. Abnormal Liver Biochemistry Is Common in Pediatric Inflammatory Bowel Disease: Prevalence and Associations. Inflamm Bowel Dis. 2015;21:2848–56. doi: 10.1097/MIB.0000000000000558. [DOI] [PubMed] [Google Scholar]