Abstract
Objective
To determine whether prophylactic indomethacin (PINDO) has more or less morbidity than delayed conservative management of the moderate-to-large patent ductus arteriosus (PDA).
Study design
We performed a prospective double cohort controlled study of infants delivered at ≤27+6 weeks gestation (n=397). From January 2005 through April 2011, all infants were treated with PINDO (n=247). From May 2011 through August 2016 no infant was treated with indomethacin until at least 8 postnatal days (Conservative epoch, n=150). Echocardiograms were performed on day 7 and at planned intervals until the PDA was small or closed. A single neonatologist prospectively collected all data.
Results
The incidence of moderate-to-large PDA on day 7 and duration of exposure to moderate-to-large PDA were significantly less in the PINDO epoch (incidence=10%, median=2 days) than the Conservative epoch (incidence=67%, median=14 days). Ligation rates were low in both epochs (PINDO=14%, Conservative=5%). In multivariate analyses PINDO infants had a significantly lower incidence of bronchopulmonary dysplasia (BPD) (RR=0.68, CI:0.46–0.89) and BPD or Death (RR=0.78, CI: 0.62–0.95) than Conservative infants. There were no differences between the epochs in Death, IVH grades 3 & 4, NEC, or ROP receiving treatment. The effects of PINDO on BPD and BPD or death were no longer significant when analyses were adjusted for presence of a moderate-to-large PDA on day 7. The significant effects of PINDO were independent of whether or not a ligation was performed.
Conclusion
PINDO decreases BPD and BPD or death compared with delayed conservative PDA management. These effects are mediated by closure of the PDA.
Keywords: newborn, premature birth, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis
Extremely preterm infants frequently develop a moderate-to-large patent ductus arteriosus (PDA) at the end of the first week. Early pharmacologic treatment of the PDA is effective in closing the PDA, decreasing the incidence of hemorrhagic pulmonary edema (1–3) and hypotension, and decreasing the need for early ventilator and inotropic support (4, 5). However, long-term benefits appear to be lacking (2, 4, 6–9). Although retrospective observational studies demonstrate an association between the presence of a PDA and long-term morbidities (necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD)), no association has been found in the randomized controlled trials (RCTs) that have explored this issue (2, 4, 6–9). Based on the evidence from the existing RCTs, the AAP Committee on Fetus and Newborn recently concluded that “routine treatment to induce closure of the ductus, either medically or surgically, in the first two weeks after birth does not improve long-term outcomes” and that “prophylactic use of indomethacin may not be justified by an expectation of better long-term outcomes” (10).
Although these RCTs failed to show any long-term benefits, it might be a mistake to conclude, based on their results, that exposure to a PDA during the first 2 weeks has no long-term consequences. Most of the prior RCTs enrolled patients based on whether the PDA was “present”, without taking into account either the magnitude or the duration of the left-to-right shunt. Recent studies have shown that the development of BPD is associated with persistent moderate-to-large PDAs but is not associated with persistent small, nonsignificant PDAs (11). In addition, the average difference between the groups in length of exposure to the PDA was less than 6 days. Therefore, it is possible that longer exposure to a moderate-to-large PDA may affect long-term morbidity.
We performed a prospective double cohort controlled study to examine whether a “conservative” approach to PDA treatment (that allows infants to be exposed to a moderate-to-large PDA shunt for at least 8 days) is associated with an increase or decrease in morbidity compared with an approach that uses prophylactic indomethacin (PINDO). Prior to May 2011, all infants in our nursery who delivered at ≤27+6 weeks gestation were treated with prophylactic indomethacin (PINDO epoch). After April 30, 2011, prophylactic indomethacin was no longer used and infants were only treated with indomethacin if the PDA persisted beyond 7 days (Conservative epoch; see Methods). We prospectively followed both groups to insure that infants treated with the Conservative approach did not have a higher incidence of long-term neonatal morbidities.
Methods
This project was approved by the Institutional Review Board of the University of California San Francisco. This study is part of an ongoing prospective study begun in 1992 to evaluate different methods of PDA treatment in extremely low birth weight infants. Infants were included in the current study if they were born between January 2005 and August 2016, delivered at ≤27 6/7 weeks gestation, and admitted to the intensive care nursery at the University of California San Francisco within 24 hours of birth. Detailed descriptions of our approach to respiratory and hemodynamic support have been previously published (5, 12–14).
During the first epoch (PINDO), prior to May 2011, all infants (n=247) were treated with a course of prophylactic indomethacin (PINDO) starting within 15 hours of birth, provided there were no contraindications. Six potential PINDO doses (a 0.2 mg/kg loading dose followed by five 0.1 mg/kg maintenance doses) were given at 24 hour intervals. An echocardiogram was performed before the third PINDO dose and doses 4–6 were given only if there was evidence (even minimal) of ductus patency on the echocardiogram. An echocardiogram was repeated at the end of the first week. Following the PINDO treatment infants with a “constricted” (small or closed) ductus (see below for criteria) were examined daily for a change in clinical symptoms indicative of a PDA (systolic murmur, widened pulse pressure, hyperdynamic precordium). If any of these occurred, an echocardiogram was performed within 24 hours.
