Abstract
Background and Objectives
For patients with cutaneous melanoma, primary tumors located in the head and neck is associated with poor outcomes. The reason for this difference and whether it is applicable to all locations within the head and neck remains unclear. We hypothesized that scalp melanoma is uniquely distinguished from other anatomic sites and is independently responsible for the poor prognosis of head and neck melanoma.
Methods
Query and analysis of a prospectively maintained melanoma database of all patients treated for primary cutaneous melanoma from 1971 – 2010.
Results
Of 11,384 patients identified, 7% (n=799) of lesions originated on the scalp. Scalp primaries were more often found in males and were associated with increased Breslow thickness and were more frequently ulcerated compared to all other anatomic sites (p=0.0001). On multivariate analysis, scalp location was an independent predictor of worse melanoma-specific (HR 1.75; CI 1.50–2.04; p<0.0001) and overall survival (HR 1.62; CI 1.41–1.86: p<0.0001).
Conclusions
This, the largest series examining scalp melanoma, confirms that scalp location is independently responsible for the negative prognosis associated with head and neck melanoma. Although the pathophysiology of this difference remains to be determined, these data argue for more rigorous surveillance of this anatomic location.
Keywords: Primary cutaneous melanoma, scalp, prognosis, outcomes
Introduction
Despite heightened public awareness and improvement in early detection through screening programs, cutaneous melanoma continues to represent one of several cancers with increasing incidence over the last several decades.[1] In particular, melanoma arising from the head and neck region as a subgroup is being diagnosed with increased frequency.[2] Primary cutaneous melanoma of the head and neck consists of lesions arising on the scalp, face, ears, and neck, each occurring with distinct individual frequencies.[3] As a group, head and neck melanoma has long been associated with a worse overall prognosis compared to cutaneous melanoma arising from all other anatomic sites.[4] Whether these differences in long term outcomes may be attributed to unique anatomic, biologic or environmental factors remains unclear.
Melanoma arising on the scalp, as a subset of head and neck melanoma, has in the past been recognized as a “high-risk” anatomic location.[5–7] Large population based studies using the Surveillance, Epidemiology and End Results (SEER) database have suggested that scalp location may be an independent factor contributing to negative outcomes, in particular worse melanoma-specific and overall survival.[8, 9] Unfortunately, these studies have been constrained by the fact that scalp and neck melanoma are a combined category in the SEER database, so delineating the true impact of scalp location alone was not possible. In smaller series, examining the relative impact of individual sites of the head and neck generated conflicting results.[10, 11]
Therefore, to our knowledge, the clinical implication of scalp location on head and neck melanoma has not been adequately defined. We hypothesized that the scalp is associated with an outcome profile that distinguishes it from all other anatomic sites and is responsible for the generally poor prognosis attributed to melanoma of the head and neck.
Methods
We queried our prospectively maintained database from 1971 through 2010 for all patients diagnosed with cutaneous melanoma and having a known primary site. In order to avoid referral bias favoring recurrent cases as well as the effect of treatment delay due to referral time, analysis was limited to patients who were treated at our institution within 4 months of their initial diagnosis. Patients with more than one primary melanoma were excluded from analysis. Anatomic sites of the primary cutaneous melanoma were classified into the following subgroups: scalp, face/neck/ear, trunk and extremity.
Treatment consisted of wide local excision with excision margins determined by recommendations current during the treatment era as well as sentinel lymph node biopsy when indicated based on histopathological findings of the initial biopsy specimen. Clinical follow up consisted of complete dermatologic and physical examination every 3 months during the first 2 years and every 4–6 months for the next 3 years and then annually thereafter. Routine blood work including complete blood count, comprehensive metabolic panel and lactate dehydrogenase as well as radiographic studies were obtained annually when indicated based on pathologic stage.
