Abstract
Background and Objectives
Primary surgical treatment of patients with early T-classification (T1-T2) oropharyngeal squamous cell carcinoma (OPSCC) has increased. We sought to determine how often these patients receive postoperative chemoradiation (CRT).
Methods
Patients with T1-T2 OPSCC in the National Cancer Database who underwent primary surgery were evaluated for receipt of postoperative CRT. Postoperative CRT use was examined among patients with high risk factors (positive margins and/or extracapsular spread (ECS)), intermediate risk factors (negative margins, no ECS, and either pT3–4 and/or N2-N3), and no apparent risk factors.
Results
Of 4,833 patients with T1-T2 OPSCC who underwent primary surgery, 43% had high risk pathologic factors, of whom only 63% received postoperative CRT. Another 31% had no apparent risk factors, of whom 16% nonetheless received postoperative CRT. On multivariable analysis, in addition to tumor and demographic factors, patients treated at community hospitals were more likely to receive postoperative CRT (O.R. 1.41 C.I. 1.18–1.87, p=0.001).
Conclusions
Variation in postoperative CRT use indicates a lack of consensus and/or knowledge about its benefits and indications. Usage of postoperative CRT regardless of pathologic risk factors suggests an area where future efforts at implementation of best practices may be targeted.
Keywords: HPV-related oropharyngeal squamous cell carcinoma (OPSCC), adjuvant chemoradiation (postoperative CRT), pathologic risk factors, National Comprehensive Cancer Network (NCCN) guidelines, hospital-level variation
INTRODUCTION
We have recently shown that the choice of primary surgical treatment for patients with T1-T2 oropharyngeal squamous cell carcinoma (OPSCC) increased from 56% of patients in 2004 to 82% of patients by 2013.1 This trend paralleled growing enthusiasm for transoral robotic surgery (TORS) and other means of transoral endoscopic head and neck surgery.2–5 In this same time period, there has also been an emerging appreciation that patients with HPV-related OPSCC, who typically present with low T-classification (T1-T2) disease, have better survival outcomes than patients with non-HPV-related OPSCC regardless of treatment choice,6,7 raising the potential to de-escalate therapy in these patients. One rationale for a primary surgical approach in early T-classification OPSCC is the opportunity to avoid acute and long-term toxicities associated with adjuvant treatment.
However, little is known about the current use of adjuvant treatments in surgically managed T1 and T2 OPSCC. Since 2005, National Comprehensive Cancer Network (NCCN) guidelines have defined risk factor groups based on pathologic features prognostic for survival and/or locoregional recurrence, and made recommendations about adjuvant treatment based on these groups. Patients at highest risk are those with positive margins and/or extracapsular spread (ECS) outside of lymph nodes.8 In this group, use of postoperative chemoradiation therapy (postoperative CRT) has been a Category 1 (highest level) recommendation until 2016 based on prospective RTOG and EORTC data and a subsequent combined analysis published in 2004–2005.9–11 Of note, tumor HPV status was not a variable in these analyses. Patients at intermediate risk include those with advanced pathologic T-stage, advanced N-stage, nodal disease in levels IV/V, perineural invasion, and vascular embolism. The recommendation (Category 2A or 2B over the years) for this group has been for postoperative radiation (RT) alone or “consider” CRT. Patients at low risk have no adverse pathologic features and are considered adequately treated with surgery alone.
The goal of this study was to identify national trends in the use of postoperative adjuvant treatment for surgically treated T1 and T2 OPSCC. Specifically, we sought to understand how often postoperative CRT is recommended based on guideline risk factor groups, as well as how often and to whom it is actually given.
MATERIALS AND METHODS
Data Source
The data source for this study was the National Cancer Database (NCDB), which is a joint program of the Commission on Cancer (CoC) and the American College of Surgeons (ACS).12 The NCDB is a hospital-based registry that collects data from over 70% of new cancer cases in the United States each year, and over 80% of cases from the oral cavity and pharynx.13 It is best used for assessing processes of care such as treatment trends.14 The source files were used in accordance with the NCDB Participant User Files (PUF) data use agreement. This study was given IRB waiver by Memorial Sloan Kettering Cancer Center.
Study Cohort
We identified all patients with clinically staged T1 and T2 OPSCC diagnosed between 2004–2013 who were ≥ 18 years old. We included International Classification of Disease for Oncology (ICD-O) codes for the oropharynx (ICDO C019, C090, C091, C098, C099, C100, C101, C102, C103, C104, C108, C109, C142). We included only patients with histologically proven SCC tissue examined by microscope rather than cytology alone; the tissue could be examined from biopsy or surgical pathology specimens. We excluded patients who had received part or all of their treatment outside of the NCDB reporting facility, whose treatment information was missing or for whom the sequence of treatments was not clear, and whose staging information was inconsistent with treatment information or could not be assessed (Figure 1).
Figure 1.
