Abstract
Background
Research relating either prenatal or concurrent measures of phthalate exposure to thyroid function in preschool children is inconclusive.
Methods
In a study of inner-city mothers and their children, metabolites of di-n-butyl phthalate, butylbenzyl phthalate, di-isobutyl phthalate, di(2-ethylhexyl) phthalate, and diethyl phthalate were measured in a spot urine sample collected from women in late pregnancy and from their children at age 3 years. We measured children's serum free thyroxine (FT4) and thyroid stimulating hormone (TSH) at age 3. Linear regression models were used to investigate the associations between phthalate metabolites, measured in maternal urine during late pregnancy and measured in child urine at age 3 and thyroid function measured at age 3.
Results
Mean concentrations (ranges) were 1.42 ng/dL (1.02 - 2.24) for FT4, and 2.62 ulU/mL (0.61 – 11.67) for TSH. In the children at age 3, among girls, FT4 decreased with increasing loge mono-n-butyl phthalate [estimated b = -0.06; 95% CI: (-0.09, -0.02)], loge mono-isobutyl phthalate [b = -0.05; 95% CI: (-0.09, -0.01)], loge monoethyl phthalate [b = -0.04; 95% CI: (-0.07, -0.01)], and loge mono(2-ethyl-5-hydroxyhexyl) phthalate [b = -0.04; 95% CI: (-0.07, -0.003)] and loge. mono(2-ethyl-5-oxy-hexyl) phthalate [b = -0.04; 95% CI: (-0.07, -0.004)] In contrast, among boys, we observed no associations between FT4 and child phthalate metabolites at age 3. On the other hand, in late gestation, FT4 increased with increasing loge mono-(2-ethylhexyl) phthalate [estimated b = 0.04; 95% CI: (0.02, 0.06)] and no sex difference was observed. We found no associations between phthalate biomarkers measured in either the child or prenatal samples and TSH at age 3.
Conclusions
The data show inverse and sex specific associations between specific phthalate metabolites measured in children at age 3 and thyroid function in preschool children. These results may provide evidence for the hypothesis that reductions in thyroid hormones mediate associations between early life phthalate exposure and child cognitive outcomes.
Introduction
Phthalates are high production chemicals found in various industrial and personal care products. Biomonitoring studies have established that phthalate exposure in the United States is ubiquitous [Centers for Disease Control and Prevention (CDC) 2017]. In humans, phthalates metabolize into monoesters which can undergo further transformations into oxidative metabolites and/or phase II conjugates and excreted primarily in urine (Heudorf et al. 2007). Phthalates have short biological half-lives and are generally eliminated within twenty-four hours (Wittassek and Angerer 2008). Urine is the preferred metric of exposure in epidemiologic studies because there is low urinary enzymatic activity; therefore, metabolite concentrations in urine reflect an individual's internal exposure rather than contaminants introduced during collection and processing (Frederiksen et al. 2007; Hogberg et al. 2008).
Evidence suggests that exposure to certain phthalates may be associated with adverse health effects in both children and adults (Autian 1973; Boas et al. 2006; Factor-Litvak et al. 2014; Huang et al. 2007; Meeker et al. 2007; Meeker and Ferguson 2011). Animal and human studies report associations between exposure to some phthalates (measured as their urinary metabolites) and reproductive and endocrine system abnormalities, and, in particular disruptions in thyroid function (Boas et al. 2010; Carlson et al. 2010; de Cock et al. 2014; Foster 2006; Hinton et al. 1986; Howarth et al. 2001; Huang et al. 2007; Kuo et al. 2015;Meeker et al. 2007; Meeker and Feguson 2011; O'Connor et al. 2002; Poon et al. 1997; Sugiyama et al. 2005; Swan et al. 2005; Swan et al. 2008; Suzuki et al. 2012). However, most of these studies were cross-sectional, assessing exposure to phthalates and thyroid function at the same point in time. Further, with only one exception, all studies assessed associations among adolescents or adults, leaving a gap in the literature regarding the associations in children. With respect to the developmental origins of health, there is also a gap in the literature regarding associations between maternal exposures to phthalates and thyroid function in their children. Adequate and timely production of maternal thyroid hormone is associated with fetal cerebral cortex development (Bernal 2000), childhood and adult metabolic function, and childhood cognitive and behavioral development (Morreale de Escobar et al. 2004; Moog et al. 2015). Sufficient production of thyroid hormones during childhood is necessary for continued brain development (Kapoor et al. 2015). Consequently, the potential thyroid disrupting effects of phthalates are especially important in young children as they may be more susceptible to environmental exposures than adults. However, aside from a study by Boas et al. (2010), which found that specific urinary phthalate metabolite concentrations during childhood were associated with decreased thyroid hormones (total and free triiodothyronine, T3) and a study which assessed exposure via the consumption of tainted foodstuffs (Wu et al. 2013), the effect of phthalate exposure on thyroid function has not been assessed in pre-school age children.
We used data from the Columbia Center for Children's Environmental Health (CCCEH) study to assess whether there is an association between prenatal and child exposure to five phthalates, di-n-butyl phthalate (DnBP), di-isobutyl phthalate (DiBP), butylbenzyl phthalate (BBzP), diethyl phthalate (DEP), and di(2-ethylhexyl) phthalate (DEHP) and thyroid function in pre-school age children.
Materials and Methods
Our study population derives from the CCCEH longitudinal birth cohort of 727 pregnant women who delivered between 1998 and 2006. Enrollment and exclusion criteria have been described previously (Perera et al. 2003). The CCCEH cohort was restricted to nonsmoking women 18–35 years of age who self-identified as either African American or Dominican and who had resided in northern Manhattan or the South Bronx in New York City for at least 1 year before pregnancy. Women were excluded if they used illicit drugs, had diabetes, hypertension, or known HIV, or had their first prenatal visit after the 20th week of pregnancy. The study was approved by the Columbia University Medical Center and CDC institutional review boards (IRBs). Study procedures were explained at enrollment, and each woman signed an IRB-approved consent form.