Clinical factors such as age and gender as well as histopathological features including Breslow thickness, presence of ulceration, number of positive lymph nodes when examined, and overall stage based on the American Joint Committee on Cancer Staging Manual[12] were compared between anatomic sites of the primary cutaneous melanoma using chi-square test. Melanoma-specific (MSS) and overall survival (OS) by primary site were analyzed and compared using Kaplan-Meier method and compared using the log-rank test. In order to account for survival differences based on stage, stage for stage survival analysis was performed. In order to identify significant independent predictors of 5-year melanoma-specific (MSS) and overall survival (OS), multivariable analysis using Cox proportional-hazards model was performed using a step-wise selection procedure to select the factors included in the final model.
Results
A total of 11,384 patients with a known primary melanoma were treated at our institution between January 1, 1971 and December 31, 2010. Of those 7% (n=799) originated on the scalp. The remainder of head and neck melanomas (face/neck/ear) consisted of 11% (n=1,241) of all melanomas treated at our center during the study period (Table 1). Males made up the majority (58%) of cases of melanoma and male gender was more common among all anatomic sites except extremity melanoma, which was more common among females. The proportion of males presenting with scalp lesions were significantly greater than any other anatomic site (80%, p=0.0001). Patients with trunk melanoma were significantly younger at the time of diagnosis compared to all other anatomic sites (p<0.0001). On average, lesions of the scalp were thicker at presentation (2.6 mm) compared to melanomas on the remainder of the head and neck (1.7 mm) (p<0.0001). In addition, presence of ulceration was less frequently identified in non-scalp head and neck locations compared to lesions of the scalp (10% vs. 16%, p=0.0001) (Table 2). Tumor-positive lymph nodes were more commonly associated with scalp primaries (20%) than any other anatomic site (p=0.0001).
Table 1.
Demographics and Histopathological Characteristics for All Patients Diagnosed with Primary Cutaneous Melanoma Stratified by Anatomic Location
| Extremity | Face/Neck/Ear | Scalp | Trunk | Total | P-value | |
|---|---|---|---|---|---|---|
| Variable | n=3112 (27) | n=1241 (11) | n=799 (7) | n=6232 (55) | n=11384 | |
|
| ||||||
| Gender | ||||||
| Male | 1404 (45) | 860 (69) | 638 (80) | 3657 (59) | 6559 (58) | <0.0001 |
| Female | 1708 (55) | 381 (31) | 161 (20) | 2575 (41) | 4825 (42) | |
| Age | ||||||
| Mean +/− SD | 51 +/− 16.6 | 56 +/− 17.0 | 54 +/− 18.0 | 48 +/− 15.9 | 50 +/− 16.6 | <0.0001 |
| Breslow | ||||||
| Mean +/− SD | 1.9 +/− 2.3 | 1.7 +/− 1.6 | 2.6 +/− 3.2 | 1.8 +/− 1.9 | 1.9 +/− 2.1 | <0.0001 |
| Ulceration | ||||||
| Absent | 1923 (62) | 787 (63) | 467 (58) | 3788 (61) | 6965 (61) | 0.0001 |
| Present | 489 (16) | 122 (10) | 125 (16) | 790 (13) | 1526 (13) | |
| Unknown | 700 (22) | 332 (27) | 207 (26) | 1654 (26) | 2893 (25) | |
| Nodal stage | ||||||
| N0 | 1280 (41) | 406 (33) | 263 (33) | 2232 (36) | 4181 (37) | <0.0001 |
| N1 | 217 (7) | 57 (5) | 75 (9) | 504 (8) | 853 (7) | |
| N2 | 130 (4) | 36 (3) | 49 (6) | 259 (4) | 474 (4) | |
| N3 | 86 (3) | 13 (1) | 42 (5) | 163 (3) | 304 (3) | |
| Unknown | 1399 (45) | 729 (59) | 370 (46) | 3074 (49) | 5572 (49) | |
| AJCC Stage | ||||||
| I/II | 2054 (66) | 798 (64) | 416 (52) | 3867 (62) | 7135 (63) | <0.0001 |
| III | 721 (23) | 216 (17) | 241 (30) | 1490 (24) | 2668 (23) | |
| IV | 315 (10) | 209 (17) | 132 (17) | 818 (13) | 1474 (13) | |
| Unknown | 22 (1) | 18 (2) | 10 (1) | 57 (1) | 107 (1) | |
AJCC = American Joint Committee on Cancer
Table 2.