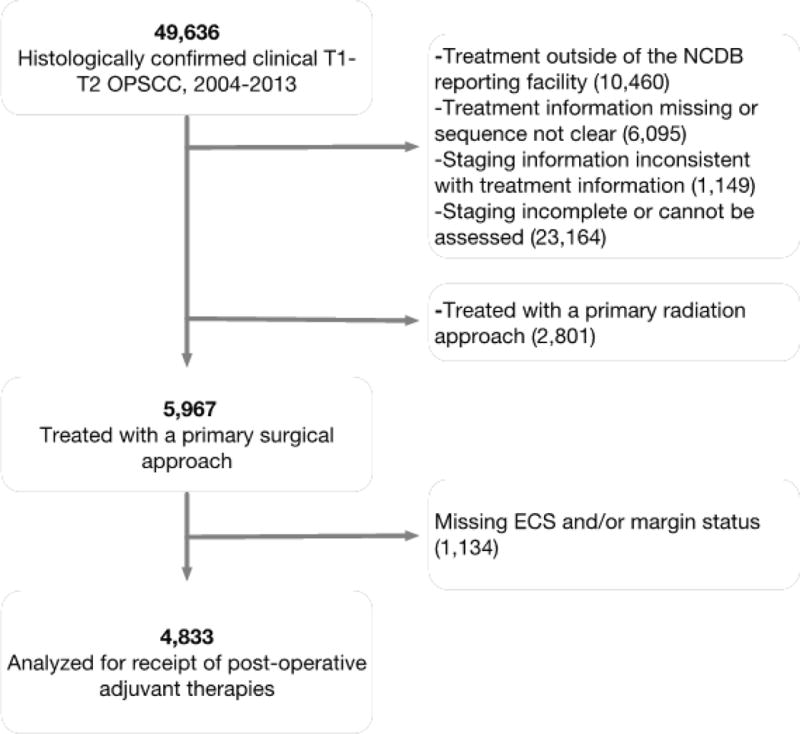
Flow diagram of patient inclusion/exclusion
We then selected patients who underwent a primary surgical approach as initial treatment. Primary surgical patients were those who underwent more than a “local tumor excision” before any radiation or chemotherapy. For tonsil and other oropharynx all categories of “pharyngectomy” were used. Base of tongue tumors (ICD C019) are listed with other tongue tumors; primary surgical patients were those who underwent at least “glossectomy.” After selecting primary surgical patients we excluded those who had incomplete information on margin status and ECS (Figure 1).
Outcomes
The outcome variable of interest was the use of postoperative CRT among patients undergoing primary surgery for T1-T2 OPSCC. Radiation was determined to be given if patients were labeled as receiving external beam radiation but no other types of treatment within 120 days of surgery. Postoperative CRT was determined to be given if patients had radiation and were labeled as receiving any type of cytotoxic chemotherapy or immunologic agent (such as cetuximab) within 120 days of surgery. We included patients who were recommended chemotherapy but refused, because the analysis aims to capture primary surgical patients who were intended to have adjuvant therapy. We also evaluated the use of postoperative CRT stratified by guideline risk factor groups: we defined a “high risk” group as patients with either positive margins and/or ECS, an “intermediate risk” group as patients with negative margins, no ECS, and either pT3–4 and/or N2-N3; and a “low risk” group as patients with negative margins, no ECS, pT1-T2, and N0-N1. Of note, other intermediate risk factors such as perineural spread and lymphovascular invasion were not available from the NCDB.
Tumor -Related Variables
We used pathologic T-classification in analyzing outcomes since all patients in the cohort underwent surgery and it is the pathologic stage that may influence the decision regarding adjuvant therapy. Similarly, we used “N-stage overall” which we defined as pathologic staging (if neck dissection performed) or clinical staging otherwise. ECS is defined in the NCDB as either negative, microscopic, macroscopic, or unknown; for this analysis ECS was determined to be positive if microscopically or macroscopically positive. Margins were defined as positive if there was microscopic or macroscopic “residual tumor.” Close margins are not recorded in the NCDB and would be considered negative by coding rules.
Additional Covariates
Patient sociodemographic factors included age, sex, race, insurance status, and comorbidities (Charlson-Deyo Comorbidity index15). Hospital volume was defined as the total number of new OPSCC cases seen over all years from 2004–2013 regardless of primary treatment, with hospitals divided into quartiles by volume for analysis. Hospital type was defined as either “academic,” which included Academic/Research programs as well as National Cancer Institute (NCI)-designated Comprehensive Cancer Centers, or “community,” which included Community Cancer Programs, Comprehensive Community Cancer Programs, Integrated Network Cancer Programs, and other specified types of programs. Less than 3% of the hospital type variable was missing; we imputed this information based on a given hospital’s designated type (academic or community) available from other patients at that hospital, by year of the missing data.
