Abstract
Genomic DNA is transiently contaminated with ribonucleotide residues during the process of DNA replication through misincorporation by the replicative DNA polymerases α, δ and ε, and by the normal replication process on the lagging strand, which uses RNA primers. These ribonucleotides are efficiently removed during replication by RNase H enzymes and the lagging strand synthesis machinery. However, when ribonucleotides remain in DNA they can distort the DNA helix, affect machineries for DNA replication, transcription and repair, and can stimulate genomic instabilities which are manifest as increased mutation, recombination and chromosome alterations. The genomic instabilities associated with embedded ribonucleotides are considered here, along with a discussion of the origin of the lesions that stimulate particular classes of instabilities.
Keywords: Ribonucleotides, genome instability, recombination, mutagenesis, DNA replication
1. Introduction
The DNA replication process is highly accurate in terms of choosing the correct base and correct sugar, and discrimination of the sugar moiety of the chosen nucleotide is an intrinsic feature of the replicative DNA polymerases [1–3]. Nonetheless, this discrimination is not perfect and given the additional challenge of higher rNTP pools compared to dNTP pools, ribonucleotides are inserted into DNA during replication at a rate of approximately one per every thousand bases replicated [4, 5]. In addition to locally distorting the DNA helix from a B-form to an A-form [6–8], ribonucleotides can hinder replication and transcription machineries and sensitize the DNA backbone to alkali [9, 10].
Generally, normal cells with intact RNase H enzymes efficiently recognize and remove ribonucleotides that are in a RNA:DNA hybrid form in a process called RER, ribonucleotide excision repair (Figure 1). Ribonucleotides that remain in DNA, usually when the RNase H enzymes are defective, ultimately result in genomic instabilities. These form three broad categories: mutagenesis with a signature of small deletions in mononucleotide or dinucleotide tracts, increased homologous recombination, and chromosome rearrangements, loss and truncations. Embedded ribonucleotides can be a target for DNA topoisomerase I (Top1) [11, 12] and some ribonucleotide-promoted instabilities are Top1-dependent (Figure 2). Top1 has a preference for ribonucleotides incorporated by DNA polymerase ε [13], resulting in an emphasis on genome instability from DNA polymerase ε errors of rNTP misincorporation. This review examines the known Top1-dependency and DNA polymerase-misincorporation for stimulation of instability, but also explores the contribution of DNA polymerases α and δ in genome instability arising from rNTP misincorporation on the lagging strand during replication. The nature of instability hotspots in light of apparently random misincorporation of ribonucleotides into DNA is also discussed.
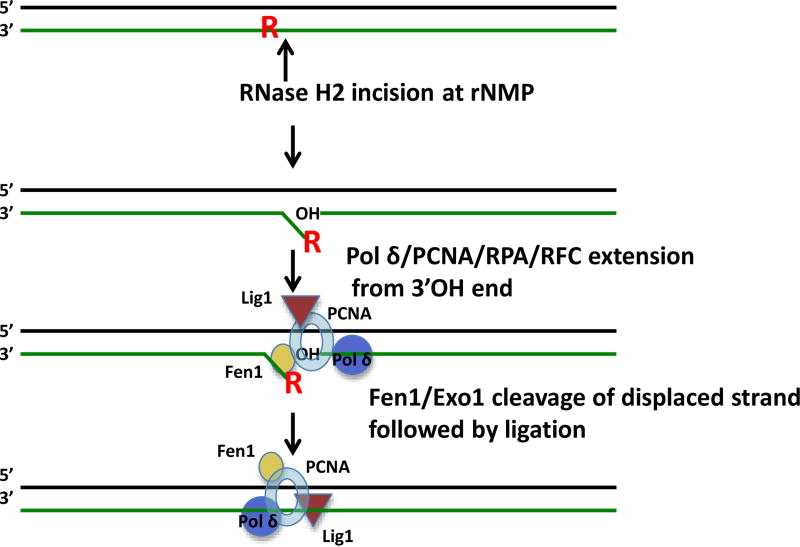
Figure 1.
The RER process. RNase H2 recognizes a rNMP residue (red R) in duplex DNA and makes an incision 5’ to the residue, creating a 3’OH end. Extension of this end by DNA Pol δ as shown or DNA pol ε (blue circle) along with associated factors PCNA (light blue open oval ring), the RFC clamp loader of PCNA and the single strand DNA binding protein RPA displaces the rNMP containing end, generating a flap. The flap is removed by the action of Fen1 nuclease as shown (yellow oval) (or Exo1 nuclease), and the nick is then sealed by Lig1 (brown triangle), completely removal of the embedded rNMP residue.
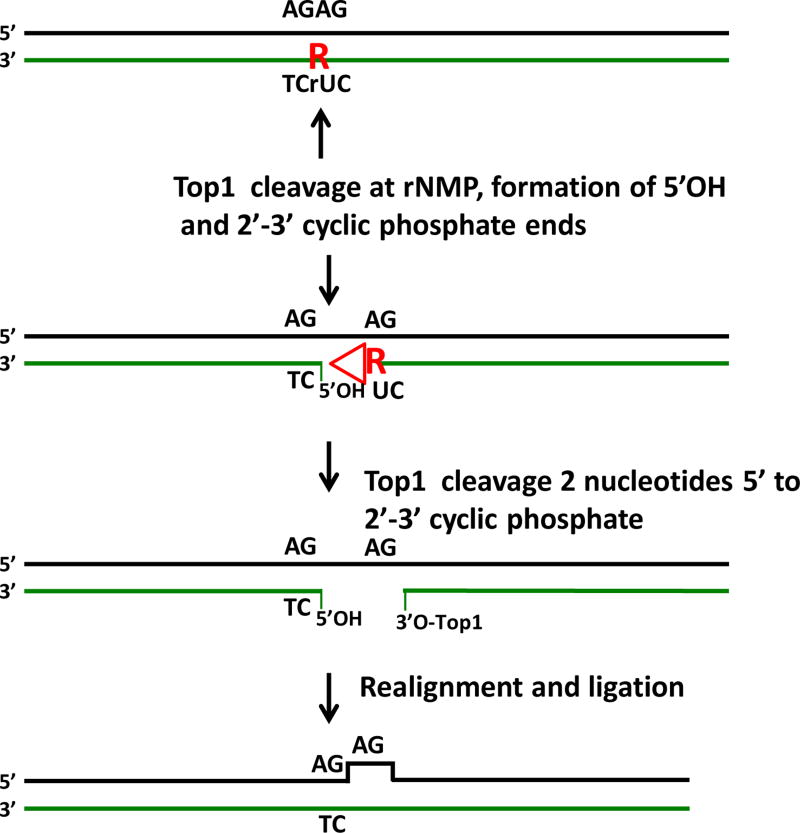
Figure 2.
