Abstract
Objective
Individuals with schizophrenia have disproportionate memory impairments when encoding relational versus item-specific information, and when using recollection versus familiarity during retrieval. It is unclear whether this pattern is unique to people with chronic schizophrenia, or if it occurs in individuals after a first episode of psychosis (FE), or when at clinical high-risk for psychosis (CHR).
Methods
We administered the Relational and Item-Specific Memory task (RiSE) to 22 CHR, 101 FE, and 58 typically developing (TD) participants. We examined group differences in item and relational encoding, and familiarity-based and recollection-based retrieval using parametric analysis and structural equation modeling (SEM). Longitudinal data allowed us to examine relations between baseline RiSE performance and change in clinical symptoms at 1-year follow-up in the FE group.
Results
Groups did not differ on familiarity. FE and CHR groups were equally impaired on overall recognition accuracy. Although recollection was impaired in both FE and CHR groups following relational encoding, only the FE group had impaired recollection following item encoding. SEM showed atypical relationships between familiarity and recollection, as well as familiarity and item recognition for both the FE and CHR groups. For FE individuals, better baseline recognition accuracy predicted less severe negative symptoms at 1-year follow-up.
Conclusions
Impaired relational and recollective memory may reflect neurodevelopmental abnormalities predating conversion to psychosis. These memory deficits appear related to negative symptom changes. In contrast, item specific recollection deficits appear to occur after the development of full psychosis. Familiarity appears to be a relatively preserved memory function across the psychosis spectrum.
Keywords: episodic memory, recollection, familiarity, clinical high risk, schizophrenia, neurocognition
1. Introduction
Episodic memory is frequently disrupted in psychosis (Heinrichs and Zakzanis, 1998) and contributes to loss of quality of life and poor functional outcomes (Green et al. 2000; Lepage et al. 2014; Milev et al. 2005). However, episodic memory is not a unitary construct. Performance depends upon effectively taking in information (encoding) and finding and using that information when needed (retrieval) (Tulving and Thomson 1973). An important division occurs between item and relational encoding. Both support long-term memory, but they differ by type of memory representation (Davachi 2006 for review). Item encoding focuses on distinct aspects of information, such as the features of a word, event or object (e.g. The bike my sister loaned me is yellow and purple). Relational encoding focuses on associative characteristics between multiple pieces of information, such as the temporal order of events, or the relative positions of multiple objects (e.g., I parked that bike behind the store, next to the tree).
Just as there are multiple ways of encoding information, there are multiple ways of retrieving it. A distinction is made between recall of information independent of context (e.g. what is needed to answer an essay question on an exam), and recognition of information within context (e.g. what is needed to answer a multiple choice question on an exam) (Raaijmakers and Shiffrin 1992 for review). Recognition memory can be achieved using both familiarity and recollection (Yonelinas et al. 2002). Familiarity is a fast signal-detection based process that evaluates memory on the basis of a sense of recency and novelty (e.g. As I came out of the store a stranger cycled past and I immediately felt that I had seen that bike before). Recollection is a slower, search-based strategy that evaluates memory on the basis of particular source details (e.g., A moment later I remembered, that bike is the one I borrowed from my sister!). Investigating these specific memory abilities can reveal areas of preserved function in disorders characterized by memory impairment. For example, people with schizophrenia experience primarily encoding and retrieval deficits (Jung and Lee 2016 for review). These patients also have disproportionate retrieval deficits for information encoded in a relational versus item-specific manner (Ragland et al. 2012a, Williams et al. 2010) and are more severely impaired when using recollection versus familiarity during retrieval (Libby et al. 2013; VanErp et al. 2008). Previous longitudinal studies show memory abilities and impairments to be generally stable in patients, even after one or more years (Censits et al. 1997, Albus et al. 2006).
