Abstract
Scope
Phytosterols are bioactive compounds in plants with similar cholesterol-lowering properties as vegetarian diets. However, information on phytosterol intake and plasma plant sterols among vegetarians is sparse.
Methods and results
We examined dietary intake and plasma concentration of plant sterols and cholesterol across five dietary patterns in the Adventist Health Study-2 Calibration Sub-study (n=861, 66% females, average age 61 y). To measure intake and plasma concentration of these compounds, we used 24-hour dietary recalls and gas-liquid chromatography-flame ionization detection, respectively. Mean (SD) total phytosterol and cholesterol intake were 363 (176) mg/d and 131 (111) mg/d; plasma β-sitosterol, campesterol, and cholesterol were 3.3 (1.7) μg/mL, 4.2 (2.3) μg/mL, and 1.9 (0.4) mg/mL, respectively. Total phytosterol intake was lowest among non-vegetarians (263 mg/d) and highest among vegans (428 mg/d) (P < 0.0001). Cholesterol intake was lowest among vegans (15.2 mg/d) and highest among non-vegetarians (124.6 mg/d) (P < 0.0001). Plasma plant sterols and cholesterol did not differ by diet. Cholesterol-adjusted plasma β-sitosterol and campesterol were significantly higher in Blacks than Whites, though no ethnic differences were observed in dietary intake of these plant sterols.
Conclusion
Dietary intake but not plasma concentration of plant sterols and cholesterol varies across distinct plant-based diets.
Keywords: beta-sitosterol, campesterol, cholesterol, phytosterols, vegetarian
INTRODUCTION
Plant sterols are bioactive compounds that occur in plant foods. Structurally, plant sterols resemble cholesterol, except for an additional methyl- or ethyl group in the side chain[1]. In humans, β-sitosterol (24-ethylcholesterol) and campesterol (24-methylcholesterol) are the predominant plant sterols occurring in blood. They are derived solely from the diet and are not synthesized endogenously within the mammalian species[2]. Over 200 plant sterols have been identified, and the most abundant are β-sitosterol (e.g., nuts and seeds, cereals, wheat germ, corn oil), campesterol (e.g., cereals, oils, vegetables) and stigmasterol (e.g., oils)[3–7]. Foods such as beans, and some fruits also contain these plant sterols. Plant sterol intakes vary from 160 – 400 mg in different populations from Europe, Japan, and Mexico and differ by gender and educational level[1, 3, 8, 9].
Plant sterols have been studied extensively for their cholesterol-lowering properties associated with coronary disease risk[10–12]. More recently, findings from animal and human studies suggest plant sterols may also have anti-cancer, anti-inflammatory, and anti-oxidation properties[3] which may confer favorable associations with chronic disease and mortality[13]. We have also shown that vegetarian dietary patterns are associated with lower risk of mortality[14], metabolic syndrome[15], cardiovascular risk factors[16, 17], and cancer[18, 19]. It is possible that the presence of plant sterols may contribute to the potential benefits derived from plant-based diets; however, limited information is available on the dietary intake and plasma concentration of plant sterols in plant-based diets.
The Adventist Health Study-2 (AHS-2) is a North American cohort[20], which provides a unique opportunity to investigate variations in the intake and plasma concentration of plant sterols, as dietary habits span a wide range of practices from vegans, lacto-ovo vegetarians (LOV), pesco vegetarians (PV), semi vegetarians (SV), to non-vegetarians (NV), as do the consumption of nuts, soy, other legumes and grains. In this report we used the AHS-2 Calibration Study cohort to examine dietary intake and plasma concentration of plant sterols across distinct plant-based dietary patterns, and hypothesized that dietary intake and plasma concentrations of plant sterols would be higher among vegetarians compared to non-vegetarians. The Institutional Review Board of Loma Linda University approved the study procedures (#5130123), and all study participants provided written consent at the time of enrollment.
MATERIALS AND METHODS
Study design and subject selection
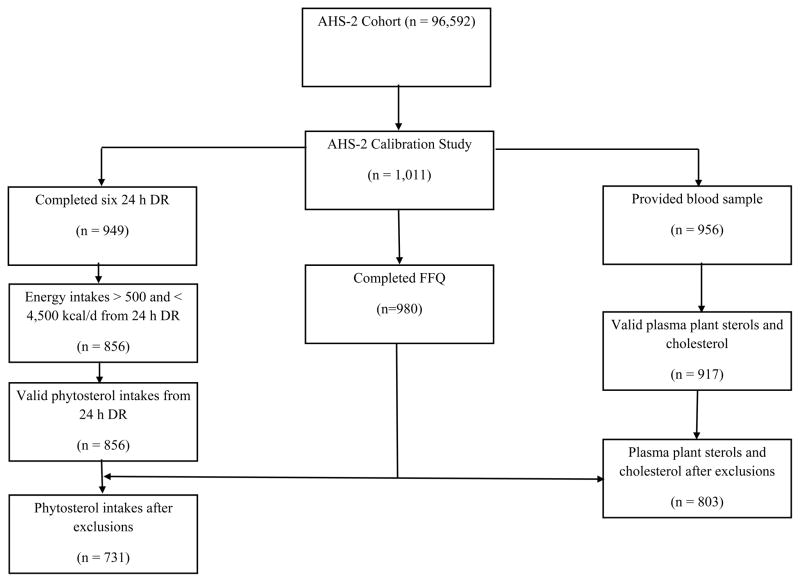
We conducted cross-sectional analyses on data from the Adventist Health Study-2 Calibration Sub-study, a representative sample of the AHS-2 cohort. Details of recruitment and selection methods of the parent cohort were described in a previous report[20]. Briefly, adult members (age 30+ years) of Seventh-day Adventist churches geographically spread throughout the US and Canada were recruited by mail from February 2002 – May 2007. Individuals who completed a comprehensive questionnaire, which included questions about medical history, dietary habits, physical activity, and demographic information, were enrolled. Approximately 27% of the cohort is black of US or Caribbean origin and the remaining participants are primarily white with a minor proportion from other races. The Calibration Sub-study participants (n = 1011) were randomly selected from the parent cohort by church and then within the church by gender and age. Participants provided six 24 h dietary recalls (DR), completed a food frequency questionnaire, and attended a clinic at their local church during which height and weight were measured, and fasting blood samples were collected. After exclusion of subjects with missing data on demographics and biometrics (n = 63), dietary patterns assessed by FFQ (n = 31), plasma cholesterol values (n = 31), or incomplete 24 h DRs (n = 62), the analytic samples for plasma plant sterol concentration and phytosterol intake analyses included 803 and 731 individuals, respectively (Figure 1).
Figure 1.