Top1 processing of rNMP residues. An embedded rNMP residue (red R) is shown in a repeated sequence AGAG:TCrUC. In the absence of the RER process Top1 can cleave at rNMP residues in duplex DNA. Top1 cleaves 3’ to the residue, and in completeting the Top1 cleavage cycle, generates a 5’OH end and a 2’-3’ cyclic phosphate end (red triangle) through nucleophilic attack by the 2’OH group of the ribose sugar. A second cleavage by Top1 2 nucleotides from the 2’–3’ cyclic phosphate end releases this blocked end and the ribose residue. If the resulting gap is in a repeated sequence as shown, slippage alignment can occur during completion of the Top1 reaction and the gap is resealed by Top1, leading to a −2 deletion on the strand that originally contained the rNMP residue.
R-loops resulting from transcription/replication collisions have been shown to cause double-strand breaks and genomic instability [14]. R-loops are the target of RNase H1, which recognizes RNA:DNA hybrids of at least three nucleotides and whose action seems to be related to R-loops arising during transcription [15]. Protection against genome instability resulting from transcription-arising R-loops has been recently reviewed [14, 16, 17] and will not be discussed here. Recent reports on genome instability in mutants lacking both RNase H1 and RNase H2 enzymes and the implications for genome destabilizing lesions will be highlighted.
2. Sources of ribonucleotides in DNA
The most common source of ribonucleotides embedded in genomic DNA comes from the replicative polymerases and the failure to completely discriminate between rNTPs and dNTPs. The discrimination pocket that is a feature of all DNA polymerases ensure high accuracy in selecting the correct nucleotide during replication [3] but nonetheless there is an error rate of approximately of 1/1000 bases replicated [5]. The highest contributor to this is DNA polymerase ε, but DNA polymerases α and δ also misincorporate ribonucleotides. This may be due to a critical residue in the steric gate of the polymerase for sugar fidelity. DNA polymerase ε has a methionine residue while the DNA polymerases α and δ have a leucine residue in the gate. The methionine residue may be more permissive for incorporation of a sugar with the 2’-OH residue [4, 18, 19]. The proofreading exonuclease of DNA polymerases ε and δ do not contribute significantly to ribonucleotide removal [4]. Under altered replication conditions, for example when the dNTP pool balance is altered, incorporation of ribonucleotides may be enhanced. Gap repair by the translesion DNA polymerases may be another source of ribonucleotides. Although the replicated tract is a very small fraction of the genome, these polymerases are far more lax in the sugar selection of the base being incorporated and also lack any proofreading editing nuclease. Ribonucleotides may also become incorporated during replication restart, which can use RNA primers for replication priming. Incomplete removal of Okazaki fragment primers is also another potential source of embedded ribonucleotides, although the contribution to the RNA:DNA hybrid load in genomic DNA has not been assessed.
Another potential source of embedded ribonucleotides, particular in nondividing human cells, is the action of HIV-1 reverse transcriptase during proviral synthesis [20]. Modification of DNA through oxidative damage of oxygen free radicals has been shown to produce ribonucleotides [21], which may be an additional source of embedded ribonucleotides, particularly under stress conditions.
3. Consequences of RNase H2 mutations
RNase H2 is an essential enzyme in mouse cells and is an early embryonic lethal in mice [22, 23]. Mutations in all three subunits of the human enzyme are causative for the autoimmune disease Aicardi-Goutieres syndrome (AGS) [24] and have also been associated with systemic lupus erythromatosis [25]. The mutations found in the AGS patients appear to be hypomorphic reductions in enzymatic activity, consistent with the essentiality of the murine enzyme. In model organisms, especially in fungal systems, RNase H2 null allele mutations are viable, which has allowed an exploration of the genome integrity consequences of loss of RNase H2 and persistent embedded ribonucleotides in DNA. RNase H2 null mutants are characterized by increased mutagenesis, increased recombination, increased loss of heterozygosity (LOH), increased chromosome instability and increased chromosome rearrangements. The genome instabilities are discussed below.
3.1 Mutagenesis
The first report that deletion of one of the subunits of RNase H2 of Saccharomyces cerevisiae led to increased spontaneous mutation was in 1999 [26], when RNase H2 was being studied for a role in primer removal during DNA replication. That study suggested that slippage deletion mutations were increased in the rnh201 null mutant, which at the time was called rnh35. An earlier report on hyper-recombination mutants of S. cerevisiae showed that the hpr4-1 mutant increased spontaneous mutation [27] and later studies revealed that hpr4-1 was an allele of RNH202 [28].
The action of the RNase H2 enzyme on genomic DNA was shown to cleave 5’ of a rNMP moiety in DNA with a preferred substrate of single ribonucleotides [29]. Detailed studies of the RNase H enzymes has shown that RNase H1 requires a minimum of three consecutive ribonucleotides for recognition and cleavage while the RNase H2 enzyme can cleave at single and multiple ribonucleotides [15, 30]. Given the overlap of RNase H2 in vivo function with the Okazaki fragment processing nuclease Fen1 and the double mutant synthetic growth defect, a model for ribonucleotide removal called RER was modeled in vitro (Figure 1) [31]. The RER process is quite efficient, as demonstrated by failure to detect significant ribonucleotide residues in genomic DNA in RNase H2 proficient cells [19] and failure to detect RNase H2-defective signature mutations in wild type cells [11].
In the absence of RNase H2 activity, not only is mutation rate increased, but a novel class of mutational events, slippage in simple repeat sequences, is detected [11, 19]. The new mutational events are deletions in simple repeats of up to 5 nucleotides in length, although the most prevalent mutations are −1 deletions in mononucleotide runs and −2 deletions in simple dinucleotide repeats, repeats that are frequently found in DNA. Mutation in many of these is dependent on Top1, which has been demonstrated to cleave at ribonucleotides in DNA [11, 28], forming nonligatable ends of a 5’-OH and a 2’-3’ cyclic phosphate (Figure 2) [11, 12]. A second Top1 cleavage 2 nucleotides from the cyclic phosphate yields ends that can be sealed by the topoisomerase reaction and a −2 deletion [11, 32]. The −1 mutations in mononucleotide runs appear to be Top1-indpendent in many cases and arise from replication errors [11]. It is not clear what determines whether a short repeat may be mutable in the absence of RNase H2 activity. First, not all simple repeats are subject to mutation [33] and second, those that are mutable can be relocated to other genomic locations and still display mutability [11], indicating that there are additional aspects to the repeat that determine its mutability. Evidence that the repeat deletion mutations are rNMP-dependent has come from studies using variants of DNA Pol ε that misinsert fewer or more rNTPs that wild type polymerase. The slippage mutation rate fluctuated according to the amount of rNMPs in DNA [19, 33], suggesting that the mutations arose from processing of rNMPs in DNA. Further studies with mutant polymerase strains has shown that Top1 cleaves preferentially at rNMPs on the leading strand, incorporated by DNA Pol ε during replication [13]. However, slippage mutations are not exclusively generated by rNMPs on the leading strand and subsequent Top1 cleavage as mutations in DNA polymerases for the lagging strand also affect mutation levels. Either these rNMPs are also a target for Top1 cleavage while on the lagging strand or they may remain in the lagging strand and in the next cell cycle would be the leading strand template and could be subsequently targeted by Top1 (Figure 3).