Infants with a persistent moderate-to-large PDA after the first week were followed with echocardiograms to determine if or when retreatment or ligation would be necessary. Echocardiograms were performed initially every 7 days for the first 2–3 weeks, then every other week until the PDA was no longer moderate-to-large in size. During the PINDO epoch, the ductus was “constricted” (small or closed) on day 7 in 90% of the infants (69% were closed, 21% were small) (Table I); in 77% of the infants, the ductus stayed small or closed from day 7 through hospital discharge (Table I). Moderate-to-large PDAs that failed to close or reopened after indomethacin treatment were ligated only if the infants were either hypotensive and required inotropic support for more than 3 days, and/or were intubated and needed ventilator support that did not improve during a 4–5 day interval. During the PINDO epoch 67% of moderate-to-large PDAs that persisted after indomethacin treatment were ligated.
Table 1.
Incidence of demographic characteristics
| Variable | Prophylactic Epoch (n=247) | Conservative Epoch (n=150) | Variable | Prophylactic Epoch (n=247) | Conservative Epoch (n=150) |
|---|---|---|---|---|---|
| Prenatal Variables: | Neonatal Variables - continued: | ||||
| Singleton (%) | 67 | 61 | Caucasian (%) | 39 | 48 |
| Preeclampsia (%) | 19 | 23 | Female (%) | 47 * | 60 |
| Chorioamnionitis (%) | 28 * | 17 | Outborn (%) | 31 * | 19 |
| Maternal diabetes mellitus (%) | 6 * | 15 | 5 minute Apgar ≤5 (%) | 31 * | 42 |
| Cesarean delivery (%) | 66 | 75 | Respiratory Distress Syndrome (%) | 94 | 93 |
| Fetal Presentation (%) | Tracheal intubation during 1st 24 hours (%) | 95 * | 82 | ||
| vertex | 64 * | 47 | Mechanical ventilation at 24 hours (%) | 60 * | 45 |
| breech | 30 | 43 | Respiratory Severity Score at 24 hours a | ||
| transverse | 5 | 10 | 0.00 to 1.49 (%) | 32 * | 26 |
| Betamethasone (%) | 1.50 to 1.99 (%) | 29 | 21 | ||
| none or ≤6 hours | 28 | 19 | ≥2.00 (%) | 40 | 53 |
| 7 to 23 hours | 8 | 11 | Fluid Intake on days 1 & 2 – ml/kg/day (m±sd) | 159±38 | 166±33 |
| 1 day to 9 days (or second course) | 49 | 57 | Bacteremia or pneumonia (%) b | 40 * | 23 |
| ≥10 days (single course) | 14 | 13 | |||
| PDA Treatment Variables: | |||||
| Neonatal Variables: | PDA status at 7 days c | ||||
| Gestational age – weeks (m±sd) | 26.1±1.2 | 26.0±1.2 | Permanently constricted d (%) | 77 * | 29 |
| Gestation ≤25 weeks (%) | 45 | 45 | Closed at 7 days (%) | 69 | 17 |
| Birth weight - grams (m±sd) | 813±197 | 802±200 | Small at 7 days (%) | 8 | 12 |
| Fenton birth weight/age z-score | PDA moderate-to-large (%) | 10 | 67 | ||
| ≥−1.0 standard deviation (%) | 84 | 82 | Constricted (small) at 7 days but reopened later (moderate-to-large) (%) | 13 | 4 |
| −1.0 to −1.28 standard deviation (%) | 4 | 11 | |||
| <1.29 standard deviation (%) | 11 * | 7 | PDA Ligation (%) | 14 * | 5 |
p-value <0.05
Respiratory Severity Score (RSS), mean airway pressure x fraction of inspired oxygen, measured at 24 hours after birth
Bacteremia, culture-positive bacteremia. Pneumonia, sudden respiratory deterioration in arterial blood gases associated with a) new progressive infiltrates in the chest radiograph that persist for more than 3 days and b) either blood leukocytosis, leukopenia, or an increase in immature neutrophil forms, and/or c) associated temperature and/or glucose instability
PDA status at 7 days: n=349; 48 infants died before day 7 (13% of the Conservative Epoch’s population died prior to 7 days; 11% of the Prophylactic Epoch’s population died prior to 7 days)
Constricted, ductus was either closed or small on echocardiogram
In May 2011, we made a change to a more conservative treatment approach. During epoch 2 (May 2011 through August 2016, n=150) PINDO was no longer used. PDAs were no longer treated with indomethacin until at least 8 days of age to allow for spontaneous closure (15). During epoch 2, all infants had an echocardiogram on postnatal day 7. Among infants with a moderate-to-large PDA that persisted beyond 7 days, 84% were treated pharmacologically (either with indomethacin alone (38%) or with acetaminophen (16, 17) followed by indomethacin (46%)). (Indomethacin dosing for infants older than 7 days: six potential 0.2 mg/kg doses were used at 0, 12, 24, 48, 72, and 96 hours; doses 5–6 were given only if there was evidence of ductus patency on the echocardiogram performed after the fourth treatment dose). Sixteen percent of the infants were not treated because their needs for respiratory support were improving despite the presence of the moderate-to-large PDA.
During epoch 2, the rate of PDA “constriction” after pharmacologic treatment was 68%. However, only 49% of the infants who received pharmacologic treatment remained “constricted” (small or closed) throughout the remainder of their hospitalization. During epoch 2 the criteria for PDA ligation were more restrictive than during epoch 1. Infants with moderate-to-large PDAs that failed to close after pharmacologic treatment were ligated only if the infants’ needs for ventilatory support were deteriorating during a 4–5 day interval or failed to improve during a 2 week interval. During epoch 2, only 22% of PDAs that continued to be moderate-to-large after indomethacin treatment were ligated.