For this analysis we focused on two distinct, but overlapping, samples. First, we studied 229 offspring at age 3 years for whom both urinary phthalate metabolites and serum FT4 and TSH were measured. This will be denoted the child sample. Second we studied 181 mother – child pairs for whom maternal urinary phthalate metabolites were measured at the end of pregnancy and child thyroid hormones (FT4 and TSH) were measured at child age 3. This will be denoted the maternal-child sample. These two groups did not differ significantly from the remaining subjects in the CCCEH cohort in terms of basic demographics (maternal age, prenatal marital status and education level, household income, proportion on Medicaid or other public assistance) or on child sex, gestational age, and birth weight (all p-values > 0.05), except that children in the maternal-child sample were more likely to be African American than Dominican (p-values < 0.001). A total of 143 children overlapped between the child and maternal-child samples.
Questionnaire and medical record data
Medical records regarding mothers' and infants' general health were collected after delivery. Data included gestational age, infant sex, birth weight, length, and head circumference, complications of pregnancy, medication use, and mode of delivery. A 45-min questionnaire was administered by a bilingual interviewer to each woman, in her home, during the third trimester of pregnancy. Information on demographics, race/ethnicity, home characteristics and residential history, history of active and passive smoking, occupational history, marital status, education and income level, prenatal alcohol and drug use, and maternal psychosocial conditions was collected (Whyatt et al. 2012).
Urine sample collection and phthalate metabolite measurements
We collected a spot urine sample from the women during the third trimester of pregnancy (mean ± standard deviation (SD), 33.8 ± 3.2 weeks of gestation; median, 33 weeks). We also collected child spot urine samples at the age 3 years visits (37.2 ± 2.4 months; 36.3 months) between 2001 and 2007. The samples were stored at Columbia University at −80°C, shipped to the CDC on dry ice, and stored at or below −70°C. Urinary phthalate metabolite concentrations were measured at the CDC in two batches: half were analyzed between 2008 and 2009; the remainder analyzed between 2010 and 2013, with analytical runs that included calibration standards, reagent blanks, and quality control samples as described previously (Kato et al. 2005). For concentrations of phthalate metabolites below the limit of detection, we were assigned a value of LOD/√2 (Hornung and Reed 1990), as we have done previously (Factor-Litvak et al. 2014). We used specific gravity to correct for urinary dilution as recommended for phthalates (Hauser et al. 2004). Specific gravity was measured using a handheld refractometer and added to regression models to control for urinary dilution (Atago PAL 10-S; Atago U.S.A. Inc., Bellevue, WA). We evaluated the following metabolites: mono(2-ethylhexyl) phthalate (MEHP),mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxy- hexyl) phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP), metabolites of DEHP, mono-n-butyl phthalate (MnBP), a metabolite of DBP, monobenzyl phthalate (MBzP), a metabolite of BBzP, mono-isobutyl phthalate (MiBP),a metabolite of DiBP, and monoethyl phthalate (MEP), a metabolite of DEP.
Serum sample collection and thyroid hormone measurements
Free-thyroxine (FT4) and thyroid stimulating hormone (TSH) were measured in sera from children at age three years. Samples were stored at −80°C in multiple aliquots, and analyzed under the supervision of Gary Bradwin in the laboratory of Dr. Nadir Rafai, Clinical and Epidemiologic Research Laboratory, Department of Laboratory Medicine, Boston Children's Hospital. FT4 was measured using a competitive immunoassay, approved by the Food and Drug Administration (FDA) for clinical use, on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN). The lowest detection limit of this assay was 0.023 ng/dL and the day-to-day imprecision values were at concentrations of 0.68, 1.64 and 3.94 ng/dL are 3.5, 3.3 and 6.6%, respectively. TSH was measured by a competitive electrochemiluminescence immunoassay, approved by the FDA for clinical use, on the 2010 Elecsys autoanalyzer. The lowest detection limit of this assay was 0.005 uIU/mL and the day-to-day imprecision values at concentrations of 0.084, 0.91 and 3.96 uIU/mL are 5.4, 2.1 and 1.8%, respectively.
Statistical analysis
We calculated summary statistics to describe demographics, phthalate metabolite concentrations and thyroid function. Chi-square and Wilcoxon rank sum test were used to detect group differences in categorical and quantitative variables, respectively, and Spearman correlation coefficients were calculated to assess the associations between quantitative variables. Potential covariates were selected on the basis of their previously reported associations with either prenatal or child phthalate exposure or child thyroid function and included weight, height (both measured at child age 3), child sex, and ethnicity (Dominican vs. African American) (Boas et al. 2010; Huang et al. 2007 Meeker et al. 2007; Meeker et al. 2011). In our samples, FT4 and TSH were not correlated with height or weight (Spearman's correlation r-values ranged from 0 to 0.05). In bivariate analyses, we examined the relationships between the potential covariates and thyroid and phthalate metabolite concentrations. Since both the phthalate metabolite concentrations and the TSH distribution were right-skewed, we applied natural logarithmic transformation to reduce the impact of extreme values and improve model fit. To examine the associations between urinary phthalate metabolite concentrations and the level of thyroid hormones FT4 and TSH, we used linear models. We performed regression analysis using the entire analytic sample as well as separately by sex of child. Based on the bivariate analysis, our final models for both the child and prenatal samples adjusted for child sex (only with the entire sample), ethnicity, and corresponding urine specific gravity. The Wald test was used to detect sex differences in the regression coefficients for associations between the phthalate metabolite concentrations and thyroid function measures.
For the 143 children who overlapped in the child and maternal–child samples, we evaluated the associations between phthalate metabolites at both measures simultaneously and either FT4 or TSH. All statistical tests were two sided with a significance level of 0.05. Analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, NC).
Results
As Table 1 indicates, African American children were more likely to have phthalate metabolite data at age 3 compared to Dominican children (p < 0.001). No other significant differences in sociodemographic and anthropometric characteristics between the child or the prenatal sample and the total cohort were seen.