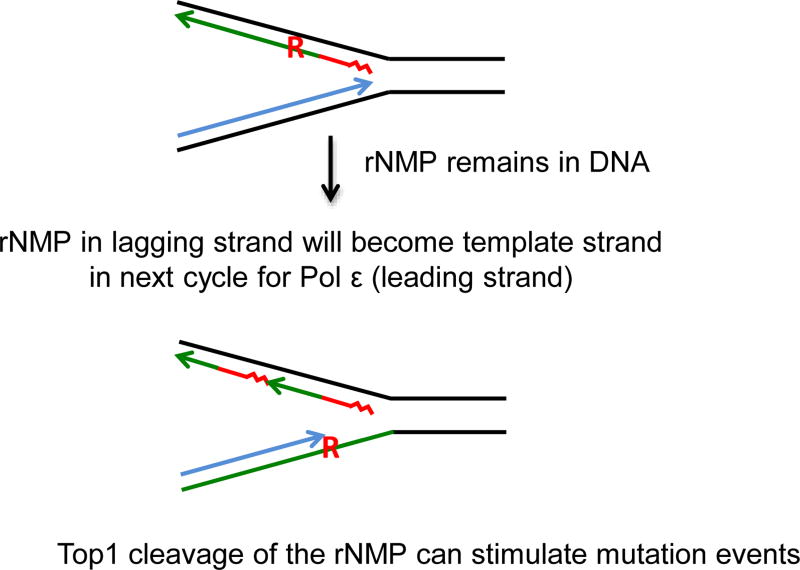
Figure 3.
Model for Top1-dependent mutagenesis from rNMPs on the lagging strand. Blue represents the leading strand during DNA replication. Red represents the lagging strand primer (squiggle) and DNA Pol α synthesis (straight line). Green represents the region replicated by DNA Pol δ. Misincorporation by DNA Pol δ results in a rNMP residue (red R). If the rNMP persists, in the next cycle it will be on the template strand for leading strand synthesis. Top1 cleavage of the rNMP during leading strand synthesis could result in a slippage mutation event.
In vitro RNase H2 not only nicks at single rNMP residues in DNA, but can also recognize and cleave at multiple tandem rNMP residues [30]. A separation of function allele in the RNH201 gene, termed rnh201-RED [30, 34], has been constructed and is completely defective in nicking at single rNMP residues but still has significant activity against tandem rNMP residues. This allele has the same rate and spectrum of spontaneous mutations as a null allele of RNH201, indicating that mutagenesis is induced by single rNMP residues in DNA.
More complex mutational events related to rNMPs have been observed under high transcription conditions [35]. In these studies short quasi-palindromic sequences were prone to mutation that increased base pairing in the palindromes in a transcription-dependent manner that also depended on RNase H1 for mutation but was independent of Top1.
All studies to date on the occurrence and nature of mutations arising from rNMPs in DNA have been performed in fungal systems where RNase H2 is not essential and mutation reporters are readily available. It is assumed that similar genome instabilities would occur in mammalian cells but this remains to be demonstrated. Moreover, the hypomorphic AGS alleles that lead to disease have not been fully studied in an experimental system for a mutator phenotype. This is limited by the fact that many AGS patients with RNase H2 mutations are compound heterozygotes, necessitating examination of both mutant alleles, and the fact that some of these alleles are in nonconserved residues.
3.2 Recombination
The hpr hyper-rec mutants were recovered on the basis of increased intrachromosomal gene conversion rates [27]. Among these mutants was hpr4-1, which was subsequently identified as an allele of RNH202 [28]. This increased recombination was partially dependent on Top1 function, but unlike the increased mutagenesis seen in RNase H2- cells, was not affect by DNA Pol ε mutants that altered rNTP misincorporation [36]. Curiously, the rnh201-RED separation of function mutant significantly reduced the recombination rate [36]. These results have suggested that tandem rNMPs in DNA, arising from errors in Okazaki fragment maturation or other events, may lead to nicks that stimulate gene conversions. This would suggest an important role for lagging strand rNMPs in creating lesions for stimulating recombination, in contrast to the preferred mutational events coming from processing of rNMPs on the leading strand by Top1 (Figure 4).
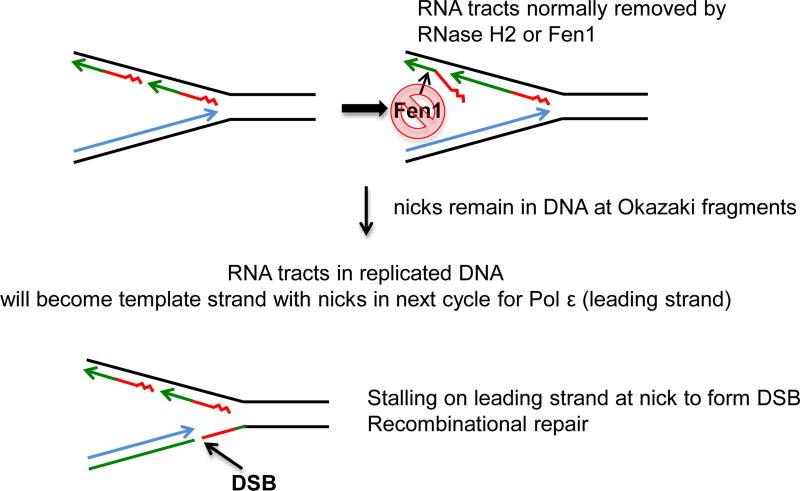
Figure 4.