There were no changes in our protocols for feeding advances, ventilator management, fluid management, or management of hypotension between the two epochs.
A single neonatologist prospectively evaluated and recorded all perinatal/neonatal risk factors and outcome measures during the hospitalization (Table I). Gestational age was determined by the date of last menstrual period and ultrasounds before 24 weeks gestation. Birth weight-for-gestational age z-scores were obtained using the growth curves from Fenton et al (18). All infants were examined with serial bedside cranial ultrasounds, initiated within the first week of life and repeated weekly or biweekly for the first 4 weeks. Imaging was repeated prior to discharge or, more frequently, if there were any abnormal findings. Serious intraventricular hemorrhage (sIVH) was defined as grades 3 or 4 IVH (using the four-level grading system) (19). Necrotizing enterocolitis (NEC) was defined as Bell’s classification II or greater. This included NEC that was treated medically or surgically, and “spontaneous perforations” that occurred before 10 days (20). Bronchopulmonary dysplasia (BPD) was defined using a modified room air challenge test between 36+0 and 36+6 weeks’ corrected age (21). Retinopathy of prematurity (ROP) was defined as stage 2 with plus disease or ≥ stage 3 that was treated with laser or bevacizumab (22). Infants who died before a diagnosis could be made were excluded from the analyses (Table II; available at www.jpeds.com).
Table 2.
online: Infants included in analyses of individual neonatal morbidities
| Morbidity a | Reasons for exclusion from the analysis | Infants included in the analysis (% of epoch’s population) | |
|---|---|---|---|
| Prophylactic Epoch (n=247) | Conservative Epoch (n=150) | ||
| Death (n=397) | None | 100 | 100 |
| IVH (grades 3 or 4) (n=380) | Infants who died ≤4 days without evidence of grade 3 or 4 IVH | 95 | 97 |
| BPD b (n=316) | Infants who died ≤36+6 weeks without evidence of BPD | 80 | 79 |
| BPD b or Death before 36 wks (n=397) | None | 100 | 100 |
| NEC c <10 days (n=337) | Infants who died <9 days without evidence of NEC | 87 | 81 |
| Any NEC c during hospitalization (n=331) | Infants who died ≤21 days without evidence of NEC | 85 | 80 |
| Treated ROP d (n=315) | Infants who died ≤40 weeks without having ROP treatment | 81 | 77 |
see Methods for definitions
BPD = failure to pass room air challenge test between 36+0 and 36+6 weeks’ corrected age
NEC = spontaneous perforations or necrotizing enterocolitis
= retinopathy of prematurity stage 2 (with plus disease) or ≥ stage 3 and treated with laser or bevacizumab
The echocardiographic studies included two dimensional imaging, M-mode, color flow mapping and Doppler interrogation as previously described (23). A moderate-to-large PDA was defined by a ductus internal diameter ≥ 1.5mm or PDA:left pulmonary artery diameter ratio ≥0.5, in addition to one or more of the following echocardiographic criteria: a) left atrium-to-aortic root (LA/Ao) ratio ≥1.6, b) ductus flow velocity ≤2.5m/sec or mean pressure gradient across the ductus ≤8mm, c) left pulmonary artery diastolic flow velocity > 0.2 m/sec, and/or d) reversed diastolic flow in the descending aorta (14, 24). Ductus that failed to meet these criteria were considered to be “constricted” (small or closed) and were never treated.
Statistical analyses
When we made the change in practice in May 2011, we anticipated that we would need 150 consecutively admitted infants to be managed with the new Conservative approach to be able to detect a 15% change in the incidence of “BPD/Death” with a power of 0.80. We based our power analysis on the incidence of “BPD/Death” that occurred during the PINDO epoch (39%, see Results).
STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) was used for all statistical analysis. Chi-Squared tests were used to compare the treatment epochs (Prophylactic and Conservative) for categorical variables. For continuous variables, Student’s t-tests were used to compare groups for parametric variables and Wilcoxon rank sum tests to compare groups for non-parametric variables. Logistic regression was used to examine the relationship between the treatment groups and the various outcome measures. Prophylactic indomethacin treatment was assigned by epoch and therefore not confounded by indication. Fifteen infants born during the PINDO epoch received their first dose of indomethacin after 24 hours (on days 2 or 3) because of initial oliguria, elevated creatinine, or coagulopathy; an additional 12 infants died prior to receiving PINDO. All infants born during the PINDO period were analyzed using the intention to treat principle.
Although infants in both epochs had similar gestational ages, birth weights, betamethasone exposure, and modes of delivery, they differed in a number of other prenatal and neonatal factors that might have affected the neonatal outcomes (Table I). We created adjusted multivariate models that were specifically designed to examine the effects of the PINDO and Conservative Treatment Epochs on the neonatal outcomes. Gestational age, respiratory severity and betamethasone exposure have all been associated with neonatal morbidities in previous studies. Therefore, we first created a basic model for each outcome that included our variable of interest (Treatment Epoch) and forced in the variables Gestational Age, Respiratory Severity Score at 24 hours, and Betamethasone Exposure. Using this model we performed a logistic regression to determine the risk ratio for the predictor variable Treatment Epoch.