Table 1. Comparisons of demographic distributions and weight and height measurements at three years of age among children in the Total cohort, the Child and Prenatal samplesa.
| Characteristic | Total Cohort 727 (%) | Child Sample 229 (%) | Prenatal Sample 181(%) |
|---|---|---|---|
| Maternal age at delivery | 25.1 (4.9) | 25.3 (5.1) | 24.9 (46) |
| Race/Ethnicity | |||
| Dominican American | 473 (65.1) | 128 (55.9) | 114 (63.0) |
| African American | 254 (34.9) | 101 (44.1) | 67 (37.0) |
| Marital status (n=721) | |||
| Never Married | 473 (66.6) | 155 (68.0) | 125 (69.1) |
| Ever Married | 246 (34.4) | 73 (32.0) | 56 (30.9) |
| Maternal education (n=713) | |||
| < High school degree | 175 (35.9) | 82 (36.4) | 66 (36.5) |
| ≥ High school degree or GED | 313 (64.1) | 143 (63.6) | 115 (63.5) |
| Sex | |||
| Girls | 376 (51.7) | 120 (52.4) | 99 (54.7) |
| Boys | 351 (48.3) | 109 (47.6) | 82 (45.3) |
| Birth Weight (kg) | 3,369.8 (476.4)b | 3,375.3 (512.1)c | 3,421.4 (478.70)d |
| Gestational Age (weeks) | 39.3 (1.4)e | 39.3 (1.5)e | 39.4 (1.3)d |
| Child age at phthalate assessment (months) | 37.1 (2.3)f | 37.2 (2.4) | 37.0 (2.1)g |
| Physical Measurement at 3 years | n=499 | n=214 | n=161 |
| Weight(kg) Mean ± SD | 16.5 ± 3.1 | 16.4 ± 3.0 | 16.2 ± 2.7 |
| Height (cm) Mean ± SD | 98.3 ± 5.0 | 98.3 ± 5.5 | 98.3 ± 5.2 |
Analyses include one subject who did not have FT4 (free thyroxine) measurements but had TSH (thyroid-stimulating hormone) measurements.
Birth weight available on n=709.
Birth weight available on n=227.
Birth weight and gestational age available on n=179.
Gestational age available on n=717.
Gestational age available on n=228.
Child age at phthalate assessment n=428
Child age at phthalate assessment n=199
The mean concentrations of FT4 in the child and prenatal samples were 1.43 (± 0.17, standard deviation) ng/dL and 1.40 (± 0.19) ng/dL, respectively. Correspondingly, the mean concentrations of TSH in the child and prenatal samples were 2.56 (± 1.21) and 2.62 (± 1.39) uIU/mL, respectively. No differences in FT4 were found between boys and girls, or between African American and Dominican participants (Table 2). However, Dominican children had significantly higher TSH concentrations than African American children in both samples (child p <0.01 and prenatal p <0.001).
Table 2.
Distribution of thyroid hormones, FT4 and TSH in the total samples and according to sex and race/ethnicity, at age three years.*
| Child Sample | Prenatal Sample | |||
|---|---|---|---|---|
| FT4 | Mean ± SD ng/dL | Range ng/dL | Mean ± SD ng/dL | Range ng/dL |
| Total Sample | (n=228) 1.43 ± 0.17 | 1.02 – 2.14 | (n=180) 1.40 ± 0.19 | 1.03 – 2.24 |
| Sex | ||||
| Girls | (n=119) 1.43 ± 0.18 | 1.02 – 2.14 | (n=98) 1.41 ± 0.19 | 1.03 – 2.14 |
| Boys | (n=109) 1.42 ± 0.16 | 1.06 – 2.03 | (n=82) 1.39 ± 0.20 | 1.06 – 2.24 |
| Race/Ethnicity | ||||
| Dominican Americans | (n=127) 1.42 ± 0.18 | 1.02 – 2.14 | (n=113) 1.40 ± 0.21 | 1.03 – 2.24 |
| African Americans | (n=101) 1.44 ± 0.16 | 1.10 – 1.75 | (n=67) 1.40 ± 0.16 | 1.10 – 1.75 |
| TSH | Mean ± SD uIU/mL | Range uIU/mL | Mean ± SD uIU/mL | Range uIU/mL |
| Total Sample | (n=229) 2.56 ± 1.21 | 0.61 – 7.04 | (n=181) 2.62 ± 1.39 | 0.61 – 11.67 |
| Sex | ||||
| Girls | (n=120) 2.59 ± 1.23 | 0.61 – 7.04 | (n=99) 2.67± 1.31 | 0.61 – 7.04 |
| Boys | (n=109) 2.54 ± 1.18 | 0.70 – 6.84 | (n=82) 256 ± 1.48 | 0.70 – 11.67 |
| Race/Ethnicity | ||||
| Dominican Americans | (n=128) 2.74 ± 1.16 | 0.68 – 7.04 | (n=114) 2.86 ± 1.48 | 0.68 – 11.67 |
| African Americans | (n=101) 2.34 ± 1.23 | 0.61 – 6.84 | (n=67) 2.20 ± 1.11 | 0.61 – 5.10 |
FT4= free thyroxine; TSH= thyroid-stimulating hormone.
Phthalate metabolites were detected in 99–100% of the urine samples, except for MEHP in which detection ranged from 80% to 90% (Table 3). Child MEHHP, MBzP, MEP, MEOHP and MECPP concentrations measured at age 3 were significantly higher in African American than in Dominican children, adjusting for specific gravity (supplemental Table 1). In the prenatal sample, adjusting for specific gravity, MBzP concentrations were significantly higher in African American than in Dominican women, while both MEHHP and MECPP concentrations were significantly higher in Dominican women. While child MEHP, MnBP, and MBzP concentrations were higher in boys than in girls and MEHHP, MIBP, MEOHP, and MCPP were somewhat higher in girls, the sex differences were not statistically significant. In contrast, prenatal MEHP concentrations were significantly higher in girls than in boys.