Comparison of Demographics and Histopathological Characteristics for Patients with Scalp vs Non-Scalp Head and Neck Melanoma
| Face/Neck/Ear | Scalp | Total | P-value | |
|---|---|---|---|---|
| Variable | n=1241 | n=799 | n=2040 | |
|
| ||||
| Gender | ||||
| Male | 860 (69) | 638 (80) | 1498 (73) | <0.0001 |
| Female | 381 (31) | 161 (20) | 542 (27) | |
| Age | ||||
| Mean +/− SD | 56 +/− 16.8 | 54 +/− 18.0 | <0.0001 | |
| Breslow | ||||
| Mean +/− SD | 1.7 +/− 1.6 | 2.6 +/− 3.2 | <0.0001 | |
| Ulceration | ||||
| Absent | 787 (63) | 467 (58) | 1254 (62) | 0.0004 |
| Present | 122 (10) | 125 (16) | 247 (12) | |
| Unknown | 332 (27) | 207 (26) | 539 (26) | |
| Nodal stage | ||||
| N0 | 406 (33) | 263 (33) | 669 (33) | <0.0001 |
| N1 | 57 (5) | 75 (9) | 132 (6) | |
| N2 | 36 (3) | 49 (6) | 85 (4) | |
| N3 | 13 (1) | 42 (5) | 55 (3) | |
| Unknown | 729 (59) | 370 (46) | 1099 (54) | |
| AJCC Stage | ||||
| I/II | 798 (64) | 416 (52) | 1214 (60) | <0.0001 |
| III | 216 (17) | 241 (30) | 457 (22) | |
| IV | 209 (17) | 132 (17) | 341 (17) | |
| Unknown | 18 (2) | 10 (1) | 28 (1) | |
AJCC = American Joint Committee on Cancer
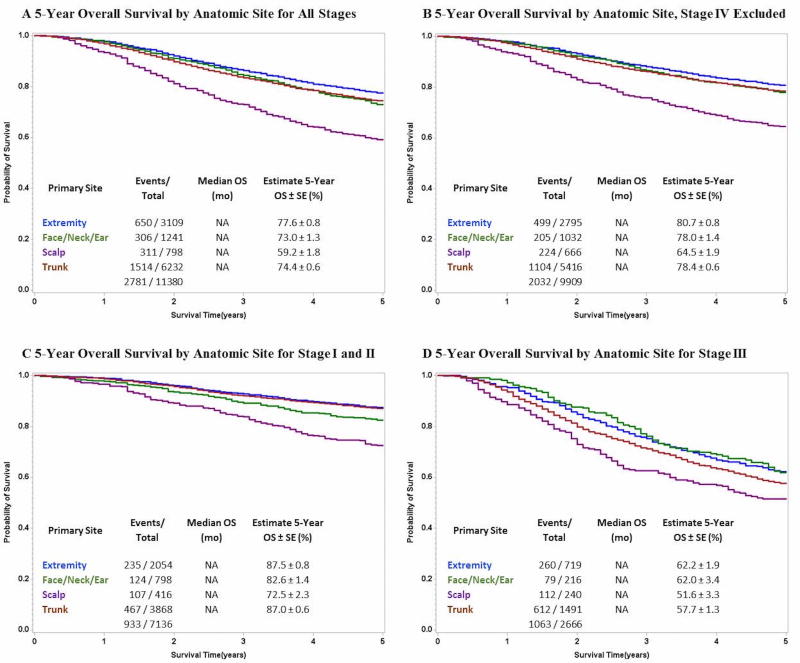
On univariate analysis, scalp location was associated with significantly reduced 5-year OS for all stages compared to all other anatomic sites (p<0.0001) (Figure 1a). When stage IV patients were excluded from analysis a statistically significant worse overall survival remained (p<0.0001) among scalp melanoma patients compared to the other groups (Figure 1b). This effect persisted when anatomic site was compared stage for stage among stage I and II patients (Figure 1c) as well as stage III patients alone (Figure 1d).