Next, we added one of the demographic variables, whose incidence differed significantly (p-value <0.05) between the two epochs, to the basic model and re-ran the logistic regression to determine how much the Treatment Epoch risk ratio was altered by the addition of the new variable to the basic model. If the addition of the new demographic variable altered the Treatment Epoch risk ratio by more than 4%, it was considered to be an important demographic variable that should be added to the Final Adjusted model. We repeated this step with each of the other demographic variables that differed significantly between the two epochs. Finally, we created the Final Adjusted model for the outcome by adding all of the important demographic variables for that outcome to the variables in the basic model (Table III). Potential outcomes estimation using STATA’s margins command was used to estimate a risk ratio for the final adjusted model.
Table 3.
Risks of neonatal morbidities during the Prophylactic and Conservative epochs
| Statistical Models | sIVH a | Death a | BPD a | BPD/Death a | NEC <10d a | Any NEC a | Treated ROP a | |||||||
| Unadjusted Model | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons |
| Risk | 0.18 | 0.19 | 0.20 | 0.24 | 0.25 | 0.36 | 0.39 | 0.50 | 0.074 | 0.12 | 0.16 | 0.17 | 0.16 | 0.18 |
| Risk Ratio | 0.95 | 0.83 | 0.71 | 0.78 | 0.64 | 0.96 | 0.86 | |||||||
| 95% CI | 0.54–1.35 | 0.51–1.13 | 0.47–0.95 * | 0.55–0.97 * | 0.20–1.08 | 0.48–1.45 | 0.43–1.29 | |||||||
| Final Adjusted Model b | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons |
| Risk | 0.18 | 0.20 | 0.21 | 0.24 | 0.25 | 0.37 | 0.40 | 0.51 | 0.08 | 0.10 | 0.17 | 0.15 | 0.17 | 0.16 |
| Risk Ratio | 0.93 | 0.88 | 0.68 | 0.78 | 0.75 | 1.09 | 1.04 | |||||||
| 95% CI | 0.55–1.32 | 0.58–1.18 | 0.46–0.89 * | 0.62–0.95 * | 0.21–1.30 | 0.53–1.65 | 0.54–1.54 | |||||||
| Final Adjusted Model plus PDA at 7 days | Pro | Cons | Pro | Cons | ||||||||||
| Risk | 0.28 | 0.32 | 0.34 | 0.39 | ||||||||||
| Risk Ratio | 0.87 | 0.86 | ||||||||||||
| 95% CI | 0.50–1.23 | 0.57–1.16 | ||||||||||||
| Final Adjusted Model plus Ligation | Pro | Cons | Pro | Cons | ||||||||||
| Risk | 0.24 | 0.38 | 0.40 | 0.51 | ||||||||||
| Risk Ratio | 0.64 | 0.78 | ||||||||||||
| 95% CI | 0.43–0.84 * | 0.62–0.94 * | ||||||||||||
p-value <0.05
Final Adjusted Models for each outcome (see Methods) were adjusted for:
sIVH = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, mechanical ventilation at 24 hours (n=380)
Death = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, fetal presentation and Fenton birth weight/age z-score (n =397)
BPD = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, chorioamnionitis, tracheal intubation during 1st 24 hours, and bacteremia or pneumonia (n=316)
BPD/Death = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, tracheal intubation during 1st 24 hours and bacteremia or pneumonia (n = 397)
NEC <10d = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, fetal presentation, maternal diabetes mellitus, 5 minute Apgar ≤5, and tracheal intubation during 1st 24 hours (n = 337)
Any NEC = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, chorioamnionitis, fetal presentation, Fenton birth weight/age z-score, and bacteremia or pneumonia (n = 331)
Treated ROP = gestational age, respiratory severity score at 24 hours after birth, antenatal betamethasone, outborn and chorioamnionitis (n = 315)
Results
Two hundred forty seven infants were admitted to the nursery during the Prophylactic epoch and 150 infants during the Conservative epoch (Table I). As expected, the incidence of moderate-to-large PDA that persisted beyond the end of the first week differed significantly between the two treatment epochs (Table I). Infants in the Conservative epoch were exposed to a moderate-to-large PDA for a significantly longer period of time than those in the PINDO epoch. The median postnatal age when infants finally achieved permanent ductus “constriction” (small/closed) in the Conservative epoch was 14 days (25–75th percentile: 7–43 days) compared with 2 days (25–75th percentile: 2–5 days) during the Prophylactic epoch (p<0.01). The difference in exposure to a moderate-to-large PDA was greatest among the most immature infants: for infants ≤25 weeks gestation, the median age when permanent ductus “constriction” was achieved was 25 days (25–75th percentile: 11–47 days) in the Conservative epoch, and 2 days (25–75th percentile; 2–12 days) in the PINDO epoch; in contrast, for infants ≥26 weeks gestation, the median age was 11 days (25–75th percentile: 7–31 days) in the Conservative epoch, and 2 days (25–75th percentile: 2–3 days) in the PINDO epoch (Figure 1).
Figure 1. Weekly incidence of Moderate-to-Large PDA shunts in the Prophylactic and Conservative Epochs.
