Abstract
BACKGROUND
Deficiency of the lysosomal enzyme galactosylcerebrosidase (GALC) causes Krabbe disease. Newborn screening for Krabbe disease is ongoing, but improved methods for follow-up analysis of screen-positive babies are needed to better advise families and to optimize treatment. We report a new assay for the enzymatic activity of GALC in lymphocytes.
METHODS
T lymphocytes were isolated from venous blood by magnetic bead technology. The assay used a close structural analog of the natural substrate and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify the amount of product with the aid of a chemically identical internal standard.
RESULTS
The analytical range of the assay (ratio of assay response for the quality control high standard to that from all non-enzymatic-dependent processes) was 20-fold greater than that for the conventional radiometric GALC assay. The LC-MS/MS could distinguish cells that were null in GALC from those that contained traces of active enzyme (down to 0.3 % of normal). There was a good correlation between the level of residual GALC activity in lymphocytes and the severity of Krabbe disease.
CONCLUSIONS
The new assay can measure small amounts of residual GALC activity in leukocytes with high accuracy compared to previous assays and can contribute, along with genotyping, biomarker analysis, and neurological imaging, a better plan for post newborn screening follow-up for Krabbe disease.
Keywords: Krabbe disease, globoid cell leukodystrophy, tandem mass spectrometry, newborn screening, lysosomal storage disease, diagnosis, biochemical genetics
INTRODUCTION
Krabbe disease (globoid cell leukodystrophy) is an often-fatal lysosomal storage disease caused by the deficiency of the enzyme galactocerebrosidase (GALC, EC 3.2.1.46), resulting in the loss of the myelin sheath in the central nervous system (1,2). Interest in newborn screening for Krabbe disease has escalated owing to the demonstration that bone marrow transplantation in children prior to the onset of severe symptoms is effective at suppressing the onset of the disease (3). New York (4), Missouri, Ohio, and Kentucky state newborn screening labsoratories now carry out newborn screening for Krabbe disease, and Illinois and New Jersey are expected to start soon.
Newborn screening for Krabbe disease is performed by direct measurement of GALC enzymatic activity in dried blood spots either by tandem mass spectrometry (MS/MS) or by fluorimetry (5). Over the past ~10 yrs, about 3.3 million newborns have been screened for Krabbe disease in New York by MS/MS. Of these tested newborns, 5 were confirmed by clinical exam follow-up to have early infantile Krabbe disease, and 12 additional patients were found to be at high risk for the development of Krabbe disease but have so far remained asymptomatic (4). The high-risk designation applies to newborns who display the lowest bracket of GALC enzymatic activity determined using a radiometric assay in lymphocytes (≦ 0.15 nmole product/hr−/mg protein) (6). All of these high-risk patients also have 2 Krabbe disease-associated alleles. Based on this data, it is generally thought that the level of residual GALC activity in leukocytes is not sufficient to explain the severity of Krabbe disease and to differentiate between symptomatic and asymptomatic patients. The value of the radiometric GALC assay is in the confirmation of diagnosis of Krabbe disease in already symptomatic patients.
Patients who are null in GALC (because of two severe mutations including a large 30-kb deletion that removes several exons of the gene (7)) are thought to develop early infantile Krabbe disease, strongly suggesting that there is not a second enzyme that can compensate for the loss of GALC enzymatic activity. We hypothesize that the severity of Krabbe disease, including the age of onset of symptoms, is dictated mainly by the degree of residual GALC activity. There is the possibility that the currently used radiometric assay for GALC is insufficiently accurate to measure small differences in residual enzymatic activity when the activity is close to zero. To study this further, we have focused our efforts on the development of a new assay of GALC enzymatic activity that has much higher resolution than the current radiometric assay so that small differences in GALC activity close to zero can be measured in a statistically confident manner. Here we describe a new GALC assay based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) that accomplishes this goal.
METHODS
T lymphocytes were isolated from venous blood by magnetic bead technology. LC-MS/MS GALC assays were carried out with a close structural analog galactosylceramide with a heptanoyl fatty acyl chain) of the natural substrate. The internal standard is chemically identical to the substrate but differentiated by deuterium substitution. Standard operating procedures for all methods in this paper are given in Supplemental File.