Figure 1.
Comparison of 5-Year Overall Survival for Cutaneous Melanoma Stratified by Anatomic Site for A) All Stages, B) with Stage IV Excluded, C) Stage I and II Only and D) Stage III Only
Multivariable analysis was performed to identify independent predictors of reduced 5-year MSS and OS. The analysis included common prognostic variables such as age, sex, primary anatomic site, tumor thickness categorized by T-stage, presence of ulceration, and lymph node status. Analysis revealed that scalp location was independently associated with worse MSS (HR 1.75; CI 1.50–2.04; p<0.0001) and OS (HR 1.62; CI 1.41–1.86; p<0.0001) compared to all other anatomic sites (Table 3). Other head and neck primary tumor locations were not significantly associated with outcomes worse than extremity, the reference site. Age, sex, T stage, ulceration and nodal status were also significant.
Table 3.
Cox Proportional-Hazards Model of 5-Year Melanoma-Specific, Overall and Distant-Disease Free Survival
| Multivariable 5-Year MSS | Multivariable 5-Year OS | Multivariable 5-Year DDFS | |
|---|---|---|---|
| HR (95% CI), P-value | HR (95% CI), P-value | HR (95% CI), P-value | |
| Variable | |||
|
| |||
| Male Sex | 1.27 (1.16–1.38), <.0001 | 1.26 (1.16–1.37), <0.0001 | 1.25 (1.14–1.36), <.0001 |
| Age (continuous) | 1.01 (1.01–1.01), <.0001 | 1.02 (1.02–1.02), <0.0001 | 1.00 (1.00–1.01), 0.0001 |
| Primary Site | |||
| Extremity (ref) | ** | ** | ** |
| Scalp | 1.75 (1.50–2.04), <.0001 | 1.62 (1.41–1.86), <.0001 | 2.06 (1.79–2.38), <.0001 |
| Face/Neck/Ear | 1.15 (0.98–1.34), 0.0930 | 1.13 (0.99–1.30), 0.0770 | 1.37 (1.19–1.59), <.0001 |
| Trunk | 1.36 (1.22–1.51), <.0001 | 1.27 (1.16–1.39), <.0001 | 1.37 (1.24–1.52), <.0001 |
| T stage | |||
| T1: 0.01–1.00 (ref) | ** | ** | ** |
| T2: 1.01–2.00 | 2.18 (1.87–2.55), <.0001 | 1.89 (1.65–2.15), <.0001 | 2.30 (2.00–2.65), <.0001 |
| T3: 2.01–4.00 | 3.93 (3.37–4.58), <.0001 | 3.11 (2.73–3.55), <.0001 | 3.68 (3.19–4.25), <.0001 |
| T4: >4.00 | 5.24 (4.43–6.20), <.0001 | 4.13 (3.57–4.78), <.0001 | 5.03 (4.29–5.9), <.0001 |
| T: Unknown | 3.23 (2.78–3.75), <.0001 | 2.41 (2.12–2.74), <.0001 | 2.94 (2.56–3.38), <.0001 |
| Ulceration | |||
| Absent (ref) | ** | ** | ** |
| Present | 1.95 (1.74–2.18), <.0001 | 1.79 (1.62–1.98), <.0001 | 1.92 (1.72–2.14), <.0001 |
| Unknown | 1.98 (1.80–2.19), <.0001 | 1.88 (1.72–2.05), <.0001 | 2.18 (1.98–2.39), <.0001 |
| Lymph Node Status | |||
| Negative (ref) | ** | ** | ** |
| Positive | 3.01 (2.68–3.37), <.0001 | 2.77 (2.50–3.07), <.0001 | 2.58 (2.31–2.88), <.0001 |
| Unknown | 1.32 (1.18–1.46), <.0001 | 1.31 (1.19–1.44), <.0001 | 1.28 (1.15–1.41), <.0001 |
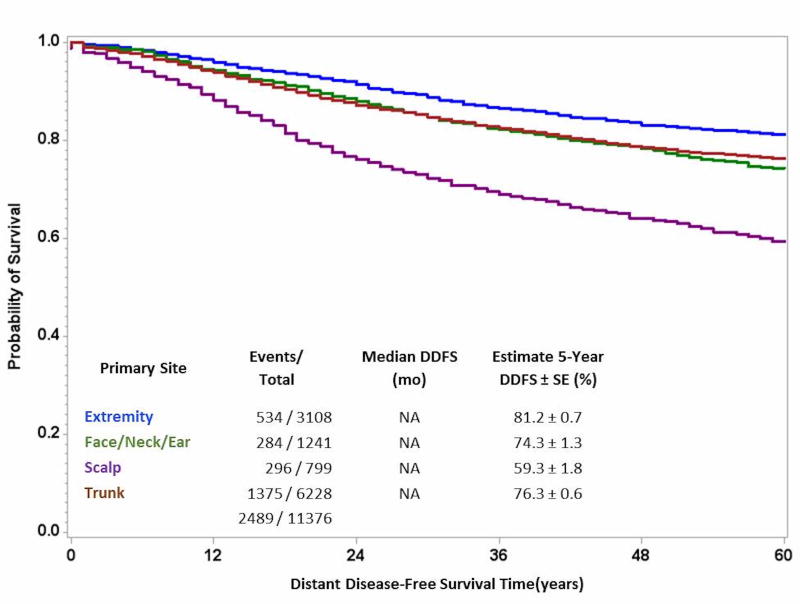