Model for hyper-recombination stimulated by rNMP residues. Blue represents the leading strand during DNA replication. Red represents the lagging strand primer (squiggle) and DNA Pol α synthesis (straight line). Green represents the region replicated by DNA Pol δ. The left panel depicts a replication fork. If the region replicated by DNA Pol α contains many rNMP residues (replication with a mutant DNA Pol1), the flap normally cleaved by the Fen1 nuclease may not be removed as the red segment contains mostly rNMP residues. As DNA ligase cannot ligate RNA to DNA, this results in nicks remaining in the lagging strand. At the next cycle of replication the nicked strand becomes the template strand and will be replicated as the leading strand. Replication to the nick on the template strand will result in a DSB. Either the initial nicks or the DNS could stimulate recombination, leading to synergistic recombination increases in the RNase H2-deficient DNA Pol1-defective double mutant.
Two recent studies have examined loss of heterozygosity (LOH) events in diploid yeast strains defective in RNase H2 [37, 38]. LOH may occur through chromosome loss, reciprocal crossing over, break-induced replication (BIR), terminal deletion or other complex events. In this section LOH through recombination is discussed while LOH through chromosome loss and rearrangements is discussed in the following section. While both groups found that LOH was increased in rnh201 mutants, they differed in finding a dependence on rNMPs misincorporated by DNA Pol ε. One group found an increase LOH in a Top1-dependent manner when more rNMPs were misincorporated and a reduction in LOH when fewer rNMPs were misincorporated [37]. This was restricted to DNA Pol ε mutants and was not found using DNA Pol δ or α mutants that increased rNMP misincorporation. The LOH events most likely are initiated by DSBs. While the precise nature if the initiating lesion is unknown other than its origin in misincorporated rNMPs and Top1 action, a recent study has shown that Top1 cleavage at rNMP residues in DNA can result in DSBs [39]. Using a different LOH assay, O’Connell et al [38] found that a DNA pol ε mutant that decreased rNMP misincorporation did not alter LOH levels while the double mutant rnh201 rnh1 had a further increase in LOH over the rnh201 level. These results suggest that multiple tandem rNMPs or R-loops stimulate LOH events. The reasons for the differing results remains unclear, but each group used very different LOH reporters at different genomic locations. Using three different assays for recombination between repeated sequences, Ii et al showed that RNase H2 defective cells were hyper-rec [40]. Whether these events are dependent on Top1 is not known.
RNA:DNA hybrids have been shown to participate in DSB repair [41–43]. Transcript RNA has been proposed to bridge a DSB and promote repair through a temporary RNA:DNA hybrid. This is a template repair that invokes a reverse transcriptase step using the RNA template [44]. The repair is dependent on an intact RNA:DNA hybrid and thus is increased when RNase H activity is diminished [42]. Both RNase H1 and RNase H2 contribute to limiting RNA-templated repair with the RNase H2 activity being more important [45]. In a related study, the double RNase H mutant, rnh1 rnh201 has a significant number of cells with Rad52 foci, a marker of DNA damage and double strand breaks and the appearance of Rad52 foci began during S phase and accumulated in G2-M stages of the cell cycle [46]. Viability of these double mutants was dependent on a functional topoisomerase I, suggesting that Top1 is needed to prevent occurrence of a lethal DNA intermediate [46]. Further studies suggested that RNA:DNA hybrids inhibit DNA repair, leading to lethal recombination events and that many such events are localized to the rDNA locus [46].
In S. pombe transient RNA:DNA hybrids have also been shown to be an important intermediate in DSB repair [43]. Following DSB formation and end processing RNA polymerase is proposed lay down a short RNA primer using the 3’ single strand DNA tail to forma a RNA:DNA hybrid. Eliminating RNase H activity stabilizes the RNA:DNA hybrid and reduces DSB repair. Over-expression of RNase H1 eliminates the RNA:DNA hybrid before repair has taken place and prevents subsequent repair steps to occur. Thus a key intermediate in DSB repair is proposed to be the RNA:DNA hybrid at the DSB. This harkens to reports of small noncoding RNAs localizing to DSB in mammalian cells [47, 48]. The short ncRNAs are regulated by the RNAi factors [49]. Whether this process also occurs in S. cerevisiae is not clear as this organism lacks an RNAi system [50]. The complex repair of DSBs with ncRNAs may help in DSB repair fidelity in promoting repair by HR and limiting DSB resection, which can be deleterious if resection is too extensive, giving the potential for deletions and rearrangements. Thus the RNase H activities preserve genome stability by enhancing accurate DSB repair.
3.3 Double-strand breaks, chromosome rearrangements and LOH
The presence of unrepaired rNMPs in DNA has been proposed to provoke DSB formation through the sequential action of Top1 at the embedded rNMP and then on the opposite strand [39]. DSB formation in this manner could lead to LOH and other genomic rearrangements that would be dependent on both Top1 and the presence and frequency of rNMPs in DNA. In this scenario LOH events would be seen only in RNase H2 deficient cells and would increase with an increase in rNMP misincorporation by the replicative DNA polymerases, especially with DNA Pol ε, as has been reported [37]. If this were the case, then some of the observed hyper-rec events seen in RNase H2 mutants, those not dependent on DNA Pol ε rNMP misincorporation levels [27, 36] may be nick-promoted and not DSB promoted.
Other genome instability events such as gross chromosomal rearrangements are increased in RNase H2-defective cells [51] when combined with other mutations in genes affecting chromatin remodeling and DSB processing. In some cases formation of genome rearrangements was dependent on a functional HR system and Top1. Chromosome instability measured by loss of a yeast artificial chromosome [52] or a chromosome fragment [36] was increased in RNase H2 defective cells, similar to other LOH assays. Chromosome instability in a mutant depleted for all RNase H activity (rnh1Δ rnh201Δ) was increased almost 10-fold over wild type levels through a mechanism that was independent of the HR factor Rad51 [53].
In a recent study examining genome-wide distribution of RNA:DNA hybrids in RNase H-deficient S. cerevisiae strains [54], specificity of the RNase H enzymes in limiting RNA:DNA hybrid formation and genome instability could be seen. RNase H2 was determined to act globally across the genome to limit hybrid formation and instability whereas RNase H1 acted on specific regions to limit hybrid formation and promote stability. Why RNase H1 showed restricted protection against genomic instability is not clear as it is localized at hybrids throughout the genome [54]. This study also suggested that RNA:DNA hybrids are the main instigator of LOH events.