RESULTS AND DISCUSSION
GALC assay reagents, cell source, and buffer
Our original MS/MS assay of GALC was based on the substrate galactosyl-ceramide with an octanoyl attached to the sphingosine backbone (8). We now prefer galactosyl-ceramide with a 7-carbon heptanoyl chain (GALC-S) because it dissolves more rapidly in buffer than the original substrate. The internal standard (GALC-IS) is chemically identical to the GALC product (GALC-P) but containins a terminal CD2CD3 in the heptanoyl chain. The use of a chemically identical internal standard accounts for all losses of GALC-P and for ionization suppression during MS/MS.
For initial studies, we decided to isolate a single type of leukocyte from whole blood, namely T-lymphocytes, by using magnetic beads containing antibody to the cell surface CD3 protein. Although there have been no reports of the relative amounts of GALC in different types of leukocytes, we were concerned that if there were differences, the measured amount of GALC enzymatic activity per milligram of cellular protein would be a function of differential cell counts. It would also be a function of differential loss of white cell types during the 1–2 d required for shipment of blood to the biochemical genetics laboratory. Recovery of T-lymphocytes using the magnetic bead technology was > 80%.
The previously published buffer for in vitro assay of GALC was citrate-phosphate, pH 4.2 (6). We measured the activity of recombinant human GALC in this buffer and also in 0.2 M sodium acetate, pH 4.0. Both buffers also contained sodium taurocholate and sodium oleate, which activate GALC (6). For reasons described below, we also tested the activity of recombinant human β-galactosidase (GLB1). In sodium acetate buffer, the MS/MS ion counts for enzymatic product obtained using lymphoblast cells from a Pompe disease patient (Coriell Biorepository, GM13793, our functional GALC control cell line) divided by the ion counts for the internal standard was 2.728 for GALC and 0.032 for GLB1. In citrate phosphate buffer, the ratios were 20.498 for GALC and 0.004 for GLB1. Thus, we preferred citrate phosphate buffer and used this for all subsequent studies.
LC-MS/MS assay of GALC
Prior to studies with blood cells we measured GALC in lysates of LCL lymphoblasts from the Coriell Cell Repository. The Pompe cell line GM13793 constituted our HIGH quality control sample for the study (GM13793-HIGH). The second cell line GM06805 (GM06805-LOW) was from a patient that assumed to be completely devoid of functional GALC enzyme because ~30% of the exons have been deleted from both copies of the GALC gene (homozygotes develop early infantile Krabbe disease (7).
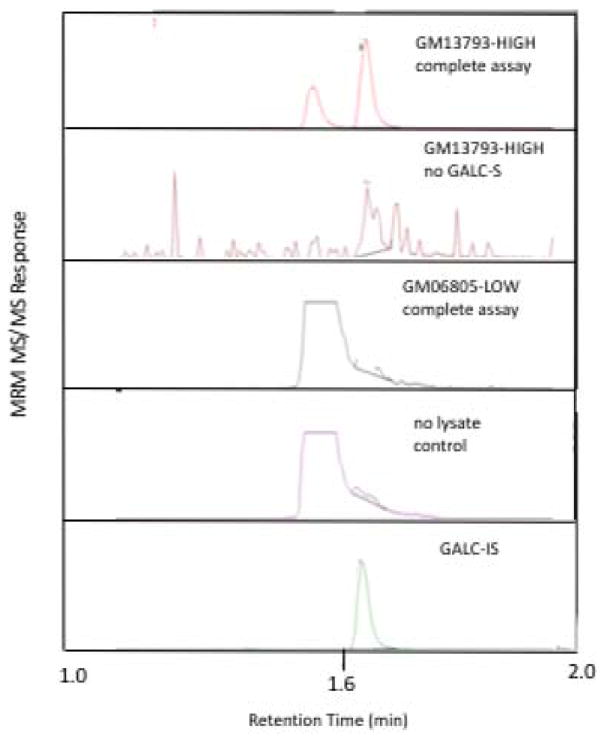
Lysates from these cells were used to perform assays containing 2 μg of cell protein in an assay cocktail consisting of 150 μM GALC-S and 5 μM GALC-IS in citrate-phosphate buffer with sodium oleate and sodium taurocholate, pH 4.2. The LC-MS/MS ion traces for GALC-P are shown in Figure 1. Table 1 contains the ion counts for the GALC-P and GALC-IS obtained by integrating the multiple reaction monitoring ion peaks versus LC retention time profile shown in Figure 1. The complete assay, in which GM13793-HIGH lysate was incubated with GALC-S, is shown in the top panel of Figure 1. The peak at ~1.67 min was due to GALC-generated GALC-P as it co-migrated with the chemically identical GALC-IS (bottom panel). The peak in the top panel eluting at ~1.57 min is GALC-P produced by non-enzymatic cleavage of GALC-S in the heated electrospray ionization source of the MS/MS instrument as it co-migrates with GALC-S (not shown). This in-source cleavage was of no consequence since the two GALC-P peaks were baseline separated, and only the 1.67 min was integrated to determine the GALC activity.