Given the significantly worse MSS and OS associated with primary lesions of the scalp, we elected to perform additional analysis to determine if this negative affect on survival could be attributed to higher rates of developing metastatic disease. As demonstrated in Figure 2, scalp location had worse distant disease-free survival compared to all other anatomic sites. Furthermore, on multivariable analysis, scalp location was independently associated with a 2-fold worse distant disease-free survival (HR 2.06; CI 1.79–2.38; p<0.0001).
Figure 2.
Comparison of 5-year Distant Disease-Free Survival Stratified by Anatomic Site of Primary Cutaneous Melanoma, All Stages
Discussion
The role of primary anatomic site as a prognostic indicator in cutaneous melanoma has been debated for decades.[5–7] Although several studies have attempted to elucidate the impact of anatomic site in the context of head and neck melanoma in the past, the relative impact of scalp location alone has remained unclear. Urist et al. published an early series on head and neck melanoma in 534 clinically stage I patients and noted that patients with scalp and neck melanoma had a worse prognosis than tumors located on the face or ear[4]. A more recent study by Leong et al. examined the impact of sentinel node status and other common prognostic factors on outcomes in a cohort of 629 patients with head and neck melanoma who underwent sentinel lymph node biopsy. This study reaffirmed that tumor site was indeed an independent predictor of mortality with scalp location being associated with the highest rate of recurrence and a more than 3-fold greater mortality than tumors of the face.[13] In the Sunbelt Melanoma Trial, among patients who underwent sentinel lymph node biopsy for melanomas > 1.0 mm in thickness and clinically negative nodal disease, scalp and non-scalp head and neck melanoma had differing clinical and histopathological features that affected long term outcomes. Of their 109 scalp melanoma patients, sentinel lymph node status was the strongest predictor of overall survival while in non-scalp melanoma patients, Breslow thickness and presence of ulceration were the primary predictors associated with reduced survival.[14] Unfortunately, while each of these series is informative, conclusions drawn are inherently limited by the small populations of patients examined. In order to address, this limitation, large population-based database analyses have been performed and have produced similar results. Using the SEER database, scalp and neck melanoma were associated with significantly decreased MSS and OS compared with other areas of the head and neck.[8, 9] The most recent of these studies by Tseng et al. featured a robust number of patients (n=27,097) of which 34% presented with melanoma of the scalp or neck. Unfortunately, SEER does not differentiate between scalp and neck melanoma, instead combining both of these anatomic sites into one category for analysis. Therefore, it is impossible to assess the true impact of scalp location alone.