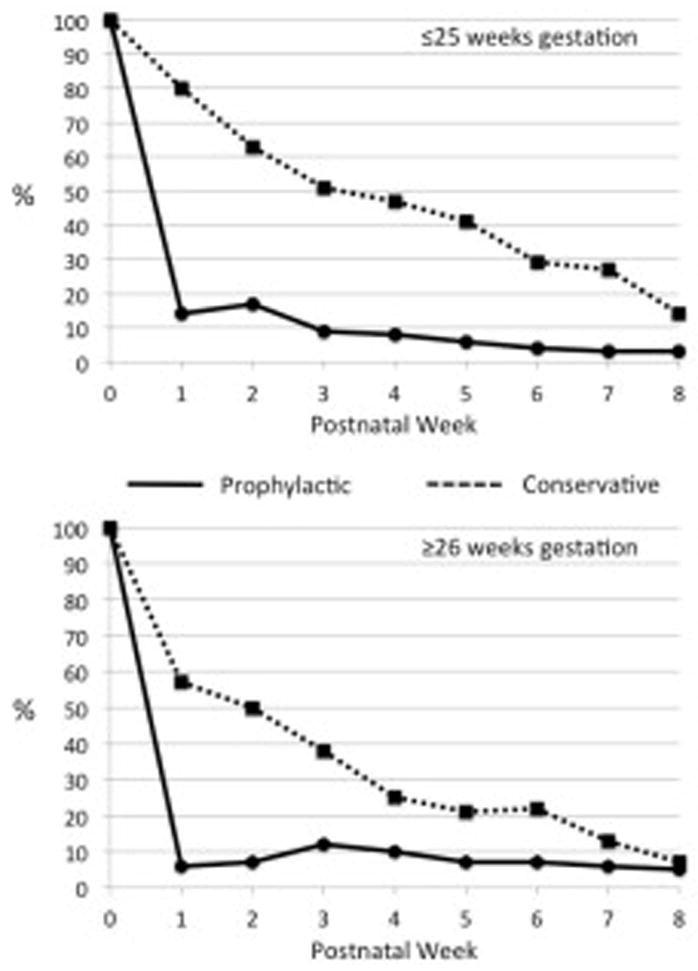
Infants were considered to have had a moderate-to-large shunt during the first week if the PDA was moderate-to-large on day 7. Infants were considered to have had a moderate-to-large shunt during each of the subsequent weeks (weeks 2–8) if the PDA was moderate-to-large for at least 4 days of the indicated week.
We created multivariate models specifically designed to compare the effects of the change from the PINDO approach with the more Conservative approach on the incidence of neonatal morbidities (Table III). In our Final Adjusted models, we found no significant differences between the two treatment approaches in the incidences of either death, grades 3 or 4 IVH, intestinal perforation or NEC occurring either before 10 days or at any time during the hospitalization, or the use of laser or bevacizumab treatment for ROP (Table III). This is consistent with the results of the previously published RCTs (2, 4, 6–9).
In contrast with what we expected, we observed a significant decrease in the rates of BPD and BPD/Death in the PINDO epoch compared with the Conservative epoch. This was true whether all infants (n=397: RR=0.784 (95%CI: 0.624–0.945)), or only infants who survived beyond 6 days (n=348: RR=0.674 (95%CI: 0.499–0.848)), were included in the analysis (; 3). The risk ratios and confidence intervals of the simple unadjusted models of BPD and BPD/Death were similar to the risk ratios and confidence intervals of their Final Adjusted models (Table III). This suggests that the effects of PINDO on BPD and BPD/Death were not confounded by any of the differences in demographic characteristics.
To examine which aspects of the PINDO approach might mediate its beneficial effects on BPD and BPD/Death, we added several potential PDA-related confounders to the Final Adjusted models (Table III). Prior observational studies have shown that infants with a moderate-to-large PDA and those who undergo a PDA ligation during the neonatal period have an increased risk for neonatal morbidity (11, 14, 25–28). In our study infants who had a persistent, moderate-to-large PDA on day 7, or who had a PDA ligation, also had an increased incidence of BPD and BPD/Death (Table IV; available at www.jpeds.com)(Figure 2).
Table 4.
online: Relationship between the predictor variables (Surgical ligation and presence of a moderate-to-large PDA on day 7) and the morbidity outcomes (BPD and BPD or death before 36 weeks)
| Death (%) | BPD (%) § | BPD or Death (%) | ||||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Ligation (%) | 12 | 11 | 54 | 26 | 58 | 33 |
| (n=349) ¶ | NS | <0.005 | <0.005 | |||
| PDA at 7d (%) | 15 | 10 | 45 | 22 | 52 | 28 |
| (n=349) ¶ | NS | <0.005 | <0.005 | |||
, 48 infants died before the echocardiogram on day 7
, 81 infants died before 36+6 weeks without evidence of BPD; therefore n=316 NS, p-value >0.05
Figure 2. Incidence of BPD or BPD/Death when the PDA is still persistently moderate-to-large on day 7, day 15 and during the 4th postnatal week.