Study design and flowchart
AHS2, Adventist Health Study-2; 24 h DR, 24-hour dietary recall; FFQ, food frequency questionnaire.
Dietary patterns
Dietary patterns in the AHS-2 cohort are well-characterized and are determined according to the frequency of reported intake of foods of animal origin from a 204-item food frequency questionnaire[21]. Vegans consume eggs/dairy, fish, and all other meats less than 1 time/mo; lacto-ovo vegetarians consume eggs/dairy 1 time/mo or more, but fish and all other meats less than 1 time/mo; pesco vegetarians consume fish 1 time/mo or more, but all other meats less than 1 time/mo; semi vegetarians consume non-fish meats 1 time/mo or more, and all meats combined (fish included) 1 time/mo or more but no more than 1 time/wk; and last, non-vegetarians consume non-fish meats 1 time/mo or more, and all meats combined (fish included) more than 1 time/wk. The food frequency questionnaire was previously validated against six 24-hour dietary recalls for intake of nutrients and selected foods/food groups. Validity correlations for red meat, poultry, fish, dairy, and eggs are 0.76, 0.76, 0.53, 0.86, and 0.64, respectively, in whites and 0.72, 0.77, 0.57, 0.82, and 0.52, respectively, in blacks[22].
Plant sterol content of foods
Plant sterol content of foods derived by the chromatography method was obtained primarily from The United States Department of Agriculture (USDA) National Nutrient Database for Standard Reference (Release 27)[23]. The USDA database provides data on β-sitosterol, campesterol, stigmasterol, and total phytosterol content of 595 foods. Foods reported by study participants were carefully matched to those in the USDA database. Where we could not find a match, we extracted plant sterol content information of these foods (n = 189) from individual published reports, which may have included data on campesterol, β-sitosterol, stigmasterol, other plant sterols, or total phytosterols[4–7, 24–34]. We report results for campesterol, β-sitosterol, stigmasterol, other phytosterols (calculated as the sum of plant stanols and other plant sterols), and total phytosterols.
Assessment of dietary intake
Trained dietitians collected from each participant six 24-hour dietary recalls by telephone, which were unannounced. The recalls were obtained in two sets (separated by approximately six months) of three variably timed interviews. Each set consisted of one Saturday, one Sunday and one weekday’s intake. Reported intake of foods, beverages, and medications were entered using Nutrition Data System for Research (NDS-R) version 4.06 or 5.0, and nutrient composition was based on the NDS-R 2008 database (NDS-R, Nutrition Coordinating Center, Minneapolis, MN, USA). Recalls where daily energy intake was < 500 kcal or > 4500 kcal (n=93) were excluded from analysis.
Plasma concentration of plant sterols and cholesterol
Dr. Dieter Lütjohann of The Institute of Clinical Chemistry and Clinical Pharmacology, University Clinics of Bonn provided estimates of plasma concentrations of cholesterol, lathosterol and the plant sterols, campesterol and β-sitosterol using gas chromatography-flame ionization detection (GCFID) method[35, 36]. Fifty μg 5α-cholestane (Serva Electrophoresis GmbH, Heidelberg, Germany) (50 μL from a stock solution mg/mL in cyclohexane), used as internal standard, was added to 100 μL of human plasma at room temperature. Alkaline hydrolysis was performed at 68°C for one hour after addition of 1.0 mL 1M ethanolic (90%) sodium hydroxide solution. After cooling to room temperature and addition of 500 μL of distilled water the unsaponified lipids were extracted twice into three ml of cyclohexane. The combined organic phases were dried under nitrogen at 65°C. The residue was dissolved in 500 μL n-decane and transferred into glass vials for GC-FID analysis. The hydroxy-groups of the sterols were derivatisised to trimethylsilyl (TMSi)-ethers by adding 40 μL TMSi-reagent (pyridine-hexamethyldisilazan-trimethylchlorosilane 9:3:1, by volume) to the n-decane solution and incubated for 1h at 90°C.
The sterols were separated on a crosslinked methyl silicone DB-XLB 122–1232 capillary column (J&W, Folsom, USA) (30m x 0.25 mm i.d. x 0.25 μm film thickness) in an Hewlett Packard (HP) gas chromatograph 6890 after splitless injection by an HP 7683 injector/auto sampler at 280°C. Hydrogen was used as carrier gas with an inlet pressure of 9.97 psi, resulting in a total gas-flow of 1.1 mL/min. The oven temperature was kept at 150°C for 3 min. and raised at a rate of 30°C/min to a final temperature of 290°C, keeping for 22.33 min. Cholesterol-TMSi ether was detected with a retention time of 15.88 min by a flame-ionization detector at 280°C with a combined constant column and make-up flow of hydrogen+nitrogen; 30 mL/min). Identity and retention times for all sterols were confirmed by using authentic cholesterol, lathosterol, sitosterol/campesterol (Sigma-Aldrich Chemie GmbH, München, Germany). Purity of the standard sterols was checked by GC-mass spectrometry spectra taken in scan-mode (50–500 m/z). Calculation of the concentrations of sterols was performed using 5α-cholestane as internal standard (50 μg) by a one-point calibration. The area amount of the corresponding sterol peak is divided by the area amount of 5α-cholestane and multiplied by the amount of 5α-cholestane added to the sample (50 μg). To validate this calculation method we used standard curves for cholesterol, campesterol and sitosterol with 5α-cholestane as internal standard. The coefficient of variation of the method for cholesterol is 2% and for lathosterol, campesterol and sitosterol were between 3 and 5%.
Statistical analysis
Descriptive analysis was performed on unadjusted phytosterol and cholesterol variables. To approximate normality, a logarithmic transformation was applied to the dietary plant sterol and phytosterol variables, and to plasma plant sterols.
Plant sterol intake per recall day (Y) was calculated as Y = Σ Xn × Sn, where X = mg of plant sterol per 100 g of food n and S = reported portion size consumed of food n in grams. Dietary intake estimates of plant sterols and cholesterol were energy-adjusted using the residual method, which produces values that are independent of energy intake. Many foods contain zero plant sterols, which result in participants having zero plant sterol intakes before energy adjustment. If the residual approach is applied these participants will end up with non-zero values. We instead performed a partitioned energy adjustment, where intake data that were initially zero were kept as zeros, and energy adjustment was applied only to non-zero values. These energy-adjusted non-zero values were then combined with the zero intake data to keep all data points on the same scale. Within each of the two sets of 24 h dietary recalls, each day was weighted appropriately to produce a synthetic week (Saturday intake + Sunday intake + 5 * weekday intake) and then divided by 7 to obtain a mean daily intake estimate. Therefore, each participant produced two repeated measures of daily intake data.