Figure 1. LC-MS/MS ion chromatograms.
Complete assay with GALC-S incubated with GM13793-HIGH cell lysate (top panel); GM13793-HIGH lysate incubated in the absence of GALC-S (second panel); GM06805-LOW lysate incubated with GALC-S (3rd panel); GALC-S incubated in buffer without cell lysate (4th panel). Panels 1–4 show the MRM ion chromatograms for GALC-P, the bottom panel is for GALC-IS.
Table 1.
LC-MS/MS GALC assay results.a
| Assay | GALC-P ion counts | GALC-IS ion counts | GALC activity (nmole/hr/mg, mean ± SD) |
|---|---|---|---|
| GM13793-HIGH lysate + GALC-S | 143518 147838 154801 |
1246449 1171068 1171068 |
0.5837 ± 0.0406 |
| no lysate control | 89 64 122 |
1231481 1150753 1156488 |
0.000364 ± 0.000119 |
| GM13793-HIGH lysate minus GALC-S | 10 8 8 |
563384 586144 532992 |
7.25 × 10−5 ± 0.979 × 10−5 |
| GM06805-LOW + GALC-S | 1129 1110 880 |
1352893 1316255 1260690 |
0.00371 ± 0.00038 |
Instrument noise was measured to be 16 based on integration of the noise region adjacent to the analyte peak over the same time window as for the peak integration.
The first control assay was the cell lysate (GM13793-HIGH) incubated without GALC-S, which showed essentially no GALC-P signal (0.007% of the activity seen with GM13793-HIGH, Figure 1 second panel, Table 1). This indicated that the lysate did not contain compounds that were isobaric with GALC-P and that eluted at the same LC retention time. The second control was GALC-S incubated in buffer without cell lysate (Figure 2, 4th panel), which had a GALC-P signal that was only 0.07% of the signal seen with cell lysate (Table 1), showing that GALC-S contained essentially no GALC-P as an impurity and that GALC-S underwent essentially no non-enzymatic breakdown to GALC-P. Figure 1 (3rd panel) is the assay with GM06805-LOW incubated with GALC-S. The signal was ~10-fold higher than that obtained in the no-lysate control, and represented 0.4% the signal seen with GM13793-HIGH (Table 1). This small increase in assay response was attributed to one or more non-GALC enzymes present in the cell lysate that were capable of generating GALC-P from GALC-S. One candidate is human β-galactosidase-1 (GLB1) since this enzyme cleaves terminal β-linked galactosyl residues from gangliosides. GLB1 deficiency causes two lysosomal storage disorders unrelated to Krabbe disease, namely GM1-gangliosidosis and Morquio B syndrome. For the assay with GM13793-HIGH the GALC-P assay response was 148,719 ion counts (mean of triplicate runs, Table 1), and thus 150-fold higher than the value seen with the GALC-null cells GM06805-LOW.
Figure 2.
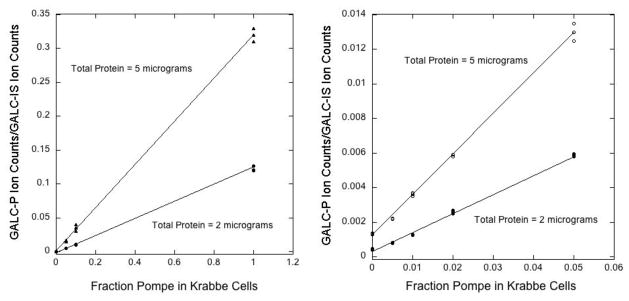
GALC-P ion counts (integral of the multiple reaction monitoring ion chromatogram) divided by the GALC-IS ion counts as a function of the fraction of Pompe disease cell lysate (GM13763-HIGH) added to the Krabbe cell lysate (GM06805-LOW). Total protein per assay was either 2 or 5 μg. The right panel is an expansion of the plot in the left panel. Assays were performed in triplicate, and all assay values are plotted. The non-zero Y-intercepts are due to a non-GALC enzyme(s) in GM06805-LOW lysate capable of a small amount of conversion of GALC-S to GALC-P.