Psychotic disorders like schizophrenia may result from neurodevelopmental abnormalities (Marenco and Weinberger, 2000). Cognitive impairments often occur in clinical high risk (CHR) individuals, who are showing early signs and symptoms but are without an Axis I diagnosis (Lencz et al. 2006). Studying CHR individuals is advantageous because they have not experienced many illness-related factors such as prolonged educational or occupational disruption, or chronic medication and treatment effects that can confound interpretation of cognitive impairments (see reviews by Ho et al., 2011, and Arnsten 2015). Although CHR research has been conducted with standard neuropsychological batteries (see de Paula et al., 2015 for review), a cognitive neuroscience approach to identify specific encoding and retrieval deficits has not been accomplished. In addition to CHR participants, we examined patients during a first episode of psychosis (FE). Most previous studies (e.g., Achim & LePage, 2003; Ragland et al., 2015; Williams et al. 2010) examined more chronically ill patients. By investigating FE participants we aim to discover if the encoding and retrieval deficits associated with chronic schizophrenia are also apparent early in the illness.
Our primary goal was to examine the magnitude and pattern of specific encoding and retrieval impairments in CHR and FE patients, in the context of what was previously observed in chronically ill patients. Based on previous work showing similar patterns of cognitive impairment between FE and chronically ill patients (Lewandowski et al. 2011), we predicted that the FE group would show prominent relational and recollective memory impairments, and moderate item and familiarity memory impairments compared to typically developing (TD) individuals. Previous CHR research found intermediate level impairments on measures of verbal memory (Hou et al., 2016, Liu et al., 2015), meta-memory (Eisenacher et al., 2015), working memory (Goghari et al., 2014), and declarative memory (see Cirillo et al., 2003 for review). Therefore, we expected the CHR group to show better performance than the FE group, but worse performance relative to TD individuals.
A secondary goal was to determine if these encoding and retrieval processes could predict severity of positive, negative, and disorganized clinical symptoms at 1-year follow-up in the FE group. Previous research found that cognitive abilities could predict future clinical symptoms in schizophrenia (see Lepage et al. 2014 for review). As memory performance impairments are particularly associated with negative and disorganized symptoms (Hill et al. 2002), and motivation, memory, cognitive organization, and cognitive abilities are deeply intertwined (Braver et al. 2014 for review), we hypothesized that better memory performance at baseline would predict less severe negative and disorganized symptoms one year later.
2. Methods
2.1 Sample
One hundred eighty-one individuals (58 TD, 101 FE, 22 CHR) participated. They were part of an ongoing longitudinal study of early psychosis (Lesh et al. 2015), although none of these memory results have been published. Clinical participants were recruited from the Early Diagnosis and Preventive Treatment (EDAPT) clinic at UC Davis Medical Center. FE participants were assessed with the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 2002), and received a psychosis spectrum diagnosis (49 schizophrenia, 19 schizoaffective, 14 bipolar disorder with psychotic features, 7 major depressive disorder with psychotic features, 1 schizophreniform, and 11 psychosis not otherwise specified). 80 were taking atypical antipsychotic medication, 2 were taking typical antipsychotic medication, and 19 were un-medicated. FE participants were within 3 years of their first psychotic break (mean = 11 months 5 days, sd = 7 months 13 days).
CHR participants had no history of psychosis and met high risk criteria based on the Structured Interview for Prodromal Syndromes (SIPS; McGlashan, 2001) (see Supplemental Material). 11 were taking atypical antipsychotic medication, and 11 were unmedicated. Participants in the TD group had no current or past Axis 1 disorders, or any first-degree relatives with a psychotic disorder. Participants were excluded for a positive drug screen at time of testing, a history of substance dependence in the past 6 months, history of severe head trauma or other neurological insult, or borderline intellectual ability (IQ < 70). IQ was assessed with Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) and groups were matched on gender, handedness and parental education (Table 1). All participants provided informed consent. The study was approved by the UC Davis Institutional Review Board.
Table 1.