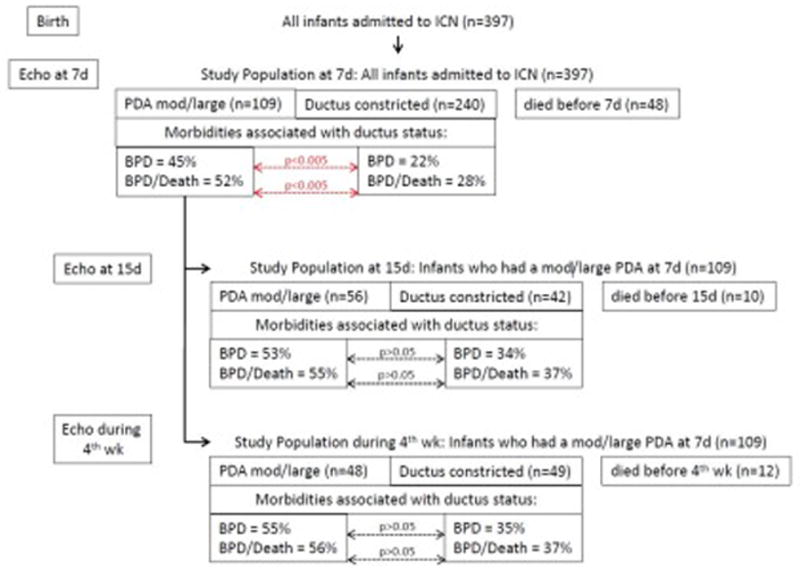
The likelihood of developing BPD or BPD/Death is examined in infants whose ductus is constricted by 7 days after birth and compared with those whose PDA is still moderate-to-large at 7 days (see Echo at 7d). Similarly, the likelihood of developing BPD or BPD/Death is examined in infants whose ductus was moderate-to-large at 7 days and was still moderate-to-large at 15 days (see Echo at 5d) or during the 4th postnatal week (see Echo during 4th week).
Although infants who had a PDA ligation had an increased incidence of BPD and BPD/Death, differences in the rates of PDA ligation between the epochs could not explain PINDO’s beneficial effects on BPD and BPD/Death (Table III). Adding the variable PDA ligation to the Final Adjusted models had no effect on the significant relationships between PINDO and the incidence of the two morbidities (Table III).
On the other hand, when we added the variable presence of a moderate-to-large PDA on day 7 to the Final Adjusted models, we found that the relationship between PINDO and the incidences of BPD and BPD/Death were no longer significant (Table III). This suggests that the effect of PINDO on these morbidities may be mediated through constriction of the PDA.
Although constriction of the PDA before day 7 was associated with a significant decrease in the incidence of BPD and BPD/Death, constriction of the PDA after day 7 was not associated with as great a beneficial effect. The incidence of BPD and BPD/Death did not appear to be significantly altered by the presence or absence of a moderate-to-large PDA on postnatal day 15 or during the 4th postnatal week if the infant had been exposed to a moderate-to-large PDA during the first 7 days (Figure 2).
Discussion
Prior retrospective observational studies that have examined the effects of the presence of a PDA and its early treatment on neonatal morbidities have been severely limited by the problem of residual confounding (i.e., the presence of a PDA and its need for treatment are also likely surrogates for immaturity and illness). In our study, the planned change in the use of indomethacin allowed us to use a double cohort controlled study design. With this study design prophylactic indomethacin was not confounded by indication, as it has been in observational retrospective studies. We found that PINDO decreases the incidences of BPD and BPD/Death. The majority of PINDO’s effects can be attributed to its ability to close the moderate-to-large PDA that persists throughout the first week when infants are managed “conservatively”.
It is interesting to note that although the incidence of BPD and BPD/Death were significantly related to the presence or absence of a moderate-to-large PDA during the first week, constriction of the PDA after 7 days did not appear to significantly alter the incidence of these two morbidities (Figure 2). Although the analyses at the later postnatal ages might be less significant because of the reduced sample sizes, one might also speculate that waiting until the end of the first week before attempting to close a moderate-to-large PDA may not have the same beneficial effects on BPD and BPD/Death as earlier closure. The nearly completed PDA-TOLERATE RCT (NCT01958320) that examines the effects of PDA treatment at the end of the first week on neonatal morbidity should be able to address this speculation.
In our study, prophylactic indomethacin was used as a surrogate instrument for PDA constriction. However, there was an imperfect correlation between prophylactic indomethacin and PDA constriction because not all infants who received PINDO constricted their ductus (23% still had a moderate-to-large PDA beyond day 7), and not all infants in the Conservative epoch had a persistent moderate-to-large PDA beyond 7 days (29% had constricted their PDA spontaneously before day 7). This imperfect correlation between prophylactic indomethacin and PDA constriction does result in misclassification bias; however, this type of misclassification would bias our estimate towards the null, meaning that the true effect of PINDO is likely greater than what we observed in our study.
Although the PINDO approach is considered more “aggressive” than the Conservative approach (10), we found that it was associated with decreased neonatal morbidity. There have been 3 other controlled trials that also have compared two epochs of “aggressive” and “conservative” PDA management (14, 29, 30). Our findings are consistent with those of Kaempf et al (29) who observed that the incidence of BPD or death was significantly increased during the “conservative” approach (29).
On the other hand, our findings appear to disagree with the results of the controlled trials by Jhaveri et al and Sung et al (14, 30). In their trials, “conservative” management was associated with less morbidity than “aggressive” management (14, 30). However, the “aggressive” approach in the Jhaveri and Sung trials relied heavily on early PDA ligation, with ligation rates of 100% and 80%, respectively. “Conservative” management was achieved through a significant reduction in the rates of ligation, to 72% and 0%, respectively(14, 30). In their study population, the reduction in ligation appeared to mediate the beneficial effects of “conservative” management because the benefits were no longer observed when the analyses were adjusted for the different ligation rates (25). Early surgical ligation is known to play a causal role in the development of BPD (28, 31, 32). Therefore, it is not surprising that a large change in the rate of ligation would be accompanied by a decrease in BPD. However, in our study (Table I), and the study by Kaempf et al (29), the ligation rates were markedly lower than in the Jhaveri and Sung studies, and the change to a “conservative” approach relied more on a change in tolerating the presence of the PDA than on a change in the rates of surgery. Although PDA ligation is associated with increased morbidity (Table IV), our rates of ligation were so low that adding PDA ligation as a variable to the Final Adjusted models had no effect on the significant relationship between PINDO and the incidence of BPD and BPD/Death (Table III). We found that the significant effects of PINDO were independent of whether or not a ligation was performed.