We explored a number of previously reported glycohydrolase inhibitors to see if the assay response in GM06805-LOW cells could be reduced toward the value seen in the no-lysate control. We also tested the inhibitor on GM13793-HIGH cells to explore the effect of the compound on GALC. The results, summarized in the supplemental material file, suggested that the residual activity in GM06805-LOW cells was due to one or more enzymes other than GALC, possibly GBL1. The result also showed that the buffer composition had a large effect on the substrate specificity of enzymes in the cell lysates capable of cleaving GALC-S.
Analytical range and accuracy of detection of small amounts of residual GALC activity
We defined the analytical range as the enzyme-dependent assay response observed with the HIGH quality control sample (GM13793-HIGH, typical of the activity in cells from a healthy individual) divided by the assay response from all enzyme-independent processes. The analytical range for the LC-MS/MS assay was discussed in detail in Supplemental File and has a value of 149. As shown in Supplemental Material, the largest contribution to the enzyme-independent assay response was conversion of GALC-S to GALC-P by one or more non-GALC components of the cell lysate.
Since the analytical range for the LC-MS/MS GALC assay was larger than that for previously reported GALC assays (see below), we tested the possibility of detecting a difference in assay response due to very small changes in the amount of GALC in cell lysates. To study this, we added small amounts of GALC-containing lysate (GM13793-HIGH) to GALC-null cell lysate (GM06805-LOW) so that the total amount of cellular protein was the same in all assays (2 or 5 μg). The results are shown in Figure 2. Assays were conducted in triplicate, and the mean assay response was obtained along with the estimated error. The LC-MS/MS ion chromatograms are shown in Supplemental Figure 2. The data showed that very small differences in GALC enzymatic activity at the low end could be observed in a statistically significant way. For example, the ratio of GALC-P to GALC-IS ion counts when 100% GM06805-LOW lysate was used was 0.0012 ± 0.000044, and it was 0.00014 ± 0.000015 for the no-lysate blank. The difference in these values was 0.0011 ± 0.000046 and was thus of high confidence. This difference was 8-fold the value for the no-lysate control. The GALC-P/GALC-IS ion count ratio observed with 100% GM13793-HIGH lysate was 0.2999, thus the 100% GM06805-LOW lysate produced 0.4% of the GALC activity as the GM13793-HIGH lysate. Under our assay conditions, this was the contribution by one or more non-GALC enzymes in the lysate (possibly GLB1).
As shown in Figure 2 (right), when 5 μg of cell lysate protein was used per assay, an increase in GALC activity above that measured with 100% null lysate (GM06805-LOW) was readily measured when this lysate was spiked with 0.5% GM13793-HIGH lysate. The activity increased linearly as the fraction of GM13793 was increased.
We believe that it will be clinically useful to compare this discrimination between small differences in GALC activity to what was feasible with the previously developed assay of GALC, which was used to support follow-up studies of Krabbe disease newborn screening-positive patients (9). The previous GALC assay made use of radiolabeled galactosyl-ceramide that contained tritium attached to C6 of the galactosyl group (6). Using the specific radioactivity of the tritiated galactosyl-ceramide substrate, the micrograms of peripheral blood mononuclear cell (mainly lymphocytes) protein per assay, and the reported GALC activity (6), ~400 dpm of tritium were detected using this radiometric assay. We have carried out this radiometric assay and observed an assay response of 406 and 433 dpm for duplicate runs using 50 μg of cell protein per assay (Supplemental Material) (6). This number agrees with the published value (6). In a control assay with omission of cell lysate, we observed an assay response of 49 and 53 dpm for duplicate runs (Supplemental Material). Thus, the analytical range for this assay was (420–50)/50 = 7.4, which was 20-fold less than that for the LC-MS/MS GALC assay. As noted above, an increment in GALC activity corresponding to 0.4% of that measured with GM13793-HIGH was observed with the LC-MS/MS with high confidence. This corresponded in the radiometric GALC assay to 0.004 × (420–50) = 1.5 dpm, which was only 3% of the dpm value measured in the no-lysate control. When we carried out the no-lysate control as 5 replicates we obtained a blank of 49 ± 3 dpm. Clearly it was not possible to measure increments of GALC activity with the radiometric assay of <2–3% of normal activity. Also of note, the radiometric assay was performed with 50 μg of cell lysate protein and required 2–3 mL of patient blood, whereas the LC-MS/MS assay was performed with 10- to 20-fold less protein and 0.5 mL of patient blood was sufficient for >15 GALC assays, thus allowing triplicate assays to be routinely performed. It is difficult to obtain > 2–3 mL of blood from newborns.