In the current study, we sought to compare a pure population of primary cutaneous scalp melanoma patients to all other anatomic sites at a single institution using a large prospectively maintained melanoma database. We found that non-scalp head and neck melanoma had similar 5-year MSS and OS compared to melanoma of the trunk and extremities. Conversely, scalp melanoma was associated with significantly worse 5-year MSS and OS. Even after excluding stage IV patients, scalp primary site, thicker Breslow, lymph node positivity, presence of ulceration, older age and male gender were all found to be associated with significantly worse overall and melanoma-specific survival. Taken together these findings suggest that scalp melanoma represents a distinct entity and is probably responsible for the generally poor prognosis associated with head and neck melanoma. In addition, scalp melanoma appears to behave in a more aggressive fashion compared to melanoma originating from other anatomic sites.
Several theories have been proposed to explain why scalp melanoma may be associated with worse outcomes compared to melanoma originating from other anatomic sites. The lymphatic drainage pattern of the scalp is complex and can often be variable and unpredictable.[15–17] To highlight this fact, it has previously been reported that the rate of false-negative sentinel lymph node biopsy in head and neck melanoma may be as high as 32%.[18] Therefore, anatomic variations in lymphatic drainage patterns of the head and neck may predispose to greater risk of distant site recurrence. This was demonstrated in the current study, as scalp location was independently associated with worse distant disease-free survival compared to all other anatomic sites. In addition, it has been hypothesized that given anatomic constraints of the scalp, patients may undergo inadequate excision predisposing to higher rates of local recurrence.[19] Finally, as shown in this study, melanoma of the scalp typically presents at more advanced stages with thicker lesions, more frequently are ulcerated and more commonly are associated with nodal involvement. Taken together, these findings may suggest that scalp lesions go unrecognized due to their location or possibly are associated with longer delay times between time of patient recognition and initial presentation to a health care provider.
Recently, mutations in the N-ras oncogene have been recognized as a common genetic mutation occurring in cutaneous melanoma with an incidence as high as 18%.[20] This mutation has been associated with specific histologic subtypes of melanoma as well as distinctive tumor locations, predominately being found in nodular melanoma arising in the setting of areas of chronic sun-damaged skin secondary to exposure to ultraviolet radiation.[20, 21] Head and neck melanoma, given their anatomic location and propensity for sun exposure, appears to be particularly susceptible to N-ras mutations. Jiveskog et al. compared frequencies of N-ras mutations between sun-exposed areas of the head and neck to unexposed regions of the body and found N-ras to be mutated in 32% of head and neck melanoma specimens, but only 7% of melanomas developing in unexposed sites.[22] N-ras mutations have also been shown to be associated with thicker lesions and have higher rates of mitotic activity. Furthermore, N-ras mutation appears to be an independent adverse prognostic factor associated with decreased MSS (HR 2.96, p=0.04).[23] Therefore, a complex combination of anatomic, biologic and environmental factors may be contributing to the aggressive nature and pathogenesis of scalp melanoma.