Table 3. Distribution of phthalate metabolites (ng/mL) in spot urine in child (n=229) and prenatal sample (n=181) *.
| Child Sample | Prenatal Sample | |||||
|---|---|---|---|---|---|---|
| Metabolite | % > LODa | Geometric Meanb (95% CI) | Range | % > LODa | Geometric Meanb (95% CI) | Range |
| MEHP | 83.0 | 3.2 (2.8, 3.7) | <LOD to 47 | 86.7 | 5.7 (4.7, 6.9) | <LOD to 213 |
| MEHHP | 99.6 | 32.8 (27.9, 38.5) | <LOD to 646 | 100 | 23.6 (19.6, 28.5) | 1.1 – 1380 |
| MnBP | 100 | 41.9 (35.8, 48.9) | 1.9 – 2020 | 100 | 37.8 (32.2, 44.5) | 1.2 – 1110 |
| MBzP | 100 | 23.0 (18.8, 28.3) | 0.3 – 2254 | 99.4 | 15.3 (12.4, 18.8) | <LOD to 550 |
| MiBP | 99.6 | 10.3 (8.7, 12.1) | <LOD to 260 | 98.9 | 8.3 (7.1, 9.6) | <LOD to 89 |
| MEP | 100 | 138.6 (116.8, 164.6) | 8.0 – 5848 | 100 | 161.8 (135.6, 193.0) | 7.9 – 3986 |
| MEOHP | 99.6 | 19.2 (16.4, 22.5) | <LOD to 345 | 100 | 19.7 (16.4, 23.7) | 0.9 – 795 |
| MECPP | 100 | 61.0 (52.9, 70.3) | 1.2– 933 | 100 | 41.6 (35.2, 49.2) | 3.2 – 1210 |
Concentrations are not adjusted for specific gravity.
Limit of detection (LOD) was 0.25–1.2 ng/mL for MEHP, 0.35– 0.7 for MEHHP; and 0.10– 0.30 ng/mL for MiBP, 0.35–0.7 ng/mL for MEOHP.
Used imputed values to calculate geometric mean
MEHP=mono(2-ethylhexyl) phthalate, MEHHP=mono(2-ethyl-5-hydroxyhexyl) phthalate, MnBP=mono-n-butyl phthalate, MBzP=monobenzyl phthalate, MiBP=mono-isobutyl phthalate, MEP=monoethyl phthalate, MEOHP=mono(2-ethyl-5-oxohexyl) phthalate, MECPP=mono(2-ethyl-5-carboxypentyl) phthalate,
In the child sample, FT4 was significantly inversely associated with loge MnBP [estimated -coefficient b= −0.04; 95% confidence interval (CI): (−0.07, −0.01)], controlling for specific gravity, sex, and ethnicity (Table 4, Figure 1). In girls, loge MEHHP [b = -0.04; 95% CI: (-0.07, -0.003)], loge MnBP [b = -0.06; 95% CI: (-0.09, -0.02)], loge MiBP [b = -0.05; 95% CI: (-0.09, -0.01)], loge MEP [b = -0.04; 95% CI: (-0.07, -0.01)], and loge MEOP [b = -0.04; 95% CI: (-0.07, -0.004)] were significantly associated with FT4. No significant associations were found between phthalate metabolite concentrations and TSH.
Table 4.
Estimated regression coefficients relating child urinary phthalate concentrations to thyroid function at child age 3 yearsa.
| FT4 | ||||
|---|---|---|---|---|
|
| ||||
| Metabolite (loge) | Totalb (n=228) | Girlsb (n=119) | Boys (n=109) | Sex Diff P value |
|
| ||||
| b (95% CI) | ||||
|
| ||||
| MEHP | 0.003 (-0.02, 0.03) | -0.001 (-0.04, 0.04) | 0.01 (-0.02, 0.04) | 0.65 |
| MEHHP | -0.02 (-0.04, 0.01) | -0.04 (-0.07, -0.003)* | 0.01 (-0.03, 0.04) | 0.06 |
| MnBP | --0.04 (-0.07,-0.01)** | -0.06 (-0.09, -0.02)** | -0.01(-0.05, 0.03) | 0.11 |
| MBzP | -0.02 (-0.03, 0.003) | -0.01(-0.04, 0.02) | -0.01 (-0.04, 0.01) | 0.08 |
| MiBP | -0.02 (-0.05, 0.002) | -0.05 (-0.09, -0.01)** | 0.001 (-0.03, 0.04) | 0.0501 |
| MEP | -0.02 (-0.04, 0.01) | -0.04 (-0.07, -0.01)** | 0.01 (-0.02, 0.04) | 0.02 |
| MEOHP | -0.02 (-0.05, 0.01) | -0.04 (-0.07, -0.004)* | 0.01 (-0.03, 0.04) | 0.07 |
| MECPP | -0.01 (-0.04, 0.02) | -0.04 (-0.08, 0.01) | 0.01 (-0.02, 0.06) | 0.06 |
|
| ||||
| Loge(TSH) | ||||
|
| ||||
| Metabolite (loge) | Total (n=229) | Girls (n=120) | Boys (n=109) | Sex Diff P value |
|
| ||||
| b (95% CI) | ||||
|
| ||||
| MEHP | 0.05 (-0.03, 0.12) | 0.02 (-0.07, 0.15) | 0.05 (-0.05, 0.15) | 0.90 |
| MEHHP | 0.004 (-0.07, 0.08) | -0.02 (-0.12, 0.08) | 0.03 (-0.08, 0.13) | 0.56 |
| MBP | -0.02 (-0.10, 0.05) | -0.04 (-0.15, 0.06) | 0.01 (-0.11, 0.12) | 0.54 |
| MBzP | 0.01 (-0.04, 0.06) | -0.01 (-0.08, 0.07) | 0.02 (-0.05, 0.10) | 0.55 |
| MiBP | -0.04 (-0.12, 0.03) | -0.02 (-0.13, 0.08) | -0.06 (-0.17, 0.04) | 0.59 |
| MEP | -0.03 (-0.09, 0.03) | -0.04 (-0.13, 0.05) | -0.02 (-0.11, 0.06) | 0.79 |
| MEOHP | 0.01 (-0.07, 0.08) | -0.03 (-0.12, 0.07) | 0.04 (-0.07, 0.15) | 0.36 |
| MECPP | -0.03 (-0.12, 0.05) | -0.11 (-0.23, 0.01) | 0.04 (-0.08, 0.17) | 0.09 |
Linear models controlled for specific gravity, ethnicity, and child sex (total analyses).
The total and female samples included one subject who did not have FT4 (free thyroxine) but had TSH (thyroid-stimulating hormone) measurement.
For MCOP, the total sample=171, female sample=88, and boy sample=83 subjects.
For MCOP, the total sample=172, female sample=89, and boy sample=83 subjects.