GALC activity in T-lymphocytes from Krabbe disease patients
We performed LC-MS/MS assays to measure the small amounts of residual GALC enzymatic activity in T-lymphocytes prepared from venous blood drawn from 8 patients. Three patients (EIKD-1, -2, -3) were infants confirmed by clinical examination to have early infantile Krabbe disease, at which time the blood was drawn for LC-MS/MS GALC assay. One patient (LOKD, Table 2) was 12-y old at the time of the blood collection and became symptomatic for Krabbe disease in the late-infantile period (stage II symptoms (10)). Three infants were identified by the New York newborn screening program but did not have symptoms of Krabbe disease (High Risk-KD-1, Moderate Risk-KD-1, Moderate Risk-KD-2). The high risk patient was placed in the high risk category by the New York Krabbe disease consortium based on a radiometric GALC activity in the lowest bracket (≦ 0.15 nmole product/hr/mg protein), whereas moderate risk patients were assigned to the moderate risk category (next bracket up). Results are shown in Table 2. Table 2 shows the repeat triplicate GALC assay results on the the 3 EIKD patients and the 1 LOKD patient after the lymphocytes were stored for 4 months at −80°C. The inter-assay variation was small in that the same difference was seen in the GALC values between the EIKD and LOKD patients as was seen earlier. The %CV for ~20 repeats of our Quality Control High (GM13763-HIGH lymphoblasts) carried out over a ~1 year period was 6%.
Table 2.
GALC activities measured by LC-MS/MS in T-lymphocytes isolated from patient blood.a
| Patient | Approximate age of onset of symptoms | Age at time of blood draw | GALC Activity (% of GM13763-HIGH) | Psychosine Psychosine in blood, nM |
|---|---|---|---|---|
| EIKD-1 | < 1 mo | < 1 mo | 0.49 ± 0.09 (0.42 ± 0.08)b | 15.7 |
| EIKD-2 | < 1 mo | < 1 mo | 0.35 ± 0.02 (0.38 ± 0.04)b | 26.1 |
| EIKD-3 | < 1 mo | < 1 mo | 0.30 ± 0.08 (0.29 ± 0.08)b | 20.4 |
| LOKD-1 (late infantile) | 10 y | 12 y | 1.72 ± 0.31 (1.79 ± 0.28)b | 6.8 |
| High Risk-KD-1 | asymptomatic, high risk | < 1 mo | 1.46 ± 0.17 | 3.0 |
| Moderate Risk-KD-1 | asymptomatic, moderate risk | < 1 mo | 11.02 ± 1.28 | 0.4 |
| Moderate Risk-KD-2 | asymptomatic, moderate risk | < 1 mo | 5.78 ± 0.24 | 0.5 |
EIKD is early-infantile Krabbe disease (onset of symptoms < 1 month), LOKD is late onset Krabbe disease, and High Risk-KD and Moderate Risk-KD patients are asymptomatic newborn (see main text for description of risk category). Error bars are the standar deviations from triplicate analyses.
Numbers in parenthesis are repeat triplicate analyses done after 4 mo of storage at −80°C of the lysates assayed originally.
All 3 early infantile Krabbe disease patients showed GALC activity in T lymphocytes that was the same as that seen in GM06805-LOW LCL lymphoblasts, which are null for GALC. On the other hand, the late onset Krabbe and the asymptomatic newborn patients had statistically significant higher levels of GALC. The number of patients analyzed was too small to make a firm conclusion that the new GALC assay can better predict the onset of Krabbe disease.