Analysis
We used descriptive and chi-square statistics to compare tumor, patient sociodemographic, and hospital factors among patients who underwent postoperative CRT versus those who did not, and to compare hospital provision of postoperative CRT according to risk factor groups. For multivariable analysis, we used hierarchical generalized linear models with a logit link to account for clustering of patients within hospitals while evaluating the influence of tumor, patient sociodemographic, and hospital factors on the binary outcome of receipt of postoperative CRT. Because the large sample size allowed us to include all variables of interest in the multivariable model, no model selection was necessary. P-values <0.05 were considered significant. Analyses were conducted using Stata Statistical Software (Release 12.1; Stata Inc., College Station, TX).
RESULTS
Characteristics of the cohort
We identified 4,833 patients who presented with T1-T2 OPSCC between 2004–2013, underwent primary surgical therapy, and had complete pathologic information for analysis (Table 1). The majority were male (78%), age 50–64 (56%), and were white (92%). The majority had private insurance (69%) and zero comorbidities on the Charlson-Deyo index (81%). More than half (59%) were treated at academic cancer programs. While 31% were treated at high volume hospitals that saw >50 patients with OPSCC, 28% were treated at very low volume hospitals where only 10 or fewer patients with OPSCC were treated over the study period. Regarding tumor factors, 25% of patients treated with primary surgery had positive margins, and 24% had ECS.
Table 1.
Receipt of postoperative CRT in patients with T1 to T2 OPSCC
| Characteristic | Overall | Receipt of Postoperative CRT | Multivariable Analysis of Postoperative CRT | ||
|---|---|---|---|---|---|
|
|
|
|
|||
|
| |||||
| No. (Column %) |
No. (Row % Compared With Data Not Shown: No Postoperative CRT)a |
pb | Adjusted OR (95% C.I.) | pb | |
| Patients | 4833 | 2120 (43.9%) | |||
|
| |||||
| Extracapsular spread | p<0.0001 | ||||
| Yes | 1208 (25%) | 814 (67.4%) | 2.75 (2.31 – 3.27) | p<0.0001 | |
| No | 3625 (75%) | 1306 (36%) | 1 [Reference] | ||
| Margins positive | p<0.0001 | ||||
| Yes | 1168 (24.2%) | 692 (59.2%) | 1.96 (1.66 – 2.32) | p<0.0001 | |
| No | 3665 (75.8%) | 1428 (39%) | 1 [Reference] | ||
| Nodal classificationc | p<0.0001 | ||||
| N0 | 1230 (25.5%) | 156 (12.7%) | 1 [Reference] | ||
| N1 | 921 (19.1%) | 368 (40%) | 3.67 (2.88 – 4.67) | p<0.0001 | |
| N2 | 2544 (52.6%) | 1497 (58.8%) | 7.50 (6.02 – 9.35) | p<0.0001 | |
| N3 | 138 (2.9%) | 99 (71.7%) | 11.85 (7.47 – 18.80) | p<0.0001 | |
| Pathologic T-classificationd | p=0.013 | ||||
| T1 | 2287 (47.3%) | 956 (41.8%) | 1 [Reference] | ||
| T2 | 2227 (46.1%) | 1009 (45.3%) | 1.09 (0.95 – 1.27) | p=0.223 | |
| T3 | 208 (4.3%) | 95 (45.7%) | 1.35 (0.94 – 1.94) | p=0.102 | |
| T4 | 111 (2.3%) | 60 (54.1%) | 1.09 (0.68 – 1.73) | p=0.732 | |
| Age at diagnosis, y | p<0.0001 | ||||
| <50 | 959 (19.8%) | 512 (53.4%) | 5.43 (2.89 – 10.21) | p<0.0001 | |
| 50–64 | 2682 (55.5%) | 1263 (47.1%) | 4.44 (2.40 – 8.21) | p<0.0001 | |
| 65–79 | 1057 (21.9%) | 329 (31.1%) | 3.66 (2.00 – 6.71) | p<0.0001 | |
| ≥80 | 135 (2.8%) | 16 (11.9%) | 1 [Reference] | ||
| Sex | p<0.0001 | ||||
| Male | 3786 (78.3%) | 1761 (46.5%) | 1.24 (1.04 – 1.48) | ||
| Female | 1047 (21.7%) | 359 (34.3%) | 1 [Reference] | p=0.017 | |
| Race | p<0.0001 | ||||
| White | 4429 (91.6%) | 1984 (44.8%) | 1 [Reference] | ||