MEHP=mono(2-ethylhexyl) phthalate, MEHHP=mono(2-ethyl-5-hydroxyhexyl) phthalate, MnBP=mono-n-butyl phthalate, MBzP=monobenzyl phthalate, MiBP=mono-isobutyl phthalate, MEP=monoethyl phthalate. MEOHP=mono(2-ethyl-5-oxohexyl) phthalate, MECPP=mono(2-ethyl-5-carboxypentyl) phthalate,
p < 0.05,
p ≤ 0.01.
Figure 1.
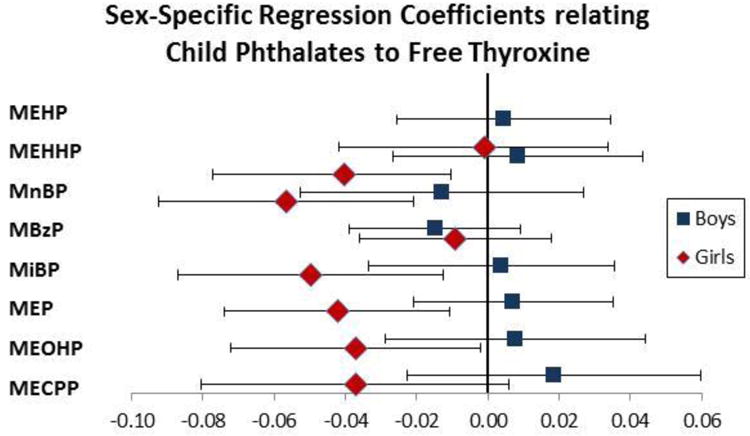
Estimated regression coefficients of child phthalate metabolites and FT4 levels at age 3 in boys (squares) and girls (diamonds). The x-axis represents the change in FT4 level associated with a log unit increase in phthalate metabolite.
MEHP=mono(2-ethylhexyl phthalate), MEHHP=mono(2-ethyl-5-hydroxyhexyl) phthalate, MnBP=monon-butyl phthalate, MBzP=monobenzyl phthalate, MiBP=mono-isobutyl phthalate, MEP=monoethyl phthalate. MEOHP=mono(2-ethyl-5-oxy- hexyl) phthalate, MECPP=mono-2-ethyl-5-carboxypentyl phthalate,
In the prenatal sample, only loge MEHP was significantly and positively associated with child FT4 in the entire sample [b = 0.04; 95% CI: (0.02, 0.07)], as well as in the sex stratified analyses (girls: [b = 0.04; 95% CI: (0.005, 0.07)]; boys: [b = 0.06; 95% CI: (0.02, 0.10)]) (Table 5).
Table 5.
Estimated regression coefficients relating prenatal urinary phthalate metabolites concentrations to thyroid function at child age 3 yearsa.
| Maternal Metabolite (loge) | Total (n=180) | Girls (n=98) | Boys (n=82) | Sex Diff P Value |
|---|---|---|---|---|
|
| ||||
| FT4 b (95% CI) | ||||
|
| ||||
| MEHP | 0.04 (0.02, 0.07)*** | 0.04 (0.005, 0.07)* | 0.06 (0.02, 0.10)** | 0.44 |
| MEHHP | 0.02 (-0.004, 0.05) | 0.02 (-0.01, 0.05) | 0.03 (-0.02, 0.09) | 0.67 |
| MnBP | -0.01 (-0.05, 0.02) | -0.02 (-0.06, 0.03) | -0.004 (-0.06, 0.05) | 0.70 |
| MBzP | -0.02 (-0.05, 0.005) | -0.02 (-0.06, 0.01) | -0.02 (-0.06, 0.02) | 0.98 |
| MiBP | 0.02 (-0.02, 0.05) | 0.01 (-0.04, 0.05) | 0.03 (-0.03, 0.09) | 0.54 |
| MEP | -0.01 (-0.04, 0.02) | 0.003 (-0.04, 0.04) | -0.02 (-0.07, 0.02) | 0.42 |
| MEOHP | 0.02 (-0.01, 0.05) | 0.02 (-0.01, 0.05) | 0.02 (-0.03, 0.08) | 0.89 |
| MECPP | 0.03 (-0.002, 0.06) | 0.03 (-0.01, 0.07) | 0.03 (-0.02, 0.09) | 0.93 |
|
| ||||
| Maternal Metabolite (loge) | Total (n=181) | Girls (n=99) | Boys (n=82) | Sex Diff P Value |
|
| ||||
| Loge(TSH) b (95% CI) | ||||
|
| ||||
| MEHP | -0.06 (-0.13, 0.001) | -0.04 (-0.12, 0.04) | -0.07 (-0.17, 0.03) | 0.65 |
| MEHHP | -0.06 (-0.13, 0.01) | -0.05 (-0.13, 0.03) | -0.05 (-0.18, 0.07) | 0.99 |
| MnBP | -0.08 (-0.17, 0.01) | -0.12 (-0.23, 0.003) | -0.02 (-0.15, 0.11) | 0.26 |
| MBzP | -0.02 (-0.08, 0.05) | -0.06 (-0.15, 0.03) | 0.03 (-0.07, 0.12) | 0.17 |
| MiBP | -0.06 (-0.15, 0.03) | -0.01 (-0.13, 0.11) | -0.13 (-0.26, 0.01) | 0.20 |
| MEP | 0.04 (-0.03, 0.12) | 0.02 (-0.08, 0.13) | 0.07 (-0.04, 0.18) | 0.54 |
| MEOHP | -0.06 (-0.13, 0.01) | -0.05 (-0.14, 0.04) | -0.05 (-0.18, 0.07) | 0.99 |
| MECPP | -0.06 (-0.14, 0.01) | -0.07 (-0.17, 0.02) | -0.01 (-0.14, 0.12) | 0.46 |
Adjusted for child age 3 specific gravity, ethnicity, and child sex (total sample).
FT4 =free thyroxine, TSH=thyroid-stimulating hormone.
MEHP=mono(2-ethylhexyl) phthalate, MEHHP=mono(2-ethyl-5-hydroxyhexyl) phthalate, MnBP=mono-n-butyl phthalate, MBzP=monobenzyl phthalate, MiBP=mono-isobutyl phthalate, MEP=monoethyl phthalate. MEOHP=mono(2-ethyl-5-oxyohexyl) phthalate, MECPP=mono(2-ethyl-5-carboxypentyl) phthalate,
p < 0.05,
p ≤ 0.01,
p ≤ 0.001.