Differences in subject characteristics by dietary pattern were determined using ANOVA on continuous, and chi-square tests on categorical variables. To determine differences in plant sterol and cholesterol intake by dietary pattern, we performed repeated measures ANCOVA using PROC MIXED with repeated measures of the plant sterol intake variables and adjusted for age, ethnicity, gender, BMI, and physical activity. Differences in plasma concentrations of plant sterols, lathosterol and cholesterol by diet were determined using PROC GLM adjusted for age, ethnicity, gender, BMI, and physical activity. We also included energy intake in all ANCOVA models, but energy was not significant and estimates changed very little if at all; thus, ANCOVA results were from analyses without energy intake as a confounder. It has been suggested that factors such as use of statin drugs, presence of metabolic syndrome, and gender may influence plasma concentration of plant sterols, and thus must be controlled for in the analysis[37]. We repeated the analyses by excluding subjects who reported taking statins and/or diabetes medications. However, results did not change appreciably from analyses when all subjects were included; therefore results reported were from analysis including all subjects.
Since ethnicity was significant in the ANCOVA models for dietary intake of β-sitosterol, campesterol, other phytosterols and total phytosterols, as well as plasma concentration of β-sitosterol and campesterol, we further examined the interaction between diet and ethnicity and performed two-way ANCOVA adjusted for age, gender, body mass index (BMI), and physical activity. For all pairwise comparisons, values in other dietary patterns were compared to that in non-vegetarians (reference) using Dunnett’s post hoc test to adjust for multiple comparisons. Analyses were carried out using SAS statistical software package release 9.4 (SAS Institute Inc., Cary, NC, USA) and figures were generated using R version 2.10.1 (http://www.r-project.org/).
RESULTS
Descriptive statistics for dietary intake of β-sitosterol, campesterol, stigmasterol, other phytosterols, total phytosterols, and cholesterol and plasma concentrations of plant sterols, lathosterol and cholesterol are shown in Table 1. Plant sterols in the diet were predominantly β-sitosterol (237 mg/d) followed by campesterol (59 mg/d), stigmasterol (44 mg/d), and other phytosterols (23 mg/d). Total phytosterol intake was on average 363 mg/d. Campesterol was higher than β-sitosterol in plasma. Mean (SD) plasma cholesterol was 192 (39) mg/dL. The analytic sample included 46% non-vegetarians, 5% semi vegetarian, 11% pesco vegetarians, 28% lacto-ovo vegetarians, and 10% vegans. Differences among selected subject characteristics by dietary pattern are presented in Table 2. Age and BMI significantly differed by dietary pattern (both p<0.0001). Average age tended to increase from non-vegetarians to vegans. As expected, BMI was lowest among vegans and highest among non-vegetarians. Energy and dietary intake of polyunsaturated (PFA) and saturated (SFA) fatty acids, and PFA to SFA (PS) ratio were significantly associated with dietary pattern (p<0.0001 for energy and fatty acids). PS ratio among vegans was double that of NVs. The proportion of Blacks and Whites was significantly different by dietary pattern (p<0.0001), with a greater proportion of Blacks being pesco vegetarians and non-vegetarians, and Whites more likely semi vegetarians, LOVs and vegans.
Table 1.
Unadjusted dietary intake and plasma concentration of phytosterols and cholesterol in a sample of adults in North America.
| Mean | SD | 5th Percentile | 50th Percentile | 95th Percentile | |
|---|---|---|---|---|---|
| Dietary intake n=856 | |||||
| β-Sitosterol (mg/d) | 237.2 | 112.6 | 93.7 | 215.7 | 383.7 |
| Campesterol (mg/d) | 58.9 | 31.0 | 22.7 | 53.1 | 98.4 |
| Stigmasterol (mg/d) | 43.7 | 31.2 | 11.5 | 35.9 | 102.2 |
| Other phytosterols (mg/d1)a | 23.3 | 13.3 | 7.6 | 20.7 | 48.6 |
| Total phytosterols (mg/d) b | 363.2 | 175.6 | 145.1 | 329.2 | 595.1 |
| Cholesterol (mg/d) | 130.8 | 110.7 | 7.9 | 106.0 | 281.6 |
| Plasma concentration, n=861 | |||||
| β-Sitosterol (μg/mL)c | 3.3 | 1.7 | 1.4 | 2.9 | 6.4 |
| Campesterol (μg/mL)c | 4.2 | 2.3 | 1.6 | 3.7 | 8.6 |
| Lathosterol (μg/mL)c | 2.2 | 1.2 | 0.8 | 2.0 | 4.4 |
| Cholesterol (mg/dL) | 191.5 | 38.6 | 136 | 188 | 261 |
Sum of plant stanols and other plant sterols.
Sum of β-sitosterol, campesterol, stigmasterol, and other phytosterols.
To convert μg/mL to mg/dL, multiply by 0.1.
Table 2.
Selected characteristics among participants of the Adventist Health Study-2 Calibration Sub-study according to dietary pattern.
| Non vegetarian | Semi vegetarian | Pesco vegetarian | Lacto-ovo vegetarian | Vegan | P-valuea | |
|---|---|---|---|---|---|---|
| n (%) | 370 (46.2) | 37 (4.6) | 91.0 (11.4) | 224 (28.0) | 79 (9.9) | |
| Age (years) | 58.8 (13.0) | 60.1 (12.7) | 60.0 (12.8) | 62.3 (14.1) | 66.4 (12.5) | <0.0001 |
| BMI (kg/m2) | 30.0 (6.9) | 27.3 (5.2) | 27.0 (5.5) | 25.5 (5.1) | 25.4 (5.7) | <0.0001 |
| Vigorous activity (min/week) | 71.0 (90.6) | 62.4 (72.7) | 94.0 (98.8) | 83.5 (101.4) | 122.7 (108.2) | 0.03 |
| Energy intake (kcal/d) | 1,566.6 (513.7) | 1,726.7 (631.3) | 1,517.2 (460.9) | 1,748.4 (506.0) | 1,651.3 (503.4) | <0.0001 |
| PFA intake (g/d) | 13.0 (6.3) | 14.6 (6.5) | 14.5 (7.6) | 17.6 (7.5) | 16.6 (6.9) | <0.0001 |
| SFA intake (g/d) | 16.1 (8.0) | 16.6 (8.8) | 11.5 (5.8) | 15.2 (7.4) | 9.7 (4.8) | <0.0001 |
| PS ratio | 1.0 (0.4) | 1.1 (0.4) | 1.5 (0.6) | 1.4 (0.6) | 2.0 (0.7) | <0.0001 |
| Gender (%) | 0.44 | |||||
| Females | 67.6 | 64.9 | 70.3 | 61.2 | 68.4 | |
| Males | 32.4 | 35.1 | 29.7 | 38.8 | 31.6 | |
| Race (%) | <0.0001 | |||||
| Whites | 47.6 | 70.3 | 40.7 | 75.9 | 55.7 | |
| Blacks | 52.4 | 29.7 | 59.3 | 24.1 | 44.3 | |
| Education (%) | 0.60 | |||||
| High school or less | 21.7 | 27.8 | 21.1 | 19.3 | 21.8 | |
| Some college | 44.2 | 30.6 | 40.0 | 38.1 | 41.0 | |
| Bachelor or above | 34.1 | 41.6 | 38.9 | 42.6 | 37.2 |
PFA = polyunsaturated fatty acid, SFA = saturated fatty acid, PS = polyunsaturated to saturated fatty acid ratio
Differences by dietary pattern were determined by analysis of variance for continuous variables reported as mean (SD), and chi square test for categorical variables.
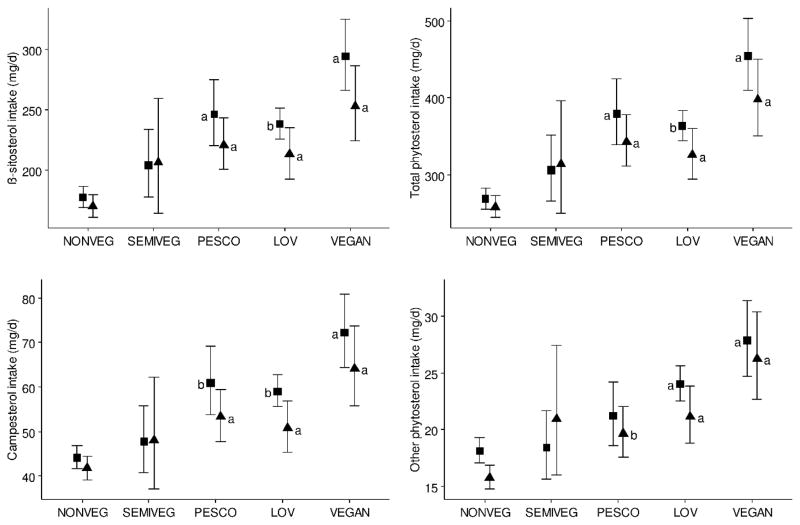
Dietary intake of phytosterols differed across diets, and generally was lowest among NVs, and highest among vegans (Table 3). Compared to non-vegetarians, PVs, LOVs and vegans had significantly higher intake of β-sitosterol (p≤0.0001 for all 3 diets), campesterol (p≤0.0001 for all 3 diets), stigmasterol (p≤0.0001 for all 3 diets), other phytosterols (p≤0.05 for all 3 diets), and total phytosterols (p≤0.0001 for all 3 diets). Total phytosterol intake was highest (428 mg/d) among vegans and lowest among non-vegetarians (263 mg/d). Cholesterol intake was significantly lower among PVs (p≤0.0001), LOVs (p≤0.0001), and vegans (p≤0.0001) compared to non-vegetarians, with vegans having the lowest (15 mg/d) and non-vegetarians the highest intake (125 mg/d) of cholesterol. Plant sterol to cholesterol ratios followed the same patterns, with ratios over 95% higher among vegans compared to non-vegetarians. In stratified analysis, we did not find ethnic differences in the intake of β-sitosterol, campesterol, other phytosterols, and total phytosterols; however, within each ethnic group, we found that intake of these phytosterols was significantly higher among PVs, LOVs, and vegans compared to non-vegetarians (Figure 2).
Table 3.
Comparison of dietary intake of phytosterols and cholesterol among adult vegetarians and non-vegetarians in North America.
| Non vegetarian n = 333 |
Semi vegetarian n = 34 |
Pesco vegetarian n = 83 |
Lacto ovo-vegetarian n = 209 |
Vegan n = 72 |
|
|---|---|---|---|---|---|
| β-Sitosterol (mg/d) | 173.4 | 201.0 | 232.8* | 227.4* | 275.3* |
| 95% CI | 166.9 – 180.3 | 178.6 – 226.1 | 216.4 – 250.4 | 216.5 – 238.9 | 254.5 – 297.9 |
| Campesterol (mg/d) | 42.9 | 46.8 | 56.8* | 55.7* | 68.3* |
| 95% CI | 41.0 – 44.8 | 40.9 – 53.5 | 52.3–61.7 | 52.6 – 58.9 | 62.5 – 74.7 |
| Stigmasterol (mg/d) | 27.7 | 32.9 | 44.4* | 38.0* | 51.0* |
| 95% CI | 26.1 – 29.4 | 27.4 – 39.5 | 39.7 – 49.8 | 35.2 – 41.0 | 45.1 – 57.6 |
| Other phytosterols (mg/d)a | 16.9 | 18.6 | 20.5** | 22.7* | 26.9* |
| 95% CI | 16.2 – 17.7 | 16.1 – 21.4 | 18.8 – 22.3 | 21.4 – 24.0 | 24.5 – 29.6 |
| Total phytosterols (mg/d2)b | 263.2 | 302.9 | 359.6* | 347.6* | 428.0* |
| 95% CI | 253.0 – 273.7 | 268.7 – 341.4 | 334.0 – 387.3 | 330.7 – 365.5 | 395.1 – 463.7 |
| Cholesterol (mg/d) | 124.6 | 96.4 | 65.5* | 48.5* | 15.2* |
| 95% CI | 112.6, 137.8 | 70.7, 131.3 | 54.1, 79.3 | 42.6, 55.2 | 12.4, 18.7 |
| β-Sitosterol:cholesterol | 2.4 | 3.4 | 10.7* | 10.8* | 64.6* |
| 95% CI | 2.0 – 2.8 | 2.1 – 5.3 | 8.0 – 14.2 | 8.9 – 13.0 | 47.6 – 87.7 |
| Campesterol:cholesterol | 0.6 | 0.8 | 2.6* | 2.6* | 15.9* |
| 95% CI | 0.5 – 0.7 | 0.5 – 1.2 | 2.0 – 3.5 | 2.2 – 3.2 | 11.6 – 21.6 |
| Stigmasterol:cholesterol | 0.4 | 0.6 | 2.0* | 1.8* | 11.8* |
| 95% CI | 0.3 – 0.5 | 0.4 – 0.9 | 1.5 – 2.8 | 1.5 – 2.2 | 8.5 – 16.4 |
All pairwise differences were determined using analysis of variance controlling for age, race, gender, BMI, and physical activity with post hoc Dunnett’s test to adjust for multiple comparisons.
Significantly different from non-vegetarians at
p≤0.0001,
p≤0.05
Sum of plant stanols and other plant sterols
Sum of β-sitosterol, campesterol, stigmasterol, and other phytosterols
Figure 2.
Comparison of mean (whiskers as 95% CI) dietary intake of β-sitosterol, campesterol, total phytosterols, and other phytosterols by dietary pattern among ▼ Blacks and ■Whites. The y-axis represents dietary intake, and the x-axis, the dietary patterns NONVEG=non-vegetarian (168 Blacks, 165 Whites), SEMIVEG=semi vegetarian (9 Blacks, 25 Whites), PESCO=pesco vegetarian (48 Blacks, 35 Whites), LOV=lacto-ovo vegetarian (43 Blacks, 166 Whites), and VEGAN (29 Blacks, 43 Whites). Pairwise differences were determined using two-way analysis of variance adjusted for age, gender, BMI, and physical activity with post hoc Dunnett’s test to correct for multiplicity. Differences were not significant by ethnicity. Within each ethnic group, difference was statistically significant compared to non-vegetarians (reference) at ap≤0.0001 or bp≤0.05.
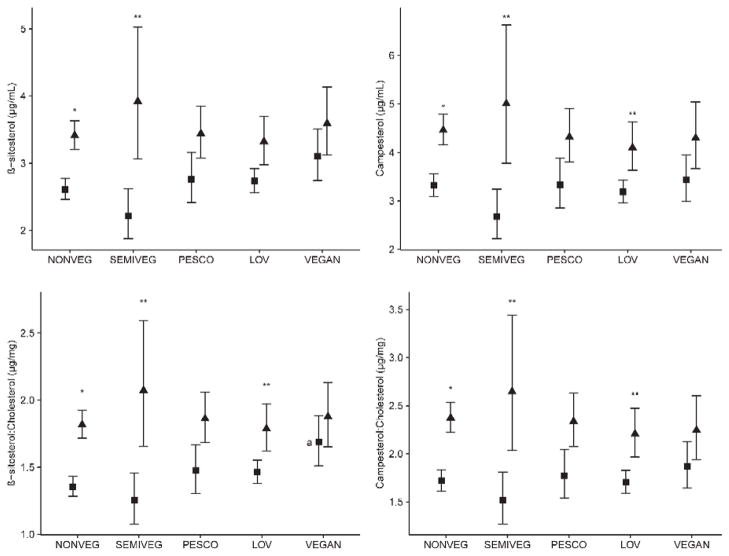
Multivariable analyses comparing plasma concentration of plant sterols, lathosterol and cholesterol by dietary pattern are shown in Table 4. Comparisons of the absolute concentration of plant sterols and lathosterol among vegetarian patterns compared to non-vegetarians were non-significant. Unexpectedly, plasma cholesterol concentration among other vegetarian diets was not significantly different from non-vegetarians. The non-significant results for plant sterol-to-cholesterol ratios were similar to those observed with absolute values, except for β-sitosterol to cholesterol ratio, which was significantly higher among vegans than non-vegetarians. On stratification by ethnic group, plasma concentrations of β-sitosterol, campesterol, and their respective ratios with cholesterol tended to be higher among Blacks than Whites (Figure 3). Compared to Whites, plasma concentrations of β-sitosterol and campesterol were significantly higher in Blacks by >30% among non-vegetarians (p≤0.0001 for both plant sterols), >75 % among SVs (p≤0.05 for both plant sterols), and >20% among LOVs (p≤0.05 for campesterol only). Differences between Blacks and Whites for β-sitosterol to cholesterol ratio and campesterol to cholesterol ratio were similar in magnitude for non-vegetarians (p≤0.0001), SVs (p≤0.05), and LOVs (p≤0.05). Plasma concentrations among Blacks did not differ by dietary pattern. Among Whites, however, β-sitosterol to cholesterol ratio among vegans was significantly higher than among non-vegetarians (p≤0.05).
Table 4.
Plasma concentrations of plant sterols, lathosterol, cholesterol, and plasma plant sterol-to-cholesterol ratios among adult vegetarians and non-vegetarians in North America.
| Non-vegetarian n = 371 |
Semi-vegetarian n = 35 |
Pesco-vegetarian n = 91 |
Lacto ovo-vegetarian n = 224 |
Vegan n = 82 |
|
|---|---|---|---|---|---|
| β-Sitosterol (μg/mL)a | 2.93 | 2.72 | 3.02 | 3.00 | 3.31 |
| 95% CI | 2.81 – 3.07 | 2.37 – 3.13 | 2.77 3.30 | 2.83 3.17 | 3.01 3.63 |
| Campesterol (μg/mL)a | 3.77 | 3.37 | 3.70 | 3.57 | 3.78 |
| 95% CI | 3.58 – 3.96 | 2.88 – 3.94 | 3.36 – 4.09 | 3.35 – 3.81 | 3.40 – 4.21 |
| Lathosterol (μg/mL)a | 1.90 | 1.81 | 1.95 | 1.97 | 2.10 |
| 95% CI | 1.81 – 2.00 | 1.54 – 2.12 | 1.77 – 2.16) | 1.84 – 2.10 | 1.89 – 2.34 |
| Cholesterol (mg/dL) | 194.8 | 183.9 | 189.9 | 189.9 | 190.5 |
| 95% CI | 190.7 – 198.8 | 171.1 – 196.7 | 181.9 – 197.9 | 184.7 – 195.1 | 181.9 – 199.1 |
| β-Sitosterol:cholesterol (μg/mg) | 1.54 | 1.51 | 1.62 | 1.61 | 1.77* |
| 95% CI | 1.48 – 1.60 | 1.33 – 1.71 | 1.50 – 1.76 | 1.53 – 1.69 | 1.62 – 1.92 |
| Campesterol:cholesterol (μg/mg) | 1.98 | 1.87 | 1.99 | 1.91 | 2.02 |
| 95% CI | 1.89 – 2.16 | 1.61 – 2.16 | 1.81 – 2.18 | 1.80 – 2.03 | 1.83 – 2.23 |
| Lathosterol:cholesterol (μg/mg) | 1.00 | 1.00 | 1.05 | 1.06 | 1.12 |
| 95% CI | 0.95 – 1.04 | 0.87 – 1.15 | 0.96–1.15 | 1.00 – 1.12 | 1.02 – 1.24 |
All pairwise differences were determined using analysis of variance controlling for age, race, gender, BMI, and physical activity with post hoc Dunnett’s test to adjust for multiple comparisons.
To convert μg/mL to mg/dL, multiply by 0.1.
Difference is statistically significant (p≤0.05) compared to non-vegetarian (reference).
Figure 3.
Comparison of mean (whiskers as 95% CI) plasma concentrations of β-sitosterol, campesterol, plasma β-sitosterol to cholesterol ratio, and plasma campesterol to cholesterol ratio by dietary pattern among ▼ Blacks and ■ Whites. The y-axis represents plasma concentrations, and the x-axis, the dietary patterns NONVEG=non-vegetarian (186 Blacks, 185 Whites), SEMIVEG=semi vegetarian (11 Blacks, 24 Whites), PESCO=pesco vegetarian (54 Blacks, 37 Whites), LOV=lacto-ovo vegetarian (58 Blacks, 166 Whites), and VEGAN (35 Blacks, 47 Whites). Pairwise differences were determined using two-way analysis of variance adjusted for age, gender, BMI, and physical activity with post hoc Dunnett’s test to correct for multiplicity. Within a specific dietary pattern, the difference between Blacks and Whites was significant at *p≤0.0001 or **p≤0.05. Within each ethnic group, difference was statistically significant compared to non-vegetarians (reference) at ap≤0.05.
DISCUSSION
Our findings in this relatively large sample of adults demonstrated that dietary intake of plant sterols varied by distinct dietary patterns, with intake of β-sitosterol, campesterol, stigmasterol, other phytosterols and total phytosterols generally highest among vegans and lowest among non-vegetarians. Absolute cholesterol intake was relatively low in this population with average intakes as low as 15 mg/d among vegans, and 125 mg/d among non-vegetarians. Plasma cholesterol, absolute plasma concentrations of β-sitosterol, campesterol, lathosterol and their ratios with cholesterol among vegetarian diets did not differ from non-vegetarians. Dietary intake of β-sitosterol and campesterol did not differ by race; however, plasma concentrations of these plant sterols tended to be higher among Blacks compared to Whites.
The associations of dietary intake of plant sterols, other phytosterols, and total phytosterols with dietary patterns were consistent with our hypothesis. The high intake of the phytosterols among vegetarians might be explained by the generally higher intakes of whole grains, tree nuts, vegetable oils, and legumes among vegetarians compared to non-vegetarians in the AHS-2 cohort[38]. Our findings, however, were contrary to a 1984 report that quantified plant sterol intake in a small sample of Adventist adults (50 NVs, 50 LOVs, and 18 vegans) selected from the previous Adventist Health Study cohort from California[39]. In that study total plant sterol intake, quantified as the sum of β-sitosterol and stigmasterol, was lowest among vegans (89 mg/d), highest among LOVs (344 mg/d), and intermediate among NVs (231 mg/d). In the current study, these values were approximately 326 mg/d, 265 mg/d, and 201 mg/d, respectively. It is likely that in the 1984 study, the dated analytical methods used to quantify plant sterols resulted in underestimated plant sterol intakes. A later 2009 publication, which compared the phytosterol content and nutrient profile of five different diets (phytosterol-deficient, DASH-based high-phytosterol, American Heart Association, Atkins Lifetime Maintenance, and vegan)[40], showed that the total phytosterol content per 2000 kcal in the vegan diet was 445 mg/d, and 500 mg/d in the DASH-based high-phytosterol diet. Both of these values are slightly lower compared to the total phytosterol intake of vegans we found in our study (518 mg per 2000 kcal/d on average); nevertheless they provide external confirmation of the relatively high phytosterol intake estimates in the AHS-2 cohort. By comparison to other populations, total phytosterol intake levels in AHS-2 were within the 3rd – 5th quintile of intake observed in the EPIC-Norfolk (265–749 mg/day)[10] and Swedish cohorts (210 – 327 mg/d)[1], and somewhat higher than the Spanish EPIC cohort (241 – 349 mg/d)[8] and adults from Finland (237 – 305 mg/d)[9].
Our findings of relatively low cholesterol intake among vegetarians were consistent with expectations given that in a previous report from our group, we reported that the intake of foods such as solid fats, butter, eggs, and other animal-based foods was higher among non-vegetarians compared to vegetarians[38]. In a 2009 report by Racete et al[40], cholesterol content in the vegan diet (8 mg/d) was even lower than the cholesterol intake of AHS-2 vegans (18 mg/d per 2000 kcal), which confirms that such low intakes of cholesterol is possible. Compared to our study population cholesterol intake was considerably higher in the EPIC-Norfolk cohort[10] as well as a population from northern Sweden[1] and Finland[9]. The lower cholesterol intake in our sample compared to other populations may be partially explained by the relatively low intake of meat in AHS-2 non-vegetarians (59 g/d) compared to the 128 g/d reported among Americans[41].
The overall null findings for the associations of absolute plasma campesterol and β-sitosterol with dietary pattern departed from expectations, though not impossible given that less than 5% of ingested plant sterols are absorbed[42] in humans. Plasma concentrations of plant sterols differ markedly from cholesterol in their intestinal absorption and biliary elimination. Specifically, absorption of plant sterols is much less and their excretion into bile is much faster than cholesterol[43–45]. For example, in a study that compared cholesterol absorption and metabolism among control and vegetarian subjects, biliary concentrations of β-sitosterol and campesterol were significantly higher among vegetarians than the controls[44]. In the same study, cholesterol absorption was also inversely correlated with fecal plant sterol, an indication that fecal plant sterol could be used as a surrogate measure of plant sterol intake[45].
Our finding that plasma cholesterol did not differ between non-vegetarians and vegans was unexpected, particularly since vegan diets tend to have lower total cholesterol compared to omnivorous diets[46, 47]. From a dietary perspective, the relatively low plasma cholesterol among the AHS-2 non-vegetarians might be reasonable because of their generally low cholesterol intake (125 mg/d) and high PS ratio (1.0), and dietary cholesterol at very high intake levels (≥650 mg/d) has been shown to be associated with high serum total cholesterol[48]. It is also possible that because cholesterol intake was particularly low among vegans, endogenous production of cholesterol may have occurred, but the cholesterol-corrected concentration of lathosterol, a surrogate plasma marker of endogenous cholesterol synthesis, was not significantly different between the groups. A more likely explanation of our results, however, may be related to the influence of phytosterols on cholesterol metabolism. In a feeding study that examined the effects of intrinsic phytosterols (naturally occurring in foods) on cholesterol metabolism, Lin et al reported that a phytosterol-abundant diet (449 mg/2000 kcal) compared to a phytosterol-poor diet (126 mg/2000 kcal) was associated with significantly lower cholesterol absorption, significantly higher excretion of total cholesterol, significantly higher cholesterol-adjusted plant sterols, and no difference in serum total cholesterol[49]. Indeed, when we assessed cholesterol-adjusted plasma plant sterols in AHS-2, we found that β-sitosterol-to-cholesterol ratio was significantly higher among vegans compared to non-vegetarians, which is consistent with results from a study that also found higher cholesterol-adjusted β-sitosterol among vegetarian compared to control subjects[44]. These observations suggest that an abundance of phytosterols may have interfered with cholesterol absorption thus producing lower than expected plasma cholesterol even among non-vegetarians.
A comparison of our results with other populations show that absolute plasma cholesterol concentration in our study subjects was lower compared to a cohort from The Netherlands[50]; whereas absolute plasma campesterol and β-sitosterol were higher compared to a sample of subjects from the LASA study with no vascular disease[50] and in control subjects from the EPIC-Norfolk cohort[51]. It is interesting to note that the absolute plasma concentrations of cholesterol and campesterol in our cohort (average age 60 y) are comparable to concentrations that have been observed in much younger populations (children and teens)[37].
A notable finding from our study is that the relationship between plasma concentration of plant sterols and vegetarian diets varied between Blacks and Whites. The vegetarians tended to have higher values in Blacks (Figure 3), despite the fact that we found no ethnic differences in plant sterol intake within the dietary patterns (Figure 2). This ethnic variation persisted even with the cholesterol-adjusted plasma β-sitosterol and campesterol values. These observations suggest a possible genetic component in the metabolism of plant sterols and other sterols in humans.
We recognize some strengths and weaknesses in this study. Although dietary patterns were determined based on consumption of animal products from FFQ, an assessment of the FFQ’s performance indicates that intake estimates of animal-based foods from the FFQ have moderate to high correlations with 24-hr dietary recalls. Having a relatively large group of subjects with diverse dietary patterns and using multiple 24-hour dietary recalls to estimate dietary intake were advantageous as these features provided variation in intake data at the group level, and allowed for adjustment of within person variation in intake, respectively. To estimate plant sterol intake, we compiled plant sterol concentration data of foods from several sources thus producing a relatively comprehensive and updated database of plant sterols. Limitations include the potential measurement error inherent in a single assessment of the FFQ and biomarkers, and the possibility of residual confounding which may have attenuated the associations observed. The study sample is representative of the full AHS-2 cohort, but lacks generalizability to the North American population because of the health-related recommendations of the Seventh-day Adventist church for its members.
In conclusion, we provide evidence that dietary intake of plant sterols and cholesterol varies among distinct vegetarian diets compared to non-vegetarians.
Acknowledgments
The authors’ contributions are as follows: KJS, EH designed research; DL conducted laboratory analysis; KJS, EH, RS collected data; GEF, DL provided laboratory materials; GEF, RS, AM provided other materials, and generated plant sterol database; KJS performed statistical analysis; KJS wrote the paper; KJS had primary responsibility for final content. All authors read and approved the final manuscript.
Financial Support
NIH/NCI Grant #U01CA152939; Unilever Research & Development, Vlaardingen, The Netherlands
Abbreviations
- NV
non-vegetarian
- SV
semi vegetarian
- PV
pesco vegetarian
- LOV
lacto-ovo vegetarian
- NDS-R
Nutrition Data System for Research
- USDA
United States Department of Agriculture
- ANOVA
analysis of variance
- ANCOVA
analysis of covariance
- BMI
body mass index
- SD
standard deviation
- PFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- PS
polyunsaturated to saturated fatty acid ratio
Footnotes
Conflict of interest: KJS and DL obtained funding from Unilever Research & Development for this project. EH, RS, GEF, and AM report no conflict of interest.
References
- 1.Klingberg S, Ellegard L, Johansson I, Hallmans G, et al. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am J Clin Nutr. 2008;87:993–1001. doi: 10.1093/ajcn/87.4.993. [DOI] [PubMed] [Google Scholar]
- 2.Li JH, Awad AB, Fink CS, Wu YW, et al. Measurement variability of plasma beta-sitosterol and campesterol, two new biomarkers for cancer prevention. Eur J Cancer Prev. 2001;10:245–249. doi: 10.1097/00008469-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004;3:1–19. doi: 10.1186/1476-511X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips KM, Ruggio DM, Ashraf-Khorassani M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J Agric Food Chem. 2005;53:9436–9445. doi: 10.1021/jf051505h. [DOI] [PubMed] [Google Scholar]
- 5.Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric. 2000;80:939–966. [Google Scholar]
- 6.Piironen V, Toivo J, Lampi AM. Plant Sterols in Cereals and Cereal Products. Cereal Chem J. 2002;79:148–154. [Google Scholar]
- 7.Piironen V, Toivo J, Puupponen-Pimiä R, Lampi AM. Plant sterols in vegetables, fruits and berries. J Sci Food Agric. 2003;83:330–337. [Google Scholar]
- 8.Escurriol V, Marí-Dell’Olmo M, Rohlfs I, Borrell C, et al. Plant sterol intake and education level in the Spanish EPIC cohort. Nutrition. 2009;25:769–773. doi: 10.1016/j.nut.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Valsta LM, Lemstrom A, Ovaskainen ML, Lampi AM, et al. Estimation of plant sterol and cholesterol intake in Finland: quality of new values and their effect on intake. Br J Nutr. 2004;92:671–678. doi: 10.1079/bjn20041234. [DOI] [PubMed] [Google Scholar]
- 10.Andersson SW, Skinner J, Ellegard L, Welch AA, et al. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: a cross-sectional study. Eur J Clin Nutr. 2004;58:1378–1385. doi: 10.1038/sj.ejcn.1601980. [DOI] [PubMed] [Google Scholar]
- 11.Escurriol V, Cofan M, Moreno-Iribas C, Larranaga N, et al. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J Lipid Res. 2010;51:618–624. doi: 10.1194/jlr.P000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genser B, Silbernagel G, De Backer G, Bruckert E, et al. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2012;33:444–451. doi: 10.1093/eurheartj/ehr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strandberg TE, Gylling H, Tilvis RS, Miettinen TA. Serum plant and other noncholesterol sterols, cholesterol metabolism and 22-year mortality among middle-aged men. Atherosclerosis. 2010;210:282–287. doi: 10.1016/j.atherosclerosis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Orlich MJ, Singh PN, Sabate J, Jaceldo-Siegl K, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173:1230–1238. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzo NS, Sabate J, Jaceldo-Siegl K, Fraser GE. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the adventist health study 2. Diabetes Care. 2011;34:1225–1227. doi: 10.2337/dc10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2) Public Health Nutr. 2012;15:1909–1916. doi: 10.1017/S1368980011003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonstad S, Stewart K, Oda K, Batech M, et al. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23:292–299. doi: 10.1016/j.numecd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlich MJ, Singh PN, Sabate J, Fan J, et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med. 2015;175:767–776. doi: 10.1001/jamainternmed.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2013;22:286–294. doi: 10.1158/1055-9965.EPI-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler TL, Fraser GE, Beeson WL, Knutsen SF, et al. Cohort profile: The Adventist Health Study-2 (AHS-2) Int J Epidemiol. 2008;37:260–265. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113:1610–1619. doi: 10.1016/j.jand.2013.06.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaceldo-Siegl K, Fan J, Sabate J, Knutsen SF, et al. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr. 2011;14:1988–1997. doi: 10.1017/S1368980011000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US, D. o. A. A. R. S. Nutrient Data Laboratory Home Page. 2014. [Google Scholar]
- 24.Awad AB, Chan KC, Downie AC, Fink CS. Peanuts as a source of beta-sitosterol, a sterol with anticancer properties. Nutr Cancer. 2000;36:238–241. doi: 10.1207/S15327914NC3602_14. [DOI] [PubMed] [Google Scholar]
- 25.Bacchetti T, Masciangelo S, Bicchiega V, Bertoli E, Ferretti G. Phytosterols, phytostanols and their esters: from natural to functional foods. Mediterranean J Nutr Metab. 2011;4:165–172. [Google Scholar]
- 26.Bradford PG, Awad AB. Modulation of signal transduction in cancer cells by phytosterols. Biofactors. 2010;36:241–247. doi: 10.1002/biof.97. [DOI] [PubMed] [Google Scholar]
- 27.Gupta AK, Savopoulos CG, Ahuja J, Hatzitolios AI. Role of phytosterols in lipid-lowering: current perspectives. QJM. 2011;104:301–308. doi: 10.1093/qjmed/hcr007. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Escrig A, Santos-Hidalgo AB, Saura-Calixto F. Common sources and estimated intake of plant sterols in the Spanish diet. J Agric Food Chem. 2006;54:3462–3471. doi: 10.1021/jf053188k. [DOI] [PubMed] [Google Scholar]
- 29.Kalogeropoulos N, Chiou A, Ioannou M, Karathanos VT, et al. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010;121:682–690. [Google Scholar]
- 30.Marangoni F, Poli A. Phytosterols and cardiovascular health. Pharmacol Res. 2010;61:193–199. doi: 10.1016/j.phrs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Normén L, Bryngelsson S, Johnsson M, Evheden P, et al. The Phytosterol Content of Some Cereal Foods Commonly Consumed in Sweden and in the Netherlands. J Food Compos Anal. 2002;15:693–704. [Google Scholar]
- 32.Normen L, Johnsson M, Andersson H, van Gameren Y, Dutta P. Plant sterols in vegetables and fruits commonly consumed in Sweden. Eur J Nutr. 1999;38:84–89. doi: 10.1007/s003940050048. [DOI] [PubMed] [Google Scholar]
- 33.Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 34.Weihrauch JL, Gardner JM. Sterol content of foods of plant origin. J Am Diet Assoc. 1978;73:39–47. [PubMed] [Google Scholar]
- 35.Lutjohann D, Brzezinka A, Barth E, Abramowski D, et al. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J Lipid Res. 2002;43:1078–1085. doi: 10.1194/jlr.m200071-jlr200. [DOI] [PubMed] [Google Scholar]
- 36.Mackay DS, Jones PJ, Myrie SB, Plat J, Lutjohann D. Methodological considerations for the harmonization of non-cholesterol sterol bio-analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;957:116–122. doi: 10.1016/j.jchromb.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 37.Chan YM, Varady KA, Lin Y, Trautwein E, et al. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr Rev. 2006;64:385–402. doi: 10.1111/j.1753-4887.2006.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 38.Orlich MJ, Jaceldo-Siegl K, Sabate J, Fan J, et al. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112:1644–1653. doi: 10.1017/S000711451400261X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair PP, Turjman N, Kessie G, Calkins B, et al. Diet, nutrition intake, and metabolism in populations at high and low risk for colon cancer. Dietary cholesterol, beta-sitosterol, and stigmasterol. Am J Clin Nutr. 1984:40. doi: 10.1093/ajcn/40.4.927. [DOI] [PubMed] [Google Scholar]
- 40.Racette SB, Spearie CA, Phillips KM, Lin X, et al. Phytosterol-deficient and high-phytosterol diets developed for controlled feeding studies. J Am Diet Assoc. 2009;109:2043–2051. doi: 10.1016/j.jada.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14:575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farquhar JW. In: Handbook of Lipids in Human Nutrition. Spiller GA, editor. CRC Press, Inc; Boca Raton, FL: 1996. pp. 101–105. [Google Scholar]
- 43.von Bergmann K, Sudhop T, Lutjohann D. Cholesterol and plant sterol absorption: recent insights. Am J Cardiol. 2005;96:10D–14D. doi: 10.1016/j.amjcard.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Vuoristo M, Miettinen TA. Absorption, metabolism, and serum concentrations of cholesterol in vegetarians: effects of cholesterol feeding. Am J Clin Nutr. 1994;59:1325–1331. doi: 10.1093/ajcn/59.6.1325. [DOI] [PubMed] [Google Scholar]
- 45.Salen G, Ahrens EH, Jr, Grundy SM. Metabolism of beta-sitosterol in man. J Clin Invest. 1970;49:952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goff LM, Bell JD, So PW, Dornhorst A, Frost GS. Veganism and its relationship with insulin resistance and intramyocellular lipid. Eur J Clin Nutr. 2005;59:291–298. doi: 10.1038/sj.ejcn.1602076. [DOI] [PubMed] [Google Scholar]
- 47.Kuchta A, Lebiedzinska A, Fijalkowski M, Galaska R, et al. Impact of plant-based diet on lipid risk factors for atherosclerosis. Cardiol J. 2016;23:141–148. doi: 10.5603/CJ.a2016.0002. [DOI] [PubMed] [Google Scholar]
- 48.Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2015;102:276–294. doi: 10.3945/ajcn.114.100305. [DOI] [PubMed] [Google Scholar]
- 49.Lin X, Racette SB, Lefevre M, Spearie CA, et al. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: a controlled feeding trial. Eur J Clin Nutr. 2010;64:1481–1487. doi: 10.1038/ejcn.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fassbender K, Lutjohann D, Dik MG, Bremmer M, et al. Moderately elevated plant sterol levels are associated with reduced cardiovascular risk--the LASA study. Atherosclerosis. 2008;196:283–288. doi: 10.1016/j.atherosclerosis.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Pinedo S, Vissers MN, von Bergmann K, Elharchaoui K, et al. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. J Lipid Res. 2007;48:139–144. doi: 10.1194/jlr.M600371-JLR200. [DOI] [PubMed] [Google Scholar]