Table 3 shows the radiometric GALC activity in lymphocytes for 14 previously identified patients by the New York Krabbe disease newborn screening program. All 14 patients were placed in the high risk category by the newborn screening program based on leukocyte radiometric GALC of ≦ 0.15 nmole product/hr/mg protein) (Table 3). In this table genotypes are given along with Krabbe disease status. Four patients were confirmed by clinical examination to have early infantile Krabbe disease. The remaining 10 patients have not displayed Krabbe disease symptoms as far as we know. All 4 patients with early infantile Krabbe disease had two severe mutations in both copies of the GALC gene (either the 30 kb deletion or a frame shift or a C-terminal extension of 42 amino acids). There was no general trend that the radiometric GALC assay gave lower enzymatic activity for symptomatic versus asymptomatic patients. In fact one of the symptomatic patients (EIKD-4) displayed a GALC activity of 0.12 nmole/hr/mg protein, which was 12-fold higher than the activity measured in EIKD-1 and was as high or higher than GALC activity in all of the asymptomatic patients. EIKD-4 is homozygous for the 30 kb deletion and should be devoid of functional GALC protein. Thus, the radiometric GALC assay gave activity values that were not correlated with strong predictions based on severe genotypes or with the severity of Krabbe disease. Estimated errors in the values in Table 3 were not available.
Table 3.
Data for 15 patients identified by the New York Krabbe disease newborn screening program.
| Patient | Radiometric GALC Activity (nmole/hr/mg protein) | Genotype | Krabbe disease status |
|---|---|---|---|
| EIKD-4 | 0.01 | p.R168C_g.30Kb Del//p.R168C_g.30 Kb Del | Infantile Krabbe disease |
| EIKD-5 | 0.05 | p.R168C_g.30 Kb Del//p.I546T_p.X670Qext42 | Infantile Krabbe disease |
| EIKD-6 | 0.02 | p.R168C_g.30Kb Del//p.R168C_g.30 Kb Del | Infantile Krabbe disease |
| EIKD-7 | 0.12 | p.R168C_g.30Kb Del//p.G360DfsX2 | Infantile Krabbe disease |
| High Risk-KD-2 | 0.06 | asymptomatic | |
| High Risk-KD-3 | 0.12 | asymptomatic | |
| High Risk-KD-4 | 0.07 | asymptomatic | |
| High Risk-KD-5 | 0.09 | asymptomatic | |
| High Risk-KD-6 | 0.12 | asymptomatic | |
| High Risk-KD-7 | 0.03 | asymptomatic | |
| High Risk-KD-8 | 0.05 | asymptomatic | |
| High Risk-KD-9 | 0.05 | asymptomatic | |
| High Risk-KD-10 | 0.06 | asymptomatic | |
| High Risk-KD-11 | 0.07 | asymptomatic |
Stability of GALC in whole blood
Freshly drawn blood must be shipped to the biochemical genetics laboratory where the GALC assay will be performed. It was thus important to study the stability of GALC in whole blood prior to isolation of T-lymphocytes. Whole blood was collected into a K2EDTA collection tube, and 0.5 mL aliquots were stored at ambient temperature (~21°C) or at 4°C for up to 5 d. At regular intervals T-lymphocytes were isolated and submitted to LC-MS/MS assay of GALC enzymatic activity. Blood stored up to 3 d at 4°C lost ≤15% of the initial GALC activity, whereas blood stored at ambient temperature for up to 3 days lost up to 30% of the initial GALC activity (Table 4). Based on this, we recommend that blood be collected, stored at 4°C until shipment the same day, and sent for arrival in < 3 d with frozen gel packs at ~4°C.
Table 4.
Whole blood GALC stability studies.
| Blood storage | GALC Activity (nmole/hr/mg) |
|---|---|
| Fresh blood (adult 1) | 3.66 |
| 3 d at 4°C storage (adult 1) | 3.91 |
| Fresh blood (adult 2) | 3.72 |
| 1 d ambient (adult 2) | 2.18 |
| 2 d ambient (adult 2) | 2.10 |
| 3 d ambient (adult 2) | 2.84 |
| Fresh blood (adult 3) | 3.55 |
| 1 d 4°C (adult 3) | 3.02 |
| 2 d 4°C (adult 3) | 3.15 |
| 3 d 4°C (adult 3) | 3.25 |
Psychosine analysis in dried blood spots from Krabbe patients
We also measured the concentration of psychosine (galactosylsphingosine) in dried blood spots from the patients listed in Table 2. Psychosine may be a physiologically-relevant substrate for GALC, and recent studies have shown that psychosine is increased in patients with early-infantile Krabbe disease but not in asymptomatic patients (11,12). All 3 early infantile Krabbe disease patients had greatly increased psychosine concentration in blood (15.7, 20.4, 26.1 nM) compared to the other samples. Of note, among the 3 asymptomatic newborns, psychosine was 6- to 8-fold increased in the patient with the lowest GALC activity among the group (1.46 % versus 5.78 and 11.2 %). Moderate Risk-KD-1 and Moderate Risk-KD-2 patients with 11.02 and 5.78 % of GALC activity, respectively, showed a low concentration of psychosine (0.4–0.5 nM), values similar to those seen in 100 random newborns (0.3–0.5 nM, data not shown). The patient with late-onset Krabbe disease also showed an intermediate increased psychosine of 6.8 nM. These results suggest that the degree of psychosine increase correlates with the level of GALC enzymatic activity. The results also showed that an increase in GALC activity of just a few percent near the low led to a lack of psychosine accumulation to any measurement extent, showing that it is critical to measure GALC enzymatic activity very accurately in the 0–5% range. The LC-MS/MS method developed in this study showed the analytical resolution to accomplish this. Further studies may prove whether measurement of psychosine or accurate GALC activity or both is best at predicting the severity of Krabbe disease.