| Black | 268 (5.5%) | 79 (29.5%) | 0.63 (0.45 – 0.88) | p=0.007 | |
| Other | 136 (2.8%) | 57 (41.9%) | 0.94 (0.62 – 1.44) | p=0.776 | |
| Insurance status | p<0.0001 | ||||
| Private | 3310 (68.5%) | 1623 (49%) | 1.54 (1.23 – 1.93) | p<0.0001 | |
| Uninsured | 187 (3.9%) | 93 (49.7%) | 1.76 (1.16 – 2.67) | p=0.008 | |
| Governmente | 1336 (27.6%) | 404 (30.2%) | 1 [Reference] | ||
| Charlson-Deyo Comorbidity Index | p<0.0001 | ||||
| 0 | 3908 (80.9%) | 1769 (45.3%) | 1 [Reference] | ||
| 1 | 754 (15.6%) | 293 (38.9%) | 0.91 (0.74 – 1.11) | p=0.330 | |
| 2 | 171 (3.5%) | 58 (33.9%) | 0.71 (0.48 – 1.07) | p=0.106 | |
| Year diagnosed | p<0.0001 | ||||
| 2004 | 225 (4.7%) | 76 (33.8%) | 1 [Reference] | ||
| 2005 | 235 (4.9%) | 112 (47.7%) | 2.07 (1.33 – 3.23) | p=0.001 | |
| 2006 | 299 (6.2%) | 149 (49.8%) | 2.39 (1.56 – 3.65) | p<0.0001 | |
| 2007 | 306 (6.3%) | 175 (57.2%) | 3.52 (2.30 – 5.38) | p<0.0001 | |
| 2008 | 480 (9.9%) | 285 (59.4%) | 3.37 (2.28 – 4.99) | p<0.0001 | |
| 2009 | 569 (11.8%) | 313 (55%) | 3.30 (2.25 – 4.84) | p<0.0001 | |
| 2010 | 580 (12%) | 235 (40.5%) | 2.33 (1.58 – 3.44) | p<0.0001 | |
| 2011 | 647 (13.4%) | 267 (41.3%) | 2.19 (1.50 – 3.21) | p<0.0001 | |
| 2012 | 719 (14.9%) | 265 (36.9%) | 1.89 (1.30 – 2.75) | p=0.001 | |
| 2013 | 773 (16%) | 243 (31.4%) | 1.46 (1.00 – 2.13) | p=0.051 | |
| Facility type | p<0.0001 | ||||
| Academic/ NCI CCC | 2848 (58.9%) | 1083 (38.0%) | 1 [Reference] | ||
| Community | 1985 (41.1%) | 1037 (52.2%) | 1.49 (1.18 – 1.87) | p=0.001 | |
| Hospital volume | p<0.0001 | ||||
| 1–10 pts | 1360 (28.1%) | 704 (51.8%) | 1.41 (0.99 – 2.01) | p=0.055 | |
| 11–20 pts | 847 (17.5%) | 359 (42.4%) | 0.99 (0.70 – 1.42) | p=0.973 | |
| 21–50 pts | 1107 (22.9%) | 454 (41%) | 1.02 (0.72 – 1.45) | p=0.911 | |
| >50 pts | 1519 (31.4%) | 603 (39.7%) | 1 [Reference] | ||
“No postoperative CRT” includes patients who underwent surgery alone, or surgery followed by radiation. For ease of visualization, this column of data is not shown in the table.
p-values in bold type are <.05
Nodal stage defined as pathologic N-stage if available, otherwise clinical stage.
Pathologic T stage used because all patients in table had primary surgery; pathologic stage related to use of adjuvant therapy
Medicare and Medicaid
Postoperative CRT
Overall 44% (2120) of patients had postoperative CRT. In 2004, 34% of patients received postoperative CRT, which rose to 59% in 2008 and then dropped to 31% by 2013 (Figure 2). Patients were more likely to receive postoperative CRT if they had ECS, positive margins, advanced nodal stage, or advanced pathologic T stage (p>0.0001 for all except T-stage p=0.013; Table 1 Column 2). Patients were more likely to receive postoperative CRT if they were younger, male, white, had private or no insurance compared to government insurance, and had fewer comorbidities (p<0.0001 for all; Table 1 Column 2). Patients were more likely to receive postoperative CRT if they were treated at community hospitals compared to academic hospitals (52% vs. 38%, p<0.0001), and at the lowest volume hospitals compared to the highest volume hospitals (52% vs. 40%, p<0.0001; Table 1 Column 2).
Figure 2.
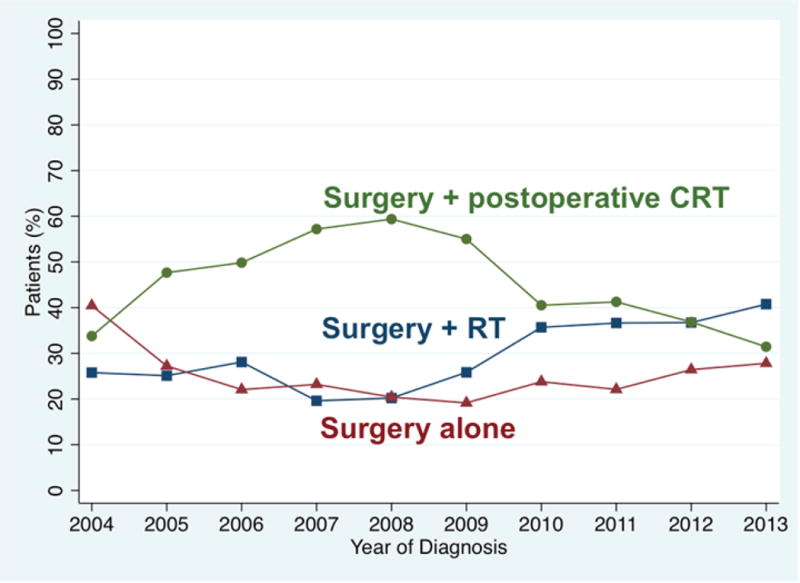
Adjuvant treatments for patients with T1-T2 OPSCC undergoing primary surgical approach, for whom ECS and margin status are known. N= 4833 including 1528 (32%) receiving surgery alone, 1185 (25%) receiving surgery and post-operative radiation (RT), and 2120 (44%) receiving postoperative CRT.