Demographics
| TD (n=58) |
FE (n=101) |
CHR (n=22) |
TD and FE |
TD and CHR |
FE and CHR |
|
|---|---|---|---|---|---|---|
| T-test [mean (SD)] | p | |||||
| Age | 19.21 (4.34) |
19.31 (3.90) |
15.32 (3.03) |
0.88 | <.01 | <.01 |
| IQ | 117.52 (11.53) |
100.00 (13.33) |
101.90 (9.96) |
<.01 | <.01 | 0.55 |
| Parent Ed |
15.15 (2.91) |
13.94 (2.60) |
13.80 (3.43) |
<.01 | 0.09 | 0.84 |
| Chi square [ % (n)] | ||||||
| Gender (male) |
60.34% (35) |
68.69% (68) |
59.09% (13) |
0.29 | 0.92 | 0.39 |
| Hand (left) |
12.07% (7) |
13.83% (13) |
23.08% (13) |
0.76 | 0.30 | 0.38 |
Clinical symptoms were assessed with the Brief Psychiatric Rating Scale (Overall, 1980), the Scale for the Assessment of Positive Symptoms, and the Scale for the Assessment of Negative Symptoms (Anderasen, 1983 a,b). Ratings were combined into positive, negative, and disorganized symptom severity dimensions (Liddle, 1987; Barch et al., 2003). For FE participants with longitudinal data, we computed change in severity for positive, negative and disorganized dimensions from baseline to 1-year follow-up (mean= 1.02 years sd=.316 years). Of the 101 FE participants, 32 had complete follow-up data. There were no significant differences in demographic or performance variables between FE participants with and without follow-up data (Supplemental Material). There were no significant group changes in positive, negative, and disorganized symptoms between baseline and follow-up.
2.2 Memory measures
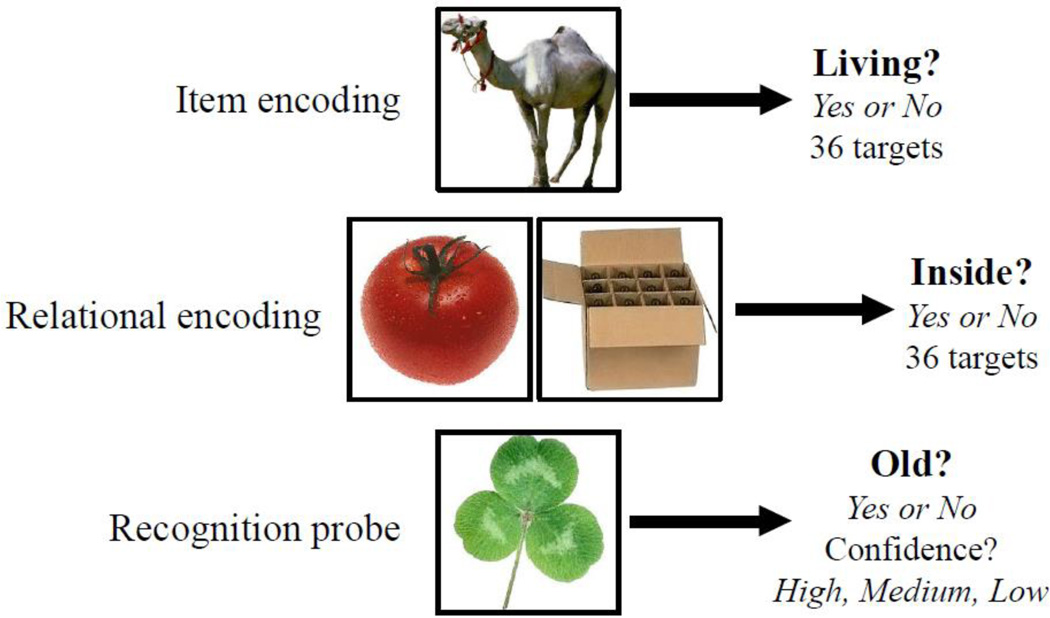
Participants completed the RiSE (Ragland et al. 2012a) following clinical assessment. RiSE is an incidental encoding paradigm, with item and relational encoding conditions. During item encoding, 36 single images are presented for 2 seconds each; participants press a button to indicate if the image is of a living object. During relational encoding, 18 pairs of stimuli are presented simultaneously for 4 seconds each; participants indicate if one of the objects can fit inside the other. Memory is tested with an item recognition task, in which 72 novel objects as well as all 36 item and 36 relationally-encoded objects are presented one at a time. Participants indicate if each object is “old” (i.e., previously studied), and their level of confidence (high, medium, or low). Participants are required to successfully complete practice trials prior to participation. Figure 1.
Figure 1.
RiSE task.