Finally, we examined associations between prenatal phthalate metabolites, child phthalate metabolites at age 3 years and child thyroid at age 3 among our participants who were included in both the child and prenatal samples (n=143). The correlations between prenatal and child age 3 phthalate metabolite concentrations ranged from 0.01 to 0.22 (supplemental Table 2). Child loge MnBP was inversely associated with FT4 in the total sample [b = -0.04; 95% CI: (-0.08, -0.003)] and in girls [b = -0.06; 95% CI: (-0.10, -0.01)], controlling for the prenatal MnBP. Similarly, child loge MEP was inversely associated with FT4 [b = -0.04; 95% CI: (-0.09, 0.002); p=0.06] in girls, controlling for the prenatal loge MEP. Prenatal loge MEHP was positively associated with FT4 in the total sample [b = 0.04; 95% CI: (0.01, 0.06)] and in boys [b = 0.04; 95% CI: (0.002, 0.08)], but marginally significant in girls [b = 0.03; 95% CI: (-0.002, 0.06); p=0.06], controlling for the child loge MEHP (supplemental Table 3).
Discussion
In the cross-sectional analysis, we found associations between several urinary phthalate metabolites and thyroid function in children at age 3 years. In girls, we found that the concentrations of the non-DEHP metabolites evaluated (MnBP, MiBP, MBzP, and MEP) were inversely associated with FT4. These child associations were all in the expected and hypothesized direction. Investigators have reported that disturbances in thyroid function are more prevalent among women (Hollowell et al. 2002; McGrogan et al. 2008). Thus, girls may be more vulnerable to the effect of thyroid disrupting chemicals even in early childhood. We also observed that prenatal MEHP (a DEHP metabolite) concentration was positively associated with child FT4 in girls and boys, a result that we did not expect. The different results obtained from the child and prenatal samples may possibly reflect the different effects of prenatal and early childhood phthalate exposure on child thyroid hormones.
We did not find associations between phthalate exposure and TSH. This is not surprising as the regulation of TSH is controlled by a variety of mechanisms, including direct regulation by thyroid hormones acting on the pituitary, as well as indirectly through the effect of thyroid hormones on thyrotropin-releasing hormone and other neuropeptides at the level of the hypothalamus (Lazar et al. 1993; Yen, 2001; Zhang and Lazar 2000). However, a more plausible reason is the variability in the measurement of TSH. For example, Andersen et al (2002) in a study of 16 adult men with repeat thyroid hormone measures found that it would require 5 independent measures of TSH to be within 25% of the ‘target value’ and 25 measures to be within 10% of the ‘target value’. A review of the literature on subclinical hypothyroidism in children found that in most studies, TSH elevations decreased over the ensuing 5-10 years without treatment (Kaplowitz 2010). We therefore conclude that a single measure of TSH, especially in children, is subject to measurement error, and that such measurement error may bias the results toward the null.
We note that the phthalate metabolite urinary concentrations in our population were similar to those in other studies of children and adolescents (Boas et al. 2010; Meeker and Ferguson 2011; Silva et al. 2004; Teitelbaum et al. 2008; Wolff et al. 2007) and specifically in studies that assessed the associations between phthalates and thyroid function (Boas et al. 2010; Meeker et al. 2007; Meeker and Ferguson 2010). The concentrations of FT4 and TSH in our sample were well within the reference ranges for children; for FT4 the range is 0.8-2 ng/dL and for TSH the range is 0.55-7.10 μIU/mL for boys and 0.46-8.10 μIU/mL for girls (www.curezone.org/upload/_I_J_Forums/Iodine/Maniek/Pediatric_Reference_Ranges_Endocrinology_0981.pdf (accessed 4/6/2017)). Only two other studies (Boas et al. 2010; Wu et al. 2013) considered these relationships in populations which include preschoolers. Boas et al. (2010) evaluated associations between phthalate metabolite concentrations, collected in 2006-2007, and thyroid hormone levels in Danish children of comparable age to those in the CCCEH study (4–9 years of age; n = 758). Similar to the CCCEH study, sex specific associations between phthalate metabolites and thyroid hormones were found. In girls, MBzP was negatively and significantly associated with FT4 while MEHP was positively and significantly associated with TSH, monohydroxyisononyl phthalate and monocarboxyisooctyl phthalate, metabolites of diisononyl phthalate (DiNP), were found to be significantly associated with a decrease in TSH. In contrast to the results from the CCCEH cohort, Boas et al. (2010) also found significant positive associations between urinary phthalate metabolites and thyroid hormones; specifically, in boys, MEHHP was positively associated with FT4 and MEP with TSH. Wu et al. (2013), in a cohort of Tanner Stage I Taiwanese children (n = 60) between the ages of two to eight, found that children exposed to DEHP and DiNP tainted foodstuffs had significantly reduced TSH levels compared with the unexposed children. In a follow-up study of these children exposed to tainted food intake, Tsai et al (2016) observed that daily DEHP intake was not associated with thyroid hormone levels among children between 6 and 10.5 years of age.
The studies by Boas et al. (2010) and Wu et al. (2013) used different study design and methods than the CCCEH study, yet the cohorts were of similar age and the studies demonstrated various, significant, phthalate-thyroid associations. Sex specific associations between phthalates and thyroid hormones are not unexpected as they are also observed for other childhood outcomes, such as behavior problems and cognition (Whyatt et al. 2012; Factor-Litvak et al. 2014), and may be related to the associations between phthalate exposures and sex steroid hormones (Martinez-Arguelles et al. 2013; Sathyanarayana, 2008). The disparate findings by Wu et al. (2013) compared to our present study may, in part, be attributable to the different mixtures and levels of DEHP and DiNP to which that population was exposed, as well as to different study designs and populations.