Tumor risk factor groups
Of the total of 2120 patients who received postoperative CRT, 62% (1304) had high risk pathologic factors (ECS and/or positive margins), 28% (583) had intermediate risk factors without high risk factors, and 11% (233) had no apparent risk factors.
Patients in different risk factor groups received variable amounts of postoperative CRT. A total of 43% (2074) of patients undergoing primary surgery had high risk pathologic factors (Table 2); of these, 63% received postoperative CRT. Another 27% (1280) of patients undergoing primary surgery had intermediate risk factors (N2-N3 and/or pT3-T4 but no ECS, negative margins); of these, 46% received postoperative CRT. An additional 31% (1479) of patients undergoing primary surgery had no apparent pathologic risk factors (no ECS, negative margins, pT1-T2, and N0-N1); of these, 16% received postoperative CRT.
Table 2.
Pathologic Guideline Indication Groups and Receipt of Postoperative CRT
| Total N (Column %) | Received Postoperative CRT N (Row %) | |
|---|---|---|
| Patients | 4833 | 2120 (43.9%) |
|
High Risk (positive margins and/or ECS) |
2074 (42.9) | 1304 (62.9) |
|
Intermediate Risk (N2-N3, pT3-T4, negative margins, no ECS) |
1280 (26.5) | 583 (45.6) |
|
Low Risk (negative margins, no ECS, N0-N1, pT1-T2) |
1479 (30.6) | 233 (15.8) |
Hospital level variation
When examined according to risk factor group and the hospital where they were treated, patients were more likely to receive postoperative CRT if they went to community hospitals or very low volume hospitals, regardless of whether they had high risk pathologic factors or no apparent risk factors (Figure 3). Wide variation can be seen across hospital types in hospitals’ provision of postoperative CRT among all patients (Figure 4A), and among patients with high risk factors or no apparent risk factors (Figures 4B, 4C).
Figure 3.
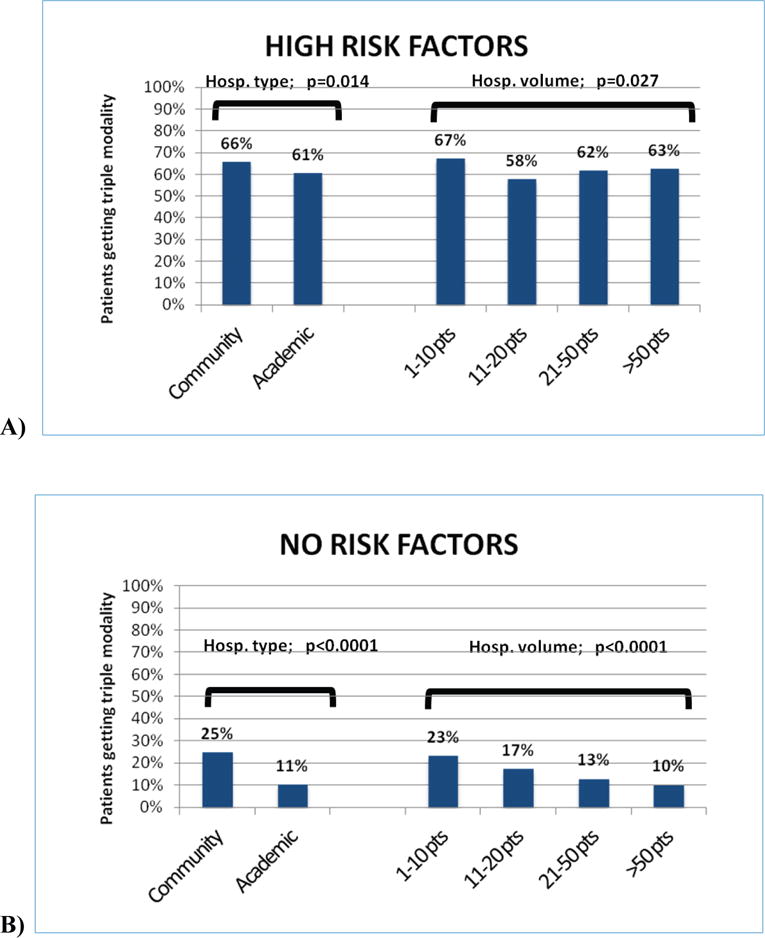
Hospitals’ provision of postoperative CRT based on pathologic risk factor groups. A) High Risk Factors Group; on the left, provision of postoperative CRT according to hospital type; on the right, provision according to hospital volume B) No Risk Factors Group; on the left, provision of postoperative CRT according to hospital type; on the right, provision according to hospital volume. P-values represent chi-square statistics.