2.3 Statistical analysis
2.3.1 Group differences
Performance was measured separately for item- and relationally-encoded objects using discriminability, recollection, and familiarity parameters. Discriminability (d’), a signal detection measure of overall recognition accuracy, was calculated as the difference between the standardized hit rate (i.e., correctly responding “old” to a previously studied items) and standardized false alarm rate (i.e., incorrectly responding “old” to a new item). Familiarity and recollection were calculated by entering confidence ratings (“high”, “medium”, “low”) for each response into a Receiver Operator Characteristics (ROC) model to obtain orthogonal estimates of these two retrieval processes (Yonelinas, 1994).
Group differences were examined with three-way group (TD, FE, CHR) by encoding condition (item-encoded, relationally-encoded) analyses of variance (ANOVA) separately for discriminability, recollection, and familiarity parameters. Subsequent two-way ANOVAs and univariate t-tests investigated main effects and higher-level interactions. Pearson’s correlation coefficients were used to identify associations between performance and positive, negative and disorganized symptoms. A two-tailed alpha level at .05 was used for significance testing and correlations were corrected for multiple comparisons using Bonfereoni corrections. Analyses were performed using SAS Version 9.2.
2.3.2 Structural Equation Modeling
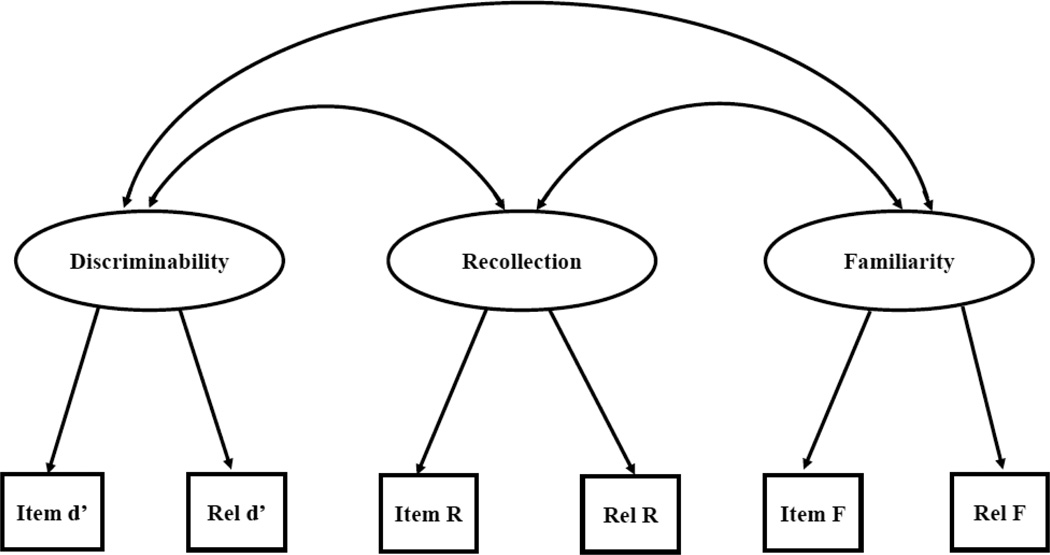
Structural Equation Modeling (SEM) was performed using MPLUS® software (Version 7, Munthen, 2012). Model fit was tested using the χ2 of exact fit, comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean squared residual (SRMR). Our basic model (Figure 2) included discriminability, recollection, and familiarity as latent variables and assumes that recollection and familiarity are independent memory abilities, discriminability reflects general memory performance, and all three latent variables relate to each other (Figure 2). These assumptions were tested using a confirmatory factor analysis. Factor loadings of the dependent measures were examined to check that no single factor dominated the latent memory variables. Model latent means and correlations were tested to determine differences between groups.
Figure 2.
- Box=measured variable
- Ellipse =latent variable
- Double headed arrow=correlation
-
Single headed arrow =direct effect
- an arrow from a latent variable to a measured variable means ‘measured by’
- an arrow from a variable to a latent variable means ‘regressed on’
2.3.3 Longitudinal changes
The ability of memory performance at baseline to predict changes in clinical dimensions at 12-month follow-up was investigated using Pearson’s correlation coefficients to identify performance measures that correlated with clinical changes. These performance variables were entered into the SEM regression models to test their ability to predict clinical changes one year later.