In our study there was a trend towards lower sIVH rates in the PINDO epoch among infants born ≤25 weeks gestation, especially among those born without adequate antenatal betamethasone exposure (data not shown); however, there was no difference in the overall rates of sIVH between the epochs (Table III). In contrast with our results, prior RCTs have observed a lower incidence of sIVH in PINDO treated infants (6). The prior RCTs were performed more than 15 years ago. Since that time sIVH rates have steadily decreased (33). We speculate that increases in the use of antenatal betamethasone, cesarean delivery, delayed cord clamping, less invasive ventilation, and decreases in sodium bicarbonate use may have rendered the beneficial effects of PINDO on sIVH less significant than they were in the era when the initial RCTs were performed (33, 34).
There are several limitations to our study. We used data from a single center. Because the incidence of moderate-to-large PDA and neonatal morbidities differ by center, our results may not be generalizable to centers where the rates differ from ours. In addition, our study was not a randomized controlled trial. We evaluated the effects of a change in practice between two consecutive time periods. Even though we adjusted our analyses for differences between the epochs, there may have been unmeasured changes in practice that could have affected the morbidity rates during the study period.
On the other hand there are also strengths to our study. The single center aspect of the study meant that the same consensus-driven, standardized approaches to respiratory, hemodynamic, fluid, nutrition and PDA evaluation and management were consistent among the infants in each of the study epochs. Although differences other than the use of prophylactic indomethacin administration existed between the two time periods (Table I), models that included these factors produced similar risk ratios and confidence intervals, suggesting that these factors were not confounders of the relationship between PINDO and BPD and BPD/Death (Table III).
Over the last decade the use of prophylactic or early PDA treatment has decreased (35). This is primarily due to the results of the prophylactic and early PDA treatment RCTs that failed to show any improvement in long-term morbidities such as BPD and BPD/Death (2, 4, 6–9). Although these RCTs may have provided information about the early, short-term effects of a PDA, they were never designed to address the consequences of persistent prolonged, hemodynamically significant PDA shunts on the newborn. The impact of a PDA is directly related to the magnitude and duration of the left-to-right shunt (11). However, most of the prior RCTs enrolled patients based on whether the PDA was “present”, without considering the magnitude of the shunt. The inclusion of patients where the ductus is patent, but not hemodynamically significant, minimizes the ability to identify any real difference between the groups. In addition, none of the prior RCTs were designed to examine the effects of prolonged exposure to the PDA shunt. All of the RCTs allowed infants in the Control group to be “rescued” or “crossed-over” to the Treatment group, thereby minimizing the difference in length of PDA exposure between the groups. The average time before infants in the Control group were “rescued” was 2–5 days. Furthermore, 40–60% of the infants in the Control groups closed their PDA spontaneously during the first 7 days. This also contributed to the small difference in exposure times between the groups. In contrast, in our study, the PINDO and “Conservative” epochs differed in their median length of exposure to a moderate-to-large PDA by 12 days.
Prior prophylactic and early treatment controlled trials have demonstrated important reductions in the risks of several short-term morbidities (4–6). Our results suggest that PINDO or early PDA treatment may have the additional benefit of reducing the incidence of BPD and BPD/Death compared with delayed conservative PDA management (Table III). Although many factors may contribute to the increasing incidence of BPD that has occurred since 2009 (33), it is interesting to note that this has occurred during the same interval that the use of PINDO and early PDA treatment has been in decline (36). Neonatologists may wish to consider these findings when they decide whether or not to use PINDO or early PDA treatment in extremely premature infants.
Acknowledgments
Supported by the U.S. Public Health Service NHLBI (HL109199) and by a gift from the Jamie and Bobby Gates Foundation. The authors declare no conflicts of interest.
We would like to thank Drs Mark Cocalis, Laura Robertson, Michael Brook, Anita Moon-Grady, and Shabnam Peyvandi for their expert help in reading and interpreting the echocardiograms.