Despite the diverse age and sex composition (adults, adolescents, male adults, and pregnant females), studies are consistent with respect to some associations between phthalate exposure and thyroid hormones. Among the three studies of children (present study, Boas et al.(2010) and Wu et al.(2013)), only the prior two measured phthalate metabolites in child urine. Results are inconsistent as to the specific phthalate metabolites associated with FT4, likely due to the different composition and concentrations of the phthalate mixtures used in products and different patterns of product use in New York and Denmark. One study conducted in newborns found no significant associations between DEHP oxidative metabolites measured in breast milk collected in the second month after birth and T4 levels obtained from heel prick blood spots sampled several days after birth (de Cock et al. 2014). Another study conducted in newborns found that prenatal MBzP concentrations were inversely associated with serum TSH in cord blood (Kuo et al. 2015). Among adults, Meeker et al. (2007) reported a significant inverse association between MEHP urinary concentrations and FT4 in adult males seen in an infertility clinic. Using data from the 2007-2008 National Health and Nutrition Examination Survey (NHANES) in US adults; significant inverse associations between DEHP metabolites and total T4, FT4, and total T3 and positive relationships with TSH were found in adults and in teenagers, although the investigators did not stratify the analyses by sex (Meeker and Ferguson, 2011). In a study of 76 pregnant women (Huang et al. 2007) urinary MnBP was negatively associated with both FT4 and total T4. In sum, however, the results suggest stronger associations in females, who are also at risk for thyroid related dysfunction in general (Fortunato et al. 2014; Hollowell et al. 2002).
Animal and experimental studies support associations between phthalate exposure and thyroid function (Breou et al. 2005; Sugiyama et al. 2005). Hinton et al. (1986) observed that rats dosed with several phthalates, including DEHP experienced a fall in T4 levels. Both Poon et al. (1997) and Howarth et al. (2001) found that administration of DEHP to rats caused histological changes in the thyroid gland. O'Connor et al. (2002) found that in rats, DnBP caused a dose-dependent, significant decrease in serum T3 and T4 concentrations. In vitro studies find that several phthalates interfere with the various thyroid functions and as such provide evidence that phthalates can alter thyroid hormones and function (Ghisari 2009; Shen et al. 2009; Shi et al. 2012; Sugiyama et al. 2005; Zoeller, 2005).
While animal, experimental, and epidemiological studies suggest that phthalate exposure is associated with deficits in thyroid function, there are limitations when comparing epidemiological studies. These include quantification of different phthalate metabolites and differences in the population structure by age, sex, race, and other sociodemographic variables which may be associated with thyroid hormone levels (Kapelari et al. 2008). Consequently, inconsistencies in results may be attributed to methodological, technical and population structure variations. However, based on the sum of the literature, as well as animal and laboratory studies, there is mounting evidence that phthalate exposure may be associated with alterations in thyroid function.
Although there is evidence to suggest that phthalates may affect the thyroid, there is limited information on the possible mechanism of actions of phthalates on the thyroid. Potential pathways may be through thyroid antagonist activity which is supported by a number of studies that show that phthalates interfere with thyroid receptors (Shen et al. 2009; Shi et al. 2012; Sugiyama et al. 2005; Zoeller, 2005). Phthalates may also act on the thyroid through alterations of the sodium-iodide symporter, as several in vitro studies suggest that phthalates may modulate the transcriptional activity of genes regulating the sodium-iodide symporter (Breous et al. 2005; Wenzel et al. 2005).
There are several limitations to our study. First, the relatively small sample size of the study sample may lower the precision of our estimates. Second, while restriction of the study sample to inner-city African American and Hispanics reduces the generalizability of the results, it also minimizes residual confounding by socioeconomic status and race. Third, we only measured phthalate metabolite concentrations at two time points, in the maternal prenatal urine and concurrently with thyroid function at age 3. It may be that other time points are important predictors of thyroid function. Fourth, phthalate metabolite measures at one point in pregnancy do not reflect long term exposure (i.e. during the entire pregnancy). This was indicated in our study by the relatively low to moderate intraclass correlation coefficients in the repeat measures during pregnancy (Whyatt et al. 2012). We did not obtain repeat urine samples over several weeks in the children. Fifth, we did not measure maternal thyroid function, and cannot evaluate associations between phthalate exposure during pregnancy and thyroid function in the mothers. Although we lacked information on whether any of the study children were diagnosed with thyroid disease, their FT4 and TSH levels were within normal limits (Kapelari et al. 2008; Oto et al. 2015).
Additionally, we were unable to determine whether exposure to maternal smoking modifies the associations, as our sample only included non-smoking women. One study (Yaghjyan et al, 2016) evaluated the metabolism of DEHP according to smoking status using NHANES data and found that smoking was not associated with the metabolism of MEHP, the primary DEHP metabolite to MEHHP, the secondary DEHP metabolite, but that current smoking was associated with a higher percent of MEHP in the entire pool of DEHP metabolites. The authors speculate that this association may be due to smoking reducing the activity of aldehyde dehydrogenase, an enzyme which catalyzes the oxidation of aldehydes, but indicate that more work needs to be done to fully assess the biological mechanisms.
Our study has major strengths. The population demographic is an age group in which, aside from Boas et al. (2010) and Wu et al. (2013), phthalate-thyroid hormone outcomes have yet to be assessed. Importantly, the cohort is composed of underrepresented minorities for which we have very little data on these environmental exposures and outcome. Urinary phthalate biomarker measurements from this sample population were within range of other study populations, which enhance the external validity of our results.
Conclusion
The CCCEH study results suggest that childhood urinary concentrations of phthalate metabolites (MnBP, MBzP, MiBP, MEHHP, and MEP) may be associated with lower FT4 in preschool age minority children. Further, these findings were stronger in females. We only found limited evidence that urinary concentrations of MEHP in the late prenatal period are positively associated with increased concentrations of FT4. Our results contribute to a growing body of literature suggesting that early life exposure to some phthalates may affect endocrine function in children.
Supplementary Material
Highlights.
In a study of inner-city mothers and their children, we measured metabolites of several phthalates in maternal prenatal urine and child urine collected at age 3. We also measured serum free thyroxine and thyroid stimulating hormone in the children at age 3.
We found inverse and sex specific associations between specific phthalate metabolites measured in children at age 3 and free thyroxine. The associations were limited to girls.
Maternal prenatal urine concentrations of MEHP, a metabolite of DEHP, were associated with increases in free thyroxine in children at age 3.
No associations were found between phthalate metabolites and thyroid stimulating hormone.
Acknowledgments
We gratefully acknowledge the technical assistance of M. Silva, E. Samandar, J. Preau, and L. Jia in measuring the urinary concentrations of phthalate metabolites.