3. Results
3.1 Group differences in memory performance
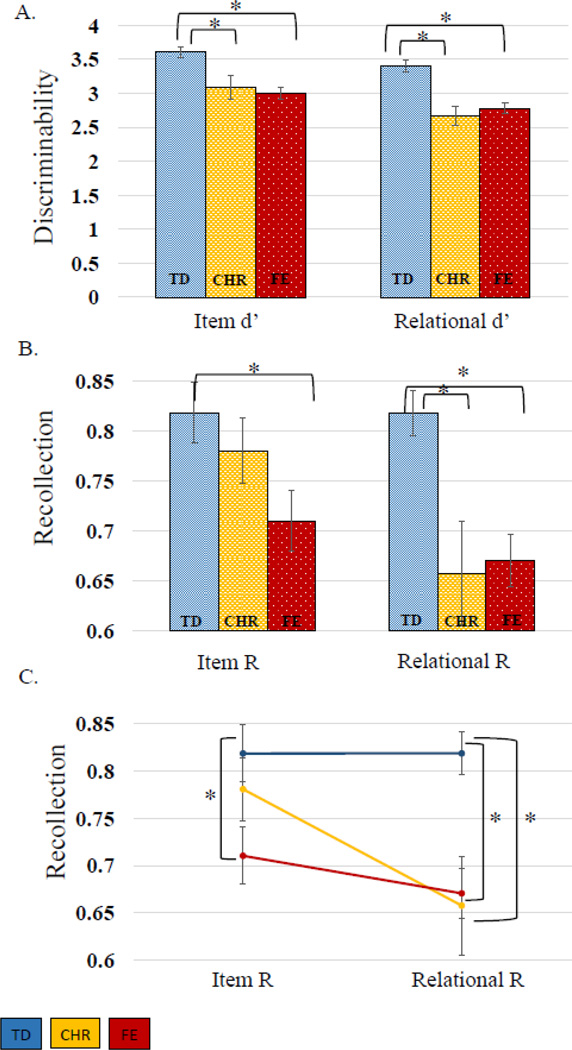
There were main effects of encoding condition [F(1,178)=50.94, p<.01) and group [F(2,178)=14.13, p<.01] on d’ accuracy, but no group-by-encoding interaction [F(2,178)=1.82, p=.16]. The effect of encoding condition was due to better discriminability following item than relational encoding (t(180)=7.42, p<.01). Group differences arose from the TD group showing better discriminability than FE (item-encoded t(150.98)=5.15, p<.01, relationally-encoded t(157)=5.11, p<.01) or CHR groups (item-encoded t(78)=3.13, p<.01, relationally-encoded t(78)=4.46, p<.01). The FE and CHR groups did not differ (item-encoded t(121)=−.39, p=.69, relationally-encoded t(121)=.56, p=.58)
For familiarity, there were no effects of encoding condition [F(1,176)=3.66, p=.06], group [F(2,176)=1.10, p=.33], or group-by-encoding interactions [F(2,176)=1.02, p=.36]. Examination of recollection revealed main effects of group [F(2,176)=5.42, p<.01], encoding condition [F(1,176)=11.39, p<.01], and a group-by-encoding interaction [F(2,176)=3.79, p=.02]. The encoding condition effect was due to better recollection following item than relational encoding [t(178)=2.76, p<.01]. Group differences were driven by the FE group, with worse recollection than the TD group [item-encoded t(143.09)=2.54, p= 0.01 relationally-encoded t(153.35)= 4.25, p<.01], but no overall differences from the CHR group [item-encoded t(58.83)=−1.58, p=.12, relationally-encoded t(120)=.21, p=.84]. The interaction arose from recollection impairments in the CHR group, relative to TD, following relational [t(78)=3.30, p<.01], but not following item encoding [t(54.1)=.84, p=.40]. (Table 2, Figure 3).
Table 2.