There are several limitations to the current study. Given the duration of the studied time period, significant variations in standard staging procedures undoubtedly occurred, which may make the study population somewhat heterogeneous. In addition, there may exist a component of referral bias among the cohort of patients studied given we are a tertiary melanoma-referral center. However, referral bias would be unlikely to selectively affect scalp melanomas, and the extent of this bias should be limited by our exclusion of patients not seen at our center within 4 months of their initial diagnosis or with multiple primary melanomas. Finally, our multivariable analysis included T and N stage independently rather than AJCC staging because of the issue of collinearity in multivariable analysis, since T and N stage are factors determining AJCC stage. Nonetheless, analysis was repeated using AJCC stage and similar results were obtained.
In summary, scalp melanoma appears to present at later stage and behave more aggressively than melanoma from all other anatomic sites. Anatomic, biologic and environmental factors may contribute to the pathogenesis of scalp melanoma. Further studies to elucidate the unique characteristics of scalp melanoma are warranted. Clinically, decisions concerning indication for sentinel lymph node biopsy in thin melanomas of the scalp should be made in light of these findings. In addition, closer follow-up may be warranted among these patients due to increased rates of recurrence. Non-scalp head and neck melanoma may not carry as negative a prognosis as previously thought. These findings support increased attention to the scalp as a component of skin surveillance in an effort to improve early detection and improve outcomes.
Conclusion
We report the largest series of scalp melanoma to date. Scalp melanoma is associated with reduced melanoma-specific and overall survival compared to non-scalp head and neck, trunk and extremity melanoma. Thus, scalp melanoma alone may be responsible for the overall poor long term outcomes of head and neck melanoma as a whole. Additional studies are warranted to ascertain unique biologic, anatomic or environmental factors contributing to the pathogenesis of scalp melanoma.
Acknowledgments
Disclosure: Research reported in this publication was supported by grants (CA189163 and CA29605) from the National Cancer Institute and by funding from the Amyx Foundation, Inc (Boise, ID), the Borstein Family Foundation (Los Angeles, CA), Dr Miriam and Sheldon G. Adelson Medical Research Foundation (Boston, MA), and the John Wayne Cancer Institute Auxiliary (Santa Monica, CA). The content of this report is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Abbreviations
- SEER
Surveillance, Epidemiology and End Results
- MSS
Melanoma-specific survival
- OS
Overall survival
- AJCC
American Joint Committee on Cancer
- DDFS
Distant Disease-Free Survival
Footnotes
Disclosure: The results and opinions expressed in this article are those of the authors, and do not reflect the opinions or official policy of the United States Army or the Department of Defense.
References
- 1.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 2.Lipsker D, Engel F, Cribier B, et al. Trends in melanoma epidemiology suggest three different types of melanoma. Br J Dermatol. 2007;157:338–343. doi: 10.1111/j.1365-2133.2007.08029.x. [DOI] [PubMed] [Google Scholar]
- 3.Shashanka R, Smitha BR. Head and neck melanoma. ISRN Surg. 2012;2012:948302. doi: 10.5402/2012/948302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urist MM, Balch CM, Soong SJ, et al. Head and neck melanoma in 534 clinical Stage I patients. A prognostic factors analysis and results of surgical treatment. Ann Surg. 1984;200:769–775. doi: 10.1097/00000658-198412000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstock MA, Morris BT, Lederman JS, et al. Effect of BANS location on the prognosis of clinical stage I melanoma: new data and meta-analysis. Br J Dermatol. 1988;119:559–565. doi: 10.1111/j.1365-2133.1988.tb03465.x. [DOI] [PubMed] [Google Scholar]