Figure 4.
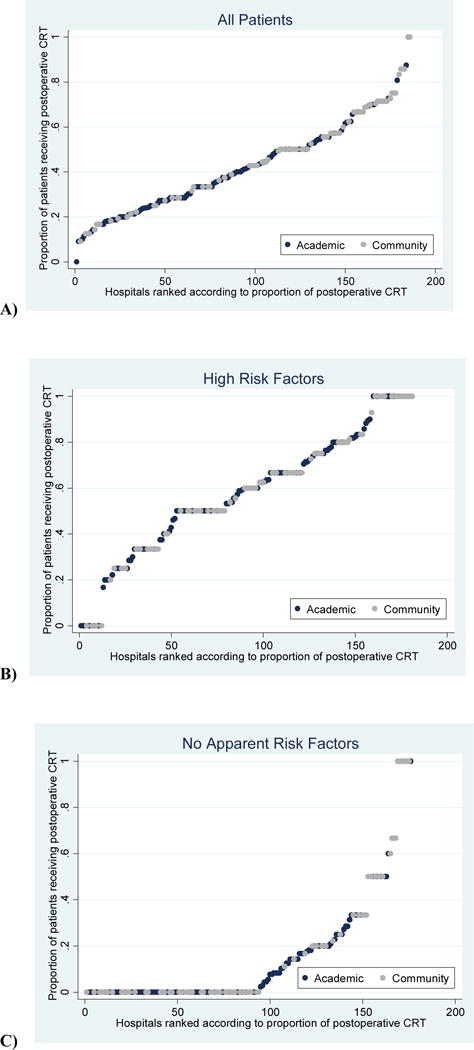
Variation in the proportion of chemotherapy given by hospitals. Note: for visual purposes, these graphs exclude hospitals seeing fewer than 6 patients over the study period, as these proportions are more likely to be at extremes of 0% or 100%. A) Hospitals’ proportion of all patients receiving postoperative CRT; B) Hospitals’ proportion of patients with high risk factors (positive margins and/or ECS) receiving postoperative CRT; C) Hospitals’ proportion of patients with no apparent risk factors (no ECS, negative margins, pT1-T2, and N0-N1) receiving postoperative CRT.
Factors associated with postoperative CRT
On multivariable analysis, both tumor and non-tumor factors predicted the use of postoperative CRT (Table 1 Column 3). Tumor factors associated with receipt of postoperative CRT were ECS (O.R. 2.75, C.I. 2.31–3.27, p<0.0001), positive margins (O.R. 1.96, C.I. 1.66–2.32, p<0.0001), and any nodal stage higher than N0 (strongest correlation N3: O.R. 11.85, C.I. 7.47–18.80, p<0.0001). Pathologic T stage was not correlated with the receipt of postoperative CRT. Treatment at a community hospital was associated with receipt of postoperative CRT (O.R. 1.49, C.I. 1.18–1.87, p=0.001), while hospital volume was not associated with postoperative CRT on multivariable analysis.
DISCUSSION AND CONCLUSION
Primary surgical therapy for patients with T1-T2 OPSCC has increased in recent years. One theoretical benefit of primary surgical treatment in appropriately selected patients is the potential to avoid toxicities related to adjuvant chemotherapy and radiation. While the NCCN has explicit guidelines regarding the use of adjuvant CRT after primary surgical treatment in OPSCC, little is currently known about the frequency and predictors of its use after primary surgery: specifically, how many of these patients actually receive it? Do characteristics other than tumor risk factor indications make patients more or less likely to receive postoperative CRT?
In this national cohort of patients undergoing primary surgery for T1-T2 OPSCC, a total of 44% of patients received postoperative CRT, of whom 65% had high risk pathologic factors (ECS and/or positive margins). Patients who had positive margins, ECS, or higher N-classification disease were more likely to receive postoperative CRT. Among all primary surgical patients, 43% had high risk pathologic factors, and 63% of these patients received postoperative CRT. Regardless of whether patients had high risk factors or no apparent risk factors, they were more likely to receive postoperative CRT at community hospitals compared to academic, and at the lowest volume hospitals compared to the highest.
Strengths of this analysis include wide representation of U.S. national treatment patterns given the broad range of hospitals in the NCDB database, robust hospital-level analysis of treatment patterns, and a time frame for analysis that covers a period of increased use of primary surgery for T1-T2 OPSCC and the emergence of level 1 data in support of adjuvant CRT.
This study has important limitations: First, many patients were excluded because of missing or poor quality data, which decreases the generalizeability of our data. Second, HPV status was only routinely recorded in the NCDB after 2010 and so we could not account for this in the analysis. Third, retrospective analyses of large secondary databases have inherent limitations related to missing variables. Specific to this study, information on perineural invasion and lymphovascular invasion are not recorded in the NCDB. This may be important because they are potential intermediate risk pathologic factors that can affect the selection of patients for postoperative CRT.