To investigate the genomic effects of RNase H2 depletion in a mouse system, conditional RNASEH2B mutations or reduced RNase H2B expression mutations were made in mice [22, 23]. Both groups observed that RNase H2 is essential in mice and complete deletions resulted in early embryonic lethality. Non-null alleles that severely reduce RNase H2 function allow embryos to develop further and showed partial rescue when p53 was deleted [23], allowing MEFs to be established. These cells grow slower, show hallmarks of DNA damage such as nicks and DSBs, activate a DNA damage response of phosphorylated histone H2AX and induction of p53 target genes, micronuclei formation and chromosome rearrangements, primarily translocations. Depletion of RNase H2 through shRNA sequences against RNase H2 subunits in HeLa cells led to slower cell growth, replication stress and activation of the DNA damage response [55]. Micronuclei formation was observed, a marker for DNA breakage. In these studies, genome instability and breakage was directly linked to the presence of ribonucleotides in DNA.
Although it has been noted that increased mutation due to persistent rNMPs in DNA stems mainly from rNTPs misincorporated by DNA Pol ε, nonetheless DNA Pol δ mutants that lead to higher rNTP misincorporation also have increased mutation rates [13]. The increased mutation rate may not be as dramatic as that seem with the DNA Pol ε mutant pol2-M644G as the rate of misincorporation is lower [19]. In addition, it is possible that rNMPs on the lagging strand are better tolerated until the next cell cycle, where they become part of the leading strand template where now they may be recognized by Top1 and subject to mutational repair (Figure 3). In contrast, at least for some HR events, the rNMP stimulation appears to occur preferentially on the lagging strand and to be stimulated by tandem rNMPs [36, 38] and the role of Top1 here is unknown. The strain bias and Top1 role in stimulating other genome instabilities such as chromosome loss and GCR events remains to be studied.
3.4 DNA damage checkpoint response
The presence of persistent ribonucleotides in DNA leads to a DNA damage response [22, 23, 28, 56]. What triggers the response is not completely clear, but seems to be related to the formation of nicks with non-ligatable ends through the action of Top1 [28]. This type of nick is subject to further Top1 action (Figure 2) [32, 57], and can lead to a DSB if a second nick can occur on the strand opposite to the initial nick [39, 58]. In yeast processing of ribonucleotides by Top1 in strains where there is an increased load of ribonucleotides through use of a DNA Pol ε that misincorporates more ribonucleotides or through mutations that prevent mutagenic processing of nicked sites of ribonucleotides results in activation of the S phase checkpoint [19, 28, 59]. Processing of ribonucleotides by RNase H2 through RNase H2 nicking is normally followed by strand displacement, flap removal and ligation (Figure 1) [31]. If ligation is attempted after nicking but before removal of the ribonucleotide, an abortive intermediate with an AMP-RNA-DNA junction is formed and must be processed by the Aprataxin protein, called Hnt3 in yeast. Under conditions of high ribonucleotide misincorporation, unrepaired nicks form which triggers an S phase checkpoint response. The slow growth and checkpoint response is eliminated when RNase H2 is mutated, as the nicked ribonucleotides substrate is not formed under these conditions [60]. Thus cleavage at ribonucleotides in DNA by RNase H2 or Top1 can be deleterious if the nick is not properly processed and ligated.
In mice, the conditional or hypomorphic mutations of RNase H2 subunits results in a DNA damage response as seen by slow growth of cells, accumulation in S and G2 phases, γ-H2AX foci accumulation and induction of p53 target genes [22, 23, 55].
In humans, mutations in RNase H2 that are causative for the autoimmune disease Aicardi-Goutieres syndrome (AGS) [24, 61] appear to have a role in other autoimmune diseases. There is overlap in phenotype of AGS and systemic lupus erythematosus (SLE) and heterozygous carriers of AGS alleles can have autoantibodies of the kind seen in systemic lupus erythematosus (SLE) even though they do have overt disease [25]. Fibroblasts from AGS and SLE patients show signs of DNA damage as increased p53 signaling and γH2AX and 53BP1 foci, which seem to stem from processing of persistent ribonucleotides in DNA. It is not clear if these mutations result in increased spontaneous mutation. Preliminary results from modeling human AGS alleles in yeast has given mixed results, with a range from no phenotype to being almost as deleterious as the null allele (Catherine Potenski and Hannah Klein, unpublished observations), suggesting some alleles are severe hypomorphic variants. These studies have been limited by the lack of significant homology between RNASEH2B and RNH202 and RNASEH2C and RNH203. Moreover, affected AGS patients usually have biallelic mutations [62] so it is necessary to be able to test both alleles in a yeast system.
4. Closing remarks and challenges
Studies of genome instabilities arising from persistent rNMPs in DNA is limited in that not all mutational events can be seen, only those that result in a loss of function of the gene under study or reversion of a particular mutation can be recovered. In various LOH and HR assays, there is again selection so only a subset of all events are detected. Nonetheless, even with these biases it is clear that novel genome instabilities, especially in terms of slippage mutagenesis in simple repeats, is enhanced in RNase H2 deficient yeast. As reporter systems for mutagenesis have yet to be applied to mouse or human cells, it is only assumed but not demonstrated that similar events that are Top1-dependent occur here too. An important question to answer is whether the human AGS mutations in RNase H2 subunits also cause genome instability. While the common RNASEH2B allele A177T causes an innate immune response in mice with some indication of DNA damage [63], it is not known if mutation or genome rearrangements occur in the mouse knock-in mutant.
Another challenge is understanding the extent of rNMP tolerance and whether there is any bias to this. This question may be related to the observation that not all simple repeats tracts in the yeast CAN1 reporter [33] are subject to mutagenesis. Indeed, it is not yet known if these sites are subject to rNTP misincorporation although rNTP misincorporation seems to be random [64]. The observation that CAN1 hotspots are portable [11] suggests that some very local property of the sequence influences both its propensity to sustain rNMP misincorporation and its ability to be subject to Top1-mediated mutagenesis. However, what makes a hotspot hot is not yet understood. Similarly, given the local hotspot binding reported for RNase H1 [54] and the potential for hotspots in the generation of breaks and LOH events, also suggests that there are chromatin or chromosome locales that predispose to ribonucleotide-stimulated genome instability. Whether this could explain the divergent conclusions regarding chromosomal LOH events and their origin as relates to ribonucleotides remains to be decided.
Based on yeast studies, there seems to be a bias in Top1 cleavage on leading versus lagging strand and stimulation of mutation versus HR events. All studies seem to be in agreement that mutation slippage events arise predominantly from rNMP misincorporation by DNA Pol ε and arise from Top1 cleavage on the leading strand [13, 59]. Moreover, slippage mutation arises from single rNMPs in DNA. HR events, including gene conversion, LOH and GCR events are also increased in RNase H2-defective strains. One study suggests that LOH and other HR events are Top1-dependent, arise from single rNMPs in DNA, and from rNMPs misincorporated on the leading strand by DNA Pol ε [37]. However, other studies using different HR and LOH reporters did not find a bias for DNA Pol ε-based, but did find evidence for multiple tandem rNMPs, either embedded in DNA or as a R-loop, that stimulated genome instability [36, 38, 54]. Further studies to discriminate between strand bias and source or the RNA:DNA hybrid will be of high priority.