- 6.Law MM, Wong JH. Evaluation of the prognostic significance of the site of origin of cutaneous melanoma. Am Surg. 1994;60:362–366. [PubMed] [Google Scholar]
- 7.Garbe C, Buttner P, Bertz J, et al. Primary cutaneous melanoma. Prognostic classification of anatomic location. Cancer. 1995;75:2492–2498. doi: 10.1002/1097-0142(19950515)75:10<2492::aid-cncr2820751015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the Surveillance, Epidemiology, and End Results (SEER) program. Arch Dermatol. 2008;144:515–521. doi: 10.1001/archderm.144.4.515. [DOI] [PubMed] [Google Scholar]
- 9.Tseng WH, Martinez SR. Tumor location predicts survival in cutaneous head and neck melanoma. J Surg Res. 2011;167:192–198. doi: 10.1016/j.jss.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien CJ, Coates AS, Petersen-Schaefer K, et al. Experience with 998 cutaneous melanomas of the head and neck over 30 years. Am J Surg. 1991;162:310–314. doi: 10.1016/0002-9610(91)90138-4. [DOI] [PubMed] [Google Scholar]
- 11.Gillgren P, Mansson-Brahme E, Frisell J, et al. A prospective population-based study of cutaneous malignant melanoma of the head and neck. Laryngoscope. 2000;110:1498–1504. doi: 10.1097/00005537-200009000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB American Joint Committee on Cancer. AJCC cancer staging manual. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 13.Leong SP, Accortt NA, Essner R, et al. Impact of sentinel node status and other risk factors on the clinical outcome of head and neck melanoma patients. Arch Otolaryngol Head Neck Surg. 2006;132:370–373. doi: 10.1001/archotol.132.4.370. [DOI] [PubMed] [Google Scholar]
- 14.Cappello ZJ, Augenstein AC, Potts KL, et al. Sentinel lymph node status is the most important prognostic factor in patients with melanoma of the scalp. Laryngoscope. 2013;123:1411–1415. doi: 10.1002/lary.23793. [DOI] [PubMed] [Google Scholar]
- 15.Fincher TR, O'Brien JC, McCarty TM, et al. Patterns of drainage and recurrence following sentinel lymph node biopsy for cutaneous melanoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2004;130:844–848. doi: 10.1001/archotol.130.7.844. [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Franc BL, Kashani-Sabet M, Singer MI. Lymphatic drainage patterns of head and neck cutaneous melanoma observed on lymphoscintigraphy and sentinel lymph node biopsy. Head Neck. 2006;28:249–255. doi: 10.1002/hed.20328. [DOI] [PubMed] [Google Scholar]
- 17.Klop WM, Veenstra HJ, Vermeeren L, et al. Assessment of lymphatic drainage patterns and implications for the extent of neck dissection in head and neck melanoma patients. J Surg Oncol. 2011;103:756–760. doi: 10.1002/jso.21865. [DOI] [PubMed] [Google Scholar]
- 18.Miller MW, Vetto JT, Monroe MM, et al. False-negative sentinel lymph node biopsy in head and neck melanoma. Otolaryngol Head Neck Surg. 2011;145:606–611. doi: 10.1177/0194599811411878. [DOI] [PubMed] [Google Scholar]
- 19.Rawlani R, Rawlani V, Qureshi HA, et al. Reducing margins of wide local excision in head and neck melanoma for function and cosmesis: 5-year local recurrence-free survival. J Surg Oncol. 2015;111:795–799. doi: 10.1002/jso.23886. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011;164:776–784. doi: 10.1111/j.1365-2133.2010.10185.x. [DOI] [PubMed] [Google Scholar]
- 21.Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–588. doi: 10.1002/humu.20481. [DOI] [PubMed] [Google Scholar]
- 22.Jiveskog S, Ragnarsson-Olding B, Platz A, Ringborg U. N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membranes or unexposed skin. J Invest Dermatol. 1998;111:757–761. doi: 10.1046/j.1523-1747.1998.00376.x. [DOI] [PubMed] [Google Scholar]
- 23.Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24:666–672. doi: 10.1111/j.1755-148X.2011.00873.x. [DOI] [PubMed] [Google Scholar]