Although receipt of postoperative CRT in the present study was related to pathologic risk factors, we found that variation in use was also dependent upon hospital characteristics even after controlling for pertinent pathologic risk factors. Very low volume and community hospitals tended to give more postoperative CRT, regardless of whether patients had high risk pathologic factors or no apparent risk factors. This raises the possibility that certain institutions are more likely to be “chemo-givers” despite indications to the contrary. On bivariate analyses these institutions were more likely to be community and very low volume ones; but it is apparent that specific hospitals of both types give variable amounts of postoperative CRT. It is therefore possible that unknown hospital characteristics besides volume and type account for much of the variation. These hypotheses deserve further inquiry, but relate to the recent finding using the NCDB that high volume and academic hospitals were less likely to give adjuvant therapies to all comers with head and neck cancers.16
When wide variation is seen in the use of a medical service, and when guideline recommendations are not well adhered to, one explanation is that the indications—the balance of benefits and harms, remain uncertain to some practitioners.17 The present analysis demonstrated a significant proportion of patients who did not receive postoperative CRT despite pathologic risk factors of positive margins and/or ECS. Although these risk factors were a category 1 indication during the study period in NCCN guidelines for patients to receive postoperative CRT, the significance of these factors for patients with HPV-associated disease has been brought into question.7,18–20 A prospective study attempts to determine the importance of ECS in this population.21 One feature of postoperative CRT that is generally agreed upon is that patients who receive all three modalities of therapy at maximal intensity tend to suffer worse functional outcomes.22,23 The wide variation in use may therefore be related to lack of consensus on the benefits of and indications for postoperative CRT, specifically in HPV positive disease, and/or concern about the harms involved with tri-modal treatment.
Conversely, some practitioners may believe that there is benefit to a primary surgical approach followed by liberal provision of postoperative CRT, on the basis that this approach might improve survival outcomes. This might be the explanation for the use of postoperative CRT seen in the present cohort in patients with either intermediate risk factors or no apparent risk factors. Explicit escalation of therapy to three modalities was the subject of another prospective study in patients with HPV negative disease (RTOG 1221), but the study was closed due to lack of accrual.24
The ECOG 3311 and other trials are currently investigating whether primary surgical therapy may be beneficial in tailoring multi-modality therapy for patients with early stage OPC based on pathologic staging factors. This approach remains experimental.25 Until these trials are finished, if a stated goal of choosing a primary surgical approach remains the possibility of avoiding the toxicities of postoperative CRT, primary treatment choice should consider pre-operative prediction of the possibility of pathologic risk factors. While a certain proportion of patients will have occult or microscopic ECS not predictable on preoperative imaging, and of unclear prognostic importance, a primary surgical approach is probably suboptimal in cases with clear evidence of gross ECS. Similarly, a low rate of margin-positive resections is anticipated for any type of operation, but when positive margins are expected at a higher rate due to size or anatomy of the tumor, a primary surgical approach is probably suboptimal. Postoperative CRT might be predicted in these cases and could have been avoided by utilizing a primary non-surgical approach.
The findings of this study have important implications, especially in an era of both increased rates of HPV-related OPSCC and increased use of primary transoral endoscopic head and neck surgery. In summary, the finding that factors other than pathology and medical comorbidities predict the use of postoperative CRT and that some variation remains unexplained by the variables included in our analysis points to potential uncertainty about the benefits, indications, and harms of postoperative CRT. If the goal of treatment is both optimal survival but also optimized patient-reported outcomes and quality of life, better data is needed to understand a) who the most appropriate candidates are for primary surgical or primary radiation treatments, with the goal of deescalation to avoid functional morbidity, and b) who should receive postoperative CRT after a primary surgical treatment approach. Wide hospital level variation in the use of postoperative CRT suggests that when new data becomes available, efforts at implementation of guideline recommendations can be bolstered to ensure that practice patterns are aligned to achieve appropriate outcomes.26
Synopsis.