Acknowledgments
I thank Duncan Smith for comments on the manuscript. Support from the National Institutes of Health R01CA146940 is acknowledged.
Abbreviations
- AGS
Aicardi-Goutieres Syndrome
- DSB
double-strand break
- HR
homologous recombination
- Top1
topoisomerase 1
- LOH
loss of heterozygosity
- ncRNA
non-coding RNA
- RER
ribonucleotide excision repair
- dNTP
deoxynucleotide triphosphate
- rNTP
ribonucleotide triphosphate
- rNMP
ribonucleotide monophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Brown JA, Suo Z. Unlocking the sugar "steric gate" of DNA polymerases. Biochemistry. 2011;50:1135–1142. doi: 10.1021/bi101915z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLucia AM, Grindley ND, Joyce CM. An error-prone family Y DNA polymerase (DinB homolog from Sulfolobus solfataricus) uses a 'steric gate' residue for discrimination against ribonucleotides. Nucleic Acids Res. 2003;31:4129–4137. doi: 10.1093/nar/gkg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyce CM. Choosing the right sugar: how polymerases select a nucleotide substrate. Proc Natl Acad Sci U S A. 1997;94:1619–1622. doi: 10.1073/pnas.94.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clausen AR, Zhang S, Burgers PM, Lee MY, Kunkel TA. Ribonucleotide incorporation, proofreading and bypass by human DNA polymerase delta. DNA Repair. 2013;12:121–127. doi: 10.1016/j.dnarep.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nick McElhinny SA, Watts BE, Kumar D, Watt DL, Lundstrom EB, Burgers PM, Johansson E, Chabes A, Kunkel TA. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc Natl Acad Sci U S A. 2010;107:4949–4954. doi: 10.1073/pnas.0914857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeRose EF, Perera L, Murray MS, Kunkel TA, London RE. Solution structure of the Dickerson DNA dodecamer containing a single ribonucleotide. Biochemistry. 2012;51:2407–2416. doi: 10.1021/bi201710q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaishree TN, van der Marel GA, van Boom JH, Wang AH. Structural influence of RNA incorporation in DNA: quantitative nuclear magnetic resonance refinement of d(CG)r(CG)d(CG) and d(CG)r(C)d(TAGCG) Biochemistry. 1993;32:4903–4911. doi: 10.1021/bi00069a027. [DOI] [PubMed] [Google Scholar]
- 8.Rychlik MP, Chon H, Cerritelli SM, Klimek P, Crouch RJ, Nowotny M. Crystal structures of RNase H2 in complex with nucleic acid reveal the mechanism of RNA-DNA junction recognition and cleavage. Mol Cell. 2010;40:658–670. doi: 10.1016/j.molcel.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovatter KR, Martinson HG. Ribonucleotide-Induced Helical Alteration in DNA Prevents Nucleosome Formation. P Natl Acad Sci USA. 1987;84:1162–1166. doi: 10.1073/pnas.84.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YF, Breaker RR. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2 '-hydroxyl group. J Am Chem Soc. 1999;121:5364–5372. [Google Scholar]
- 11.Kim N, Huang SN, Williams JS, Li YC, Clark AB, Cho JE, Kunkel TA, Pommier Y, Jinks-Robertson S. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science. 2011;332:1561–1564. doi: 10.1126/science.1205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekiguchi J, Shuman S. Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I. Mol Cell. 1997;1:89–97. doi: 10.1016/s1097-2765(00)80010-6. [DOI] [PubMed] [Google Scholar]
- 13.Williams JS, Clausen AR, Lujan SA, Marjavaara L, Clark AB, Burgers PM, Chabes A, Kunkel TA. Evidence that processing of ribonucleotides in DNA by topoisomerase 1 is leading-strand specific. Nat Struct Mol Biol. 2015;22:291–297. doi: 10.1038/nsmb.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Muse T, Aguilera A. Transcription-replication conflicts: how they occur and how they are resolved. Nat Rev Mol Cell Bio. 2016;17:553–563. doi: 10.1038/nrm.2016.88. [DOI] [PubMed] [Google Scholar]
- 15.Cerritelli SM, Crouch RJ. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 2009;276:1494–1505. doi: 10.1111/j.1742-4658.2009.06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillard H, Aguilera A. Transcription as a Threat to Genome Integrity. Annu Rev Biochem. 2016;85:291–317. doi: 10.1146/annurev-biochem-060815-014908. [DOI] [PubMed] [Google Scholar]
- 17.Kim N, Jinks-Robertson S. Transcription as a source of genome instability. Nat Rev Genet. 2012;13:204–214. doi: 10.1038/nrg3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lujan SA, Williams JS, Clausen AR, Clark AB, Kunkel TA. Ribonucleotides are signals for mismatch repair of leading-strand replication errors. Mol Cell. 2013;50:437–443. doi: 10.1016/j.molcel.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nick McElhinny SA, Kumar D, Clark AB, Watt DL, Watts BE, Lundstrom EB, Johansson E, Chabes A, Kunkel TA. Genome instability due to ribonucleotide incorporation into DNA. Nat Chem Biol. 2010;6:774–781. doi: 10.1038/nchembio.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy EM, Amie SM, Bambara RA, Kim B. Frequent Incorporation of Ribonucleotides during HIV-1 Reverse Transcription and Their Attenuated Repair in Macrophages. Journal of Biological Chemistry. 2012;287:14280–14288. doi: 10.1074/jbc.M112.348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randerath K, Reddy R, Danna TF, Watson WP, Crane AE, Randerath E. Formation of Ribonucleotides in DNA Modified by Oxidative Damage Invitro and Invivo - Characterization by P-32 Postlabeling. Mutat Res. 1992;275:355–366. doi: 10.1016/0921-8734(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 22.Hiller B, Achleitner M, Glage S, Naumann R, Behrendt R, Roers A. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. Journal of Experimental Medicine. 2012;209:1419–1426. doi: 10.1084/jem.20120876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reijns MA, Rabe B, Rigby RE, Mill P, Astell KR, Lettice LA, Boyle S, Leitch A, Keighren M, Kilanowski F, Devenney PS, Sexton D, Grimes G, Holt IJ, Hill RE, Taylor MS, Lawson KA, Dorin JR, Jackson AP. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell. 2012;149:1008–1022. doi: 10.1016/j.cell.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, Ali M, Semple C, Aicardi J, Babul-Hirji R, Baumann C, Baxter P, Bertini E, Chandler KE, Chitayat D, Cau D, Dery C, Fazzi E, Goizet C, King MD, Klepper J, Lacombe D, Lanzi G, Lyall H, Martinez-Frias ML, Mathieu M, McKeown C, Monier A, Oade Y, Quarrell OW, Rittey CD, Rogers RC, Sanchis A, Stephenson JB, Tacke U, Till M, Tolmie JL, Tomlin P, Voit T, Weschke B, Woods CG, Lebon P, Bonthron DT, Ponting CP, Jackson AP. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet. 2006;38:910–916. doi: 10.1038/ng1842. [DOI] [PubMed] [Google Scholar]