This work was supported by National Institute of Environmental Health Sciences (NIEHS) grants R01ES013543, R01ES014393, and R01ES08977 and by NIEHS/U.S. Environmental Protection Agency grant P50 ES09600/RD 83214101.
The findings expressed in this article are the opinions of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Funding Statement: National Institute of Environmental Health Sciences (NIEHS) grants R01ES013543, R01ES014393, and R01ES08977 and by NIEHS/United States Environmental Protection Agency grant P50 ES09600/RD 83214101. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen S, Pedersen KM, Bruun NB, Laurberg P. Narrow individual variabions in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. Journal of Clinical Endocrinology and Metabolism. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect. 1973;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J. Thyroid Hormones in Brain Development and Function. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2000. [Google Scholar]
- Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eur J Endocrinol. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118:1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KR. United States Consumer Product Safety Commission (Memorandum) Bethesda, MD, USA: 2010. Toxicity review of di(2-ethylhexyl) phthalate (DEHP) [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals. 2017 Updated Tables, January 2017. Available at https://www.cdc.gov/exposurereport/
- de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants - a Dutch prospective cohort study. Environ Health. 2014;13:106. doi: 10.1186/1476-069X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, Whyatt RM. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One. 2014;10(9):e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- Fortunato RS, Ferreira AC, Hecht F, Dupuy C, Carvalho DP. Sexual dimorphism and thyroid dysfunction: a matter of oxidative stress? J Endocrinol. 2014;221:R31–40. doi: 10.1530/JOE-13-0588. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, et al. Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect. 1986;70:195–210. doi: 10.1289/ehp.8670195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed DL. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Howarth JA, Price SC, Dobrota M, Kentish PA, Hinton RH. Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol Lett. 2001;121:35–43. doi: 10.1016/s0378-4274(01)00313-7. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22:2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Ghisari M, Bonefeld-Jorgensen EC. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett. 2009;189:67–77. doi: 10.1016/j.toxlet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kapelari K, Kirchlechner C, Högler W, Schweitzer K, Virgolini I, Moncayo R. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocr Disord. 2008;8:15. doi: 10.1186/1472-6823-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz PB. Subclinical hypothyroidism in children: normal variation or sign of a failing thyroid gland? International Journal of Pediatric Endocrinology. 2010 doi: 10.1155/2010/281453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Fanibunda SE, Desouza LA, Guha SK, Vaidya VA. Perspectives on thyroid hormone action in adult neurogenesis. J Neurochem. 2015;133(5):599–616. doi: 10.1111/jnc.13093. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Kuo FC, Su SW, Wu CF, Huang MC, Shiea J, Chen BH, Chen YL, et al. Relationship of urinary phthalate metabolites with serum thyroid hormones in pregnant women and their newborns: a prospective birth cohort in Taiwan. PLoS One. 2015;10:e0123884. doi: 10.1371/journal.pone.0123884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA. Thyroid Hormone Receptors: Multiple Forms, Multiple Possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Campioli E, Culty M, Zirkin BR, Papadopoulos V. Fetal origin of endocrine dysfunction in the adult: the phthalate model. J Steroid Biochem Mol Biol. 2013;137:5–17. doi: 10.1016/j.jsbmb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di (2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in US adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan A, Seaman HE, Wright JW, de Vries CS. The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol. 2008;69:687–96. doi: 10.1111/j.1365-2265.2008.03338.x. [DOI] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.09.070. http://dx.doi.org/10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(Suppl 3):U25–37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Frame SR, Ladics GS. Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol Sci. 2002;69:92–108. doi: 10.1093/toxsci/69.1.92. [DOI] [PubMed] [Google Scholar]
- Oto Y, Muroya K, Hanakawa J, Asakura Y, Adachi M. The ratio of serum free triiodothyronine to free thyroxine in children: a retrospective database survey of healthy short individuals and patients with severe thyroid hypoplasia or central hypothyroidism. Thyroid Res. 2015;8:1–6. doi: 10.1186/s13044-015-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, Chu I. Subchronic oral toxicity of di-noctyl phthalate and di(2-Ethylhexyl) phthalate in the rat. Food Chem Toxicol. 1997;35:225–239. doi: 10.1016/s0278-6915(96)00064-6. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S. Phthalates and children's health. Curr Probl Pediatr Adolesc Health Care. 2008;38:34–49. doi: 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, et al. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett. 2009;19:9–14. doi: 10.1016/j.toxlet.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Shi W, Zhang FX, Hu GJ, Hao YQ, Zhang XW, Liu HL, et al. Thyroid hormone disrupting activities associated with phthalate esters in water sources from Yangtze River Delta. Environ Int. 2012;42:117–123. doi: 10.1016/j.envint.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama SI, Shimada N, Miyoshi H, Yamauchi K. Detection of Thyroid System–Disrupting Chemicals Using in Vitro and in Vivo Screening Assays in Xenopus laevis. Toxicol Sci. 2005;88:367–374. doi: 10.1093/toxsci/kfi330. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35:236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tsai YA, Lin CL, Hou JW, Huang PC, Lee MC, Chen BH, et al. Effects of high di(2-ethylhexyl) phthalate (dehp) exposure due to tainted food intake on pre-pubertal growth characteristics in a Taiwanese population. Environ Res. 2016;149:197–205. doi: 10.1016/j.envres.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Franz C, Breous E, Loos U. Modulation of iodide uptake by dialkyl phthalate plasticisers in FRTL-5 rat thyroid follicular cells. Mol Cell Endocrinol. 2005;244:63–71. doi: 10.1016/j.mce.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120:290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Androl. 2008;31:131–138. doi: 10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MT, Wu CF, Chen BH, Chen EK, Chen YL, Shiea J, et al. Intake of phthalate-tainted foods alters thyroid functions in Taiwanese children. PLoS One. 2013;8:e55005. doi: 10.1371/journal.pone.0055005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghjyan L, Carlsson NP, Ghita GL, Chang SH. Associations of individual characteristics and lifestyle factors with metabolism of di-2-ethylhexyl phthalate in NHANES 2001-2012. Environmental Research. 2016;149:23–31. doi: 10.1016/j.envres.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- Zoeller RT. Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol. 2005;242:10–15. doi: 10.1016/j.mce.2005.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