Primary surgical treatment of patients with early T-classification (T1-T2) oropharyngeal squamous cell carcinoma (OPSCC) has increased. We sought to determine how often and why these patients receive postoperative chemoradiation (CRT). We analyzed patients who received postoperative CRT with T1-T2 OPSCC in the National Cancer Database who underwent primary surgery. On our multivariable analysis, in addition to tumor factors and some demographic factors, patients treated at community hospitals were more likely to receive postoperative CRT, but there was no difference by hospital volume. We concluded that the variation in the use of postoperative CRT indicates a lack of consensus and/or knowledge about its benefits and indications.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Discipline: Head and Neck Cancer
Conflict of Interest: None declared (all Authors)
Author Contributions:
Overall content guarantors: B Roman; M Cohen
Conceptualization: B Roman; S Baxi; J Cracchiolo; D Pfister; S McBride; I Ganly; J Shah; S Patel; L Morris; M Cohen
Methodology: B Roman; S Baxi; J Cracchiolo; L Morris; M Cohen
Software: B Roman; S Baxi; J Cracchiolo; M Cohen
Validation: B Roman; S Baxi; J Cracchiolo; M Cohen
Formal Analysis: B Roman; S Baxi; J Cracchiolo; M Cohen
Investigation: B Roman; S Baxi; J Cracchiolo; M Cohen
Resources: B Roman; M Cohen
Data curation: B Roman; M Cohen
Writing- original draft: B Roman; S Baxi; J Cracchiolo; M Cohen
Writing- review and editing: B Roman; S Baxi; J Cracchiolo; D Pfister; S McBride; I Ganly; J Shah; S Patel; L Morris; M Cohen
Visualization: B Roman; S Baxi; J Cracchiolo; L Morris; M Cohen
Supervision: B Roman; M Cohen
Project Administration: B Roman; M Cohen; T. Blackwell
Funding acquisition: B Roman; S Baxi; J Cracchiolo; D Pfister; S McBride; I Ganly; J Shah; S Patel; L Morris; M Cohen
References
- 1.Cracchiolo JR, Baxi SS, Morris LG, et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer. 2016 Mar 11; doi: 10.1002/cncr.29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holsinger FC. New Approaches: Robotics and Endoscopic Head and Neck Surgery. In: Harrison LB, Sessions RB, Kies M, editors. Head and Neck Cancer: A Multidisciplinary Approach. 4th. 2013. [Google Scholar]
- 3.Chen AY. A shifting paradigm for patients with head and neck cancer: transoral robotic surgery (TORS) Oncology (Williston Park) 2010 Oct;24(11):1030, 1032. [PubMed] [Google Scholar]
- 4.Mydlarz WK, Chan JY, Richmon JD. The role of surgery for HPV-associated head and neck cancer. Oral oncology. 2015 Apr;51(4):305–313. doi: 10.1016/j.oraloncology.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Lorincz BB, Jowett N, Knecht R. Decision management in transoral robotic surgery (tors): Indications, individual patient selection, and role in the multidisciplinary treatment of head and neck cancer from a european perspective. Head & neck. 2015 Mar 31; doi: 10.1002/hed.24059. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010 Jul 1;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. The Laryngoscope. 2012 Sep;122(Suppl 2):S13–33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Nework. Head and Neck Cancers NCCN Guidelines Version 1.2005. http://www.nccn.org/professionals/2005.
- 9.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. 2004 May 6;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2004 May 6;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 11.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head & neck. 2005 Oct;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons. National Cancer Database (NCDB) 2015 https://www.facs.org/quality-programs/cancer/ncdb.
- 13.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Annals of surgical oncology. 2008 Mar;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. Journal of surgical oncology. 2009 Jun 15;99(8):488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen MM, Roman SA, Yarbrough WG, Burtness BA, Sosa JA, Judson BL. Trends and variations in the use of adjuvant therapy for patients with head and neck cancer. Cancer. 2014 Jul 15; doi: 10.1002/cncr.28870. [DOI] [PubMed] [Google Scholar]
- 17.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999 Oct 20;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 18.Sinha P, Lewis JS, Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012 Jul 15;118(14):3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 19.Sinha P, Piccirillo JF, Kallogjeri D, Spitznagel EL, Haughey BH. The role of postoperative chemoradiation for oropharynx carcinoma: A critical appraisal of the published literature and National Comprehensive Cancer Network guidelines. Cancer. 2015 Jan 14; doi: 10.1002/cncr.29242. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell JH, Ferris RL, Gooding W, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. 2013 Sep 15;119(18):3302–3308. doi: 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 21.Clinicaltrials.gov. Post Operative Adjuvant Therapy De-intensification Trial for Human Papillomavirus-related, p16+ Oropharynx Cancer (ADEPT) 2012 https://clinicaltrials.gov/ct2/show/NCT01687413. Accessed 4/20/2016.
- 22.Hutcheson KA, Holsinger FC, Kupferman ME, Lewin JS. Functional outcomes after TORS for oropharyngeal cancer: a systematic review. Eur Arch Otorhinolaryngol. 2015 Feb;272(2):463–471. doi: 10.1007/s00405-014-2985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonhardt FD, Quon H, Abrahao M, O’Malley BW, Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head & neck. 2012 Feb;34(2):146–154. doi: 10.1002/hed.21688. [DOI] [PubMed] [Google Scholar]
- 24.Radiation Therapy Oncology Group. RTOG 1221 Protocol Information. 2015 http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1221. Accessed May 26th, 2015.
- 25.Adelstein DJ, Ridge JA, Brizel DM, et al. Transoral resection of pharyngeal cancer: summary of a National Cancer Institute Head and Neck Cancer Steering Committee Clinical Trials Planning Meeting, November 6–7, 2011, Arlington, Virginia. Head & neck. 2012 Dec;34(12):1681–1703. doi: 10.1002/hed.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011;6:26. doi: 10.1186/1748-5908-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]