Abbreviations
- RCT
randomized controlled trial
- PDA
patent ductus arteriosus
- PINDO
prophylactic indomethacin treatment
- NEC
necrotizing enterocolitis
- ROP
retinopathy of prematurity
- NEC
necrotizing enterocolitis
- sIVH
serious intraventricular/intracranial hemorrhage (grades 3 or 4)
- BPD
bronchopulmonary dysplasia
- CPAP
continuous positive nasal airway pressure
- BiPAP
biphasic nasal ventilation
- STATA
Stata Statistical Software, StataCorp. 2015
- RR
risk ratio
- CI
confidence interval
- RSS
Respiratory Severity Score
- BPD/Death
Bronchopulmonary dysplasia or Death before 36 weeks
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al Faleh K, Smyth J, Roberts R, Solimano A, Asztalos E, Schmidt B. Prevention and 18-month outcome of serious pulmonary hemorrhage in extremely low birth weight infants: results from the triall of indomethacin prophylaxis in preterms. Pediatrics. 2008;121(2):e233–8. doi: 10.1542/peds.2007-0028. [DOI] [PubMed] [Google Scholar]
- 2.Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F99–F104. doi: 10.1136/archdischild-2013-304695. [DOI] [PubMed] [Google Scholar]
- 3.Aranda JV, Clyman R, Cox B, Van Overmeire B, Wozniak P, Sosenko I, et al. A randomized, double-blind, placebo-controlled trial on intravenous ibuprofen L-lysine for the early closure of nonsymptomatic patent ductus arteriosus within 72 hours of birth in extremely low-birth-weight infants. Am J Perinatol. 2009;26(3):235–45. doi: 10.1055/s-0028-1103515. [DOI] [PubMed] [Google Scholar]
- 4.Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003;(2):CD003745. doi: 10.1002/14651858.CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebowitz M, Koo J, Wickremasinghe A, Allen IE, Clyman RI. Effects of Prophylactic Indomethacin on Vasopressor-Dependent Hypotension in Extremely Preterm Infants. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;(7):CD000174. doi: 10.1002/14651858.CD000174.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160(6):929–35. e1. doi: 10.1016/j.jpeds.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2015;2:CD003481. doi: 10.1002/14651858.CD003481.pub6. [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011;(7):CD004213. doi: 10.1002/14651858.CD004213.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Benitz WE Committee On F, Newborn. Patent Ductus Arteriosusin Preterm Infants. Pediatrics. 2016;137(1):1–6. doi: 10.1542/peds.2015-3730. [DOI] [PubMed] [Google Scholar]
- 11.Schena F, Francescato G, Cappelleri A, Picciolli I, Mayer A, Mosca F, et al. Association between Hemodynamically Significant Patent Ductus Arteriosus and Bronchopulmonary Dysplasia. J Pediatr. 2015;166(6):1488–92. doi: 10.1016/j.jpeds.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Liebowitz MC, Clyman RI. Predicting the Need for Home Oxygen Therapy in Preterm Infants Born Before 28 Weeks’ Gestation. Am J Perinatol. 2016;33(1):34–9. doi: 10.1055/s-0035-1555122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clyman RI, Wickremasinghe A, Merritt TA, Solomon T, McNamara P, Jain A, et al. Hypotension following patent ductus arteriosus ligation: the role of adrenal hormones. J Pediatr. 2014;164:1449–55. doi: 10.1016/j.jpeds.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157(3):381–7. 7e1. doi: 10.1016/j.jpeds.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117(4):1113–21. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]
- 16.Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128(6):e1618–21. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 17.El-Khuffash A, Jain A, Corcoran D, Shah PS, Hooper CW, Brown N, et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatric Research. 2014;76(3):238–44. doi: 10.1038/pr.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight < 1500 grams. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 20.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–11. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 22.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 23.Keller RL, Tacy TA, Fields S, Ofenstein JP, Aranda JV, Clyman RI. Combined treatment with a non-selective nitric oxide synthase inhibitor (L-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatric Research. 2005;58:1216–21. doi: 10.1203/01.pdr.0000183659.20335.12. [DOI] [PubMed] [Google Scholar]
- 24.El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F419–22. doi: 10.1136/adc.2003.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickremasinghe AC, Rogers EE, Piecuch RE, Johnson BC, Golden S, Moon-Grady AJ, et al. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr. 2012;161(6):1065–72. doi: 10.1016/j.jpeds.2012.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. J Pediatr. 2007;150(3):229–34. doi: 10.1016/j.jpeds.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 27.Sellmer A, Bjerre JV, Schmidt MR, McNamara PJ, Hjortdal VE, Host B, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98(6):F505–10. doi: 10.1136/archdischild-2013-303816. [DOI] [PubMed] [Google Scholar]
- 28.Clyman R, Cassady G, Kirklin JK, Collins M, Philips JB. The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized controlled trial. J Pediatr. 2009;154(6):873–6. doi: 10.1016/j.jpeds.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G. What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants? J Perinatol. 2012;32(5):344–8. doi: 10.1038/jp.2011.102. [DOI] [PubMed] [Google Scholar]
- 30.Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, et al. Mandatory Closure Versus Nonintervention for Patent Ductus Arteriosus in Very Preterm Infants. J Pediatr. 2016;177:66–71. e1. doi: 10.1016/j.jpeds.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Chang LY, McCurnin D, Yoder B, Shaul PW, Clyman RI. Ductus arteriosus ligation and alveolar growth in preterm baboons with a patent ductus arteriosus. Pediatric Research. 2008;63(3):299–302. doi: 10.1203/PDR.0b013e318163a8e4. [DOI] [PubMed] [Google Scholar]
- 32.Waleh N, McCurnin DC, Yoder BA, Shaul PW, Clyman RI. Patent ductus arteriosus ligation alters pulmonary gene expression in preterm baboons. Pediatric Research. 2011;69(3):212–6. doi: 10.1203/PDR.0b013e3182084f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314(10):1039–51. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lea CL, Smith-Collins A, Luyt K. Protecting the premature brain: current evidence- based strategies for minimising perinatal brain injury in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2016 doi: 10.1136/archdischild-2016-311949. [DOI] [PubMed] [Google Scholar]
- 35.Slaughter JL, Reagan PB, Bapat RV, Newman TB, Klebanoff MA. Nonsteroidal anti- inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr. 2016;175(6):775–83. doi: 10.1007/s00431-016-2705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lokku A, Mirea L, Lee SK, Shah PS Canadian Neonatal N. Trends and Outcomes of Patent Ductus Arteriosus Treatment in Very Preterm Infants in Canada. Am J Perinatol. 2016 doi: 10.1055/s-0036-1593351. [DOI] [PubMed] [Google Scholar]