- 25.Gunther C, Kind B, Reijns MA, Berndt N, Martinez-Bueno M, Wolf C, Tungler V, Chara O, Lee YA, Hubner N, Bicknell L, Blum S, Krug C, Schmidt F, Kretschmer S, Koss S, Astell KR, Ramantani G, Bauerfeind A, Morris DL, Cunninghame Graham DS, Bubeck D, Leitch A, Ralston SH, Blackburn EA, Gahr M, Witte T, Vyse TJ, Melchers I, Mangold E, Nothen MM, Aringer M, Kuhn A, Luthke K, Unger L, Bley A, Lorenzi A, Isaacs JD, Alexopoulou D, Conrad K, Dahl A, Roers A, Alarcon-Riquelme ME, Jackson AP, Lee-Kirsch MA. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest. 2015;125:413–424. doi: 10.1172/JCI78001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu JZ, Qian Y, Frank P, Wintersberger U, Shen BH. Saccharomyces cerevisiae RNase H(35) functions in RNA primer removal during lagging-strand DNA synthesis, most efficiently in cooperation with Rad27 nuclease. Molecular and Cellular Biology. 1999;19:8361–8371. doi: 10.1128/mcb.19.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilera A, Klein HL. Genetic-Control of Intrachromosomal Recombination in Saccharomyces-Cerevisiae .1. Isolation and Genetic-Characterization of Hyper-Recombination Mutations. Genetics. 1988;119:779–790. doi: 10.1093/genetics/119.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potenski CJ, Niu H, Sung P, Klein HL. Avoidance of ribonucleotide-induced mutations by RNase H2 and Srs2-Exo1 mechanisms. Nature. 2014;511:251–254. doi: 10.1038/nature13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rydberg B, Game J. Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. P Natl Acad Sci USA. 2002;99:16654–16659. doi: 10.1073/pnas.262591699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chon H, Sparks JL, Rychlik M, Nowotny M, Burgers PM, Crouch RJ, Cerritelli SM. RNase H2 roles in genome integrity revealed by unlinking its activities. Nucleic Acids Res. 2013;41:3130–3143. doi: 10.1093/nar/gkt027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparks JL, Chon H, Cerritelli SM, Kunkel TA, Johansson E, Crouch RJ, Burgers PM. RNase H2-initiated ribonucleotide excision repair. Mol Cell. 2012;47:980–986. doi: 10.1016/j.molcel.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparks JL, Burgers PM. Error-free and mutagenic processing of topoisomerase 1-provoked damage at genomic ribonucleotides. EMBO J. 2015;34:1259–1269. doi: 10.15252/embj.201490868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho JE, Kim N, Li YC, Jinks-Robertson S. Two distinct mechanisms of Topoisomerase 1-dependent mutagenesis in yeast. DNA Repair (Amst) 2013;12:205–211. doi: 10.1016/j.dnarep.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerritelli SM, Crouch RJ. The Balancing Act of Ribonucleotides in DNA. Trends Biochem Sci. 2016;41:434–445. doi: 10.1016/j.tibs.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim N, Cho JE, Li YC, Jinks-Robertson S. RNA:DNA hybrids initiate quasi-palindrome-associated mutations in highly transcribed yeast DNA. PLoS Genet. 2013;9:e1003924. doi: 10.1371/journal.pgen.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epshtein A, Potenski CJ, Klein HL. Increased spontaneous recombination in RNase H2-deficient cells arises from multiple contiguous rNMPs and not from single rNMP residues incorporated by DNA polymerase epsilon. Microb Cell. 2016;3:248–254. doi: 10.15698/mic2016.06.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conover HN, Lujan SA, Chapman MJ, Cornelio DA, Sharif R, Williams JS, Clark AB, Camilo F, Kunkel TA, Argueso JL. Stimulation of Chromosomal Rearrangements by Ribonucleotides. Genetics. 2015;201:951–961. doi: 10.1534/genetics.115.181149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Connell K, Jinks-Robertson S, Petes TD. Elevated Genome-Wide Instability in Yeast Mutants Lacking RNase H Activity. Genetics. 2015;201:963–975. doi: 10.1534/genetics.115.182725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang SN, Williams JS, Arana ME, Kunkel TA, Pommier Y. Topoisomerase I-mediated cleavage at unrepaired ribonucleotides generates DNA double-strand breaks. EMBO J. 2016 doi: 10.15252/embj.201592426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ii M, Ii T, Mironova LI, Brill SJ. Epistasis analysis between homologous recombination genes in Saccharomyces cerevisiae identifies multiple repair pathways for Sgs1, Mus81-Mms4 and RNase H2. Mutat Res-Fund Mol M. 2011;714:33–43. doi: 10.1016/j.mrfmmm.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keskin H, Meers C, Storici F. Transcript RNA supports precise repair of its own DNA gene. RNA Biol. 2016;13:157–165. doi: 10.1080/15476286.2015.1116676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keskin H, Shen Y, Huang F, Patel M, Yang T, Ashley K, Mazin AV, Storici F. Transcript-RNA-templated DNA recombination and repair. Nature. 2014;515:436–439. doi: 10.1038/nature13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohle C, Tesorero R, Schermann G, Dobrev N, Sinning I, Fischer T. Transient RNA-DNA Hybrids Are Required for Efficient Double-Strand Break Repair. Cell. 2016;167:1001–1013. doi: 10.1016/j.cell.2016.10.001. e1007. [DOI] [PubMed] [Google Scholar]
- 44.Meers C, Keskin H, Storici F. DNA repair by RNA: Templated, or not templated, that is the question. DNA Repair (Amst) 2016;44:17–21. doi: 10.1016/j.dnarep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keskin H, Storici F. Defects in RNase H2 Stimulate DNA Break Repair by RNA Reverse Transcribed into cDNA. Microrna. 2015;4:109–116. doi: 10.2174/2211536604666150820120129. [DOI] [PubMed] [Google Scholar]