Prospects for Krabbe Disease Diagnosis and Prediction
The main purpose of this report was to show that it was possible to develop an assay of GALC that had a much higher accuracy than the currently used radiometric GALC assay. The preliminary data on a small number of patients supports the new LC-MS/MS GALC as having the potential to predict which patients will develop Krabbe disease early in life, later on, or not at all.
Supplementary Material
Acknowledgments
This work was supported by Grants from the National Institutes of Health (R01 DK067859) and from the Legacy of Angels Foundation (www.tloaf.org).
Abbreviations
- GALC
human galactocerebrosidase
- GLB1
human β-galactosidase-1
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
References
- 1.Wenger DA, Rafi MA. Krabbe disease: One Hundred years from the bedside to the bench to the bedside. J Neurosci Res. 2016;94:982–9. doi: 10.1002/jnr.23743. [DOI] [PubMed] [Google Scholar]
- 2.Escolar ML, West T, Dallavecchia A, Poe MD, LaPoint K. Clinical managemenet of Krabbe disease. J Neurosci Res. 2016;94:1118–25. doi: 10.1002/jnr.23891. [DOI] [PubMed] [Google Scholar]
- 3.Escolar ML, Poe MD, Provenzale MJ, Richards KC, Allison J, Wood S, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. NEJM. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 4.Orsini JJ, Kay DM, Saavedra-Matiz CA, Wenger DA, Duffner PK, et al. Newborn screening for Krabbe disease in New York State: the first eight years’ experience. Genet Med. 2016;18:239–48. doi: 10.1038/gim.2015.211. [DOI] [PubMed] [Google Scholar]
- 5.Gelb MH, Scott CR, Turecek T. Newborn screening for lysosomal storage diseasees. Clin Chem. 2015;61:335–46. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenger DA, Sattler M, Clark C, McKelvey H. An improved method for the identification of patients and carriers of Krabbe’s disease. Clin Chim Acta. 1974;56:199–206. doi: 10.1016/0009-8981(74)90228-9. [DOI] [PubMed] [Google Scholar]
- 7.Luzi P, Rafi MA, Wenger DA. Characterization of the large deletion in the GALC gene found in patients with Krabbe disease. Hum Mol Genet. 1995;4:2335–8. doi: 10.1093/hmg/4.12.2335. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–96. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffner PK, Caggana M, Orsini JJ, Wenger DA, Patterson MC, Crosley CJ, et al. Newborn screening for Krabbe disease: the New York State model. Pediatr Neurol. 2009;40:245–52. doi: 10.1016/j.pediatrneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Escolar ML, Poe MD, Martin HR, Kurtzberg J. A stagging system for infantile Krabbe disease to predict the outcome after unrelated umbilical cord blood transplantation. Pediatr. 2006;118:e879–89. doi: 10.1542/peds.2006-0747. [DOI] [PubMed] [Google Scholar]
- 11.Matern D, Turgeon CC, Orsini JJ, Sanders KA, Hesemann JL, Oglesbee, et al. Measurement of psychosine in dried blood spots-a possible improvement to newborn screeningn programs for Krabbe disease. J Inherit Metab Dis. 2015;38:923–9. doi: 10.1007/s10545-015-9822-z. [DOI] [PubMed] [Google Scholar]
- 12.Chuang WL, Pacheco J, Zhang XK, Martin MM, Biski CK, Keutzer JM, et al. Determination of psychosine concentration in dried blood spots from newborns that were identified by newborn screening to be at risk for Krabbe disease. Clin Chim Acta. 2013;419:73–6. doi: 10.1016/j.cca.2013.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.