Group means.
| Mean (SD) | TD | CHR | FE |
|---|---|---|---|
| Discriminability | |||
| Item- encoded |
3.61 (.61) |
3.08 (.82) |
3.00 (.87) |
| Relationally- encoded |
3.40 (.65) |
2.67 (.65) |
2.77 (.79) |
| Recollection | |||
| Item- encoded |
.82 (.23) |
.78 (.15) |
.71 (.30) |
| Relationally- encoded |
.82 (.17) |
.66 (.24) |
.67 (.27) |
Figure 3.
A. Discriminability means compared across groups. B. and C. Recollection means compared across groups. *=p<.01
3.2 SEM results
Our SEM revealed a good overall fit (CFI=.883, SRMR=.037) (model 1a; see supplemental material for additional test output for all referenced models). Factor loadings showed no significant differences in the item or relational memory measures’ contributions to the latent variables (p<.01) and each path was significant (p<.05).
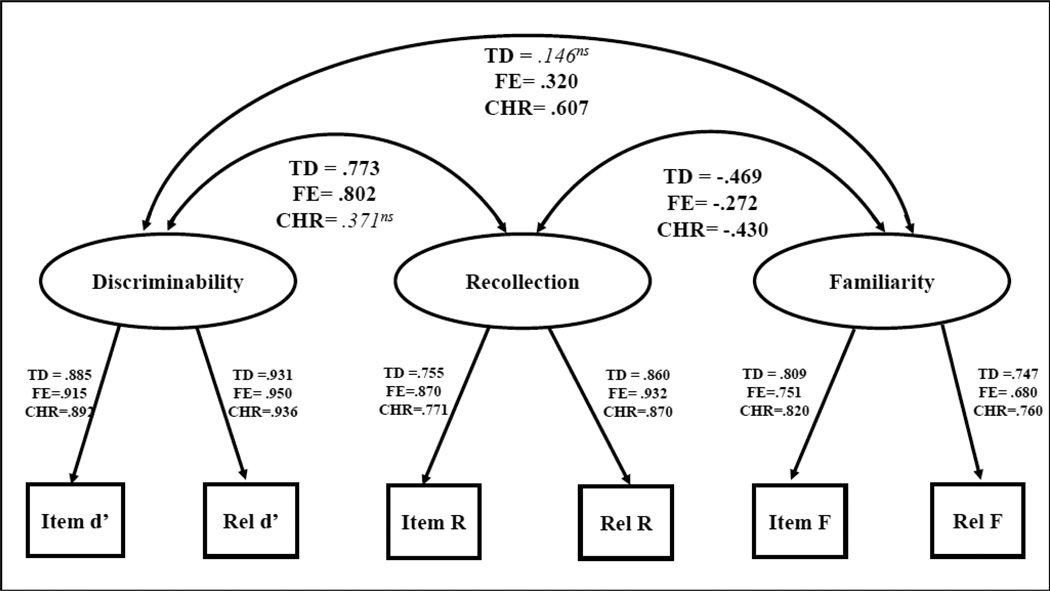
Model fit was significantly improved when latent variables were allowed to be free across groups (model 2b—latent means different across the three groups [CFI=.789, SRMR=.383], compared to model 2a—full invariance across groups [CFI=.758, SRMR= .494], p<.05, and model 2c—latent means and latent variances/covariances different across the three groups [CFI=.812, SRMR=.220] compared to 2b, p<.05) justifying examination of individual correlations and pairwise comparisons. We next determined correlations between latent variables for each group as shown in Figure 4 (model 3, latent means invariant between groups, variances and covariances free across groups [CFI=.773, SRMR=.332]). Recollection and familiarity were negatively correlated in the TD group (−.469, p<.05). Recollection and familiarity were also negatively correlated in the FE group ( −.272, p<.05), though the strength of that association was significantly weaker than for the TD group (p<.05). The CHR group showed a negative correlation between recollection and familiarity (−.430, p<.05), which was not different from either the TD or FE groups. Familiarity and discriminability abilities were not associated with each other in the TD group. However, improved familiarity was correlated with improved discriminability for both patient groups (FE=320, p<05, CHR=607, p<05). Better recollection was correlated with better discriminability for both the TD (.773, p<05) and FE (.802, p<05) groups, but not for the CHR group.
Figure 4.
- non significant correlations are shown in italics
- Significant = p<.05
- ns=non significant
3.3 Longitudinal changes