- 46.Amon JD, Koshland D. RNase H enables efficient repair of R-loop induced DNA damage. Elife. 2016;5 doi: 10.7554/eLife.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francia S, Cabrini M, Matti V, Oldani A, d’Adda di Fagagna F. DICER, DROSHA and DNA damage response RNAs are necessary for the secondary recruitment of DNA damage response factors. J Cell Sci. 2016;129:1468–1476. doi: 10.1242/jcs.182188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francia S, Michelini F, Saxena A, Tang D, de Hoon M, Anelli V, Mione M, Carninci P, d’Adda di Fagagna F. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature. 2012;488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury D, Choi YE, Brault ME. Charity begins at home: non-coding RNA functions in DNA repair. Nat Rev Mol Cell Biol. 2013;14:181–189. doi: 10.1038/nrm3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Billmyre RB, Calo S, Feretzaki M, Wang X, Heitman J. RNAi function, diversity, and loss in the fungal kingdom. Chromosome Res. 2013;21:561–572. doi: 10.1007/s10577-013-9388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen-Soltero S, Martinez SL, Putnam CD, Kolodner RD. A saccharomyces cerevisiae RNase H2 interaction network functions to suppress genome instability. Mol Cell Biol. 2014;34:1521–1534. doi: 10.1128/MCB.00960-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahba L, Amon JD, Koshland D, Vuica-Ross M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol Cell. 2011;44:978–988. doi: 10.1016/j.molcel.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wahba L, Gore SK, Koshland D. The homologous recombination machinery modulates the formation of RNA-DNA hybrids and associated chromosome instability. Elife. 2013;2:e00505. doi: 10.7554/eLife.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmer AD, Koshland D. Differential roles of the RNases H in preventing chromosome instability. Proc Natl Acad Sci U S A. 2016;113:12220–12225. doi: 10.1073/pnas.1613448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pizzi S, Sertic S, Orcesi S, Cereda C, Bianchi M, Jackson AP, Lazzaro F, Plevani P, Muzi-Falconi M. Reduction of hRNase H2 activity in Aicardi-Goutieres syndrome cells leads to replication stress and genome instability. Hum Mol Genet. 2015;24:649–658. doi: 10.1093/hmg/ddu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazzaro F, Novarina D, Amara F, Watt DL, Stone JE, Costanzo V, Burgers PM, Kunkel TA, Plevani P, Muzi-Falconil M. RNase H and Postreplication Repair Protect Cells from Ribonucleotides Incorporated in DNA. Molecular Cell. 2012;45:99–110. doi: 10.1016/j.molcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang SY, Ghosh S, Pommier Y. Topoisomerase I alone is sufficient to produce short DNA deletions and can also reverse nicks at ribonucleotide sites. J Biol Chem. 2015;290:14068–14076. doi: 10.1074/jbc.M115.653345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pommier Y, Sun Y, Huang SN, Nitiss JL. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat Rev Mol Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams JS, Smith DJ, Marjavaara L, Lujan SA, Chabes A, Kunkel TA. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol Cell. 2013;49:1010–1015. doi: 10.1016/j.molcel.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tumbale P, Williams JS, Schellenberg MJ, Kunkel TA, Williams RS. Aprataxin resolves adenylated RNA-DNA junctions to maintain genome integrity. Nature. 2014;506:111–115. doi: 10.1038/nature12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reijns MA, Jackson AP. Ribonuclease H2 in health and disease. Biochem Soc Trans. 2014;42:717–725. doi: 10.1042/BST20140079. [DOI] [PubMed] [Google Scholar]
- 62.Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, Artuch R, Montalto SA, Bacino CA, Barroso B, Baxter P, Benko WS, Bergmann C, Bertini E, Biancheri R, Blair EM, Blau N, Bonthron DT, Briggs T, Brueton LA, Brunner HG, Burke CJ, Carr IM, Carvalho DR, Chandler KE, Christen HJ, Corry PC, Cowan FM, Cox H, D’Arrigo S, Dean J, De Laet C, De Praeter C, Dery C, Ferrie CD, Flintoff K, Frints SG, Garcia-Cazorla A, Gener B, Goizet C, Goutieres F, Green AJ, Guet A, Hamel BC, Hayward BE, Heiberg A, Hennekam RC, Husson M, Jackson AP, Jayatunga R, Jiang YH, Kant SG, Kao A, King MD, Kingston HM, Klepper J, van der Knaap MS, Kornberg AJ, Kotzot D, Kratzer W, Lacombe D, Lagae L, Landrieu PG, Lanzi G, Leitch A, Lim MJ, Livingston JH, Lourenco CM, Lyall EG, Lynch SA, Lyons MJ, Marom D, McClure JP, McWilliam R, Melancon SB, Mewasingh LD, Moutard ML, Nischal KK, Ostergaard JR, Prendiville J, Rasmussen M, Rogers RC, Roland D, Rosser EM, Rostasy K, Roubertie A, Sanchis A, Schiffmann R, Scholl-Burgi S, Seal S, Shalev SA, Corcoles CS, Sinha GP, Soler D, Spiegel R, Stephenson JB, Tacke U, Tan TY, Till M, Tolmie JL, Tomlin P, Vagnarelli F, Valente EM, Van Coster RN, Van der Aa N, Vanderver A, Vles JS, Voit T, Wassmer E, Weschke B, Whiteford ML, Willemsen MA, Zankl A, Zuberi SM, Orcesi S, Fazzi E, Lebon P, Crow YJ. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;81:713–725. doi: 10.1086/521373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackenzie KJ, Carroll P, Lettice L, Tarnauskaite Z, Reddy K, Dix F, Revuelta A, Abbondati E, Rigby RE, Rabe B, Kilanowski F, Grimes G, Fluteau A, Devenney PS, Hill RE, Reijns MA, Jackson AP. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. EMBO J. 2016;35:831–844. doi: 10.15252/embj.201593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clausen AR, Lujan SA, Burkholder AB, Orebaugh CD, Williams JS, Clausen MF, Malc EP, Mieczkowski PA, Fargo DC, Smith DJ, Kunkel TA. Tracking replication enzymology in vivo by genome-wide mapping of ribonucleotide incorporation. Nat Struct Mol Biol. 2015;22:185–191. doi: 10.1038/nsmb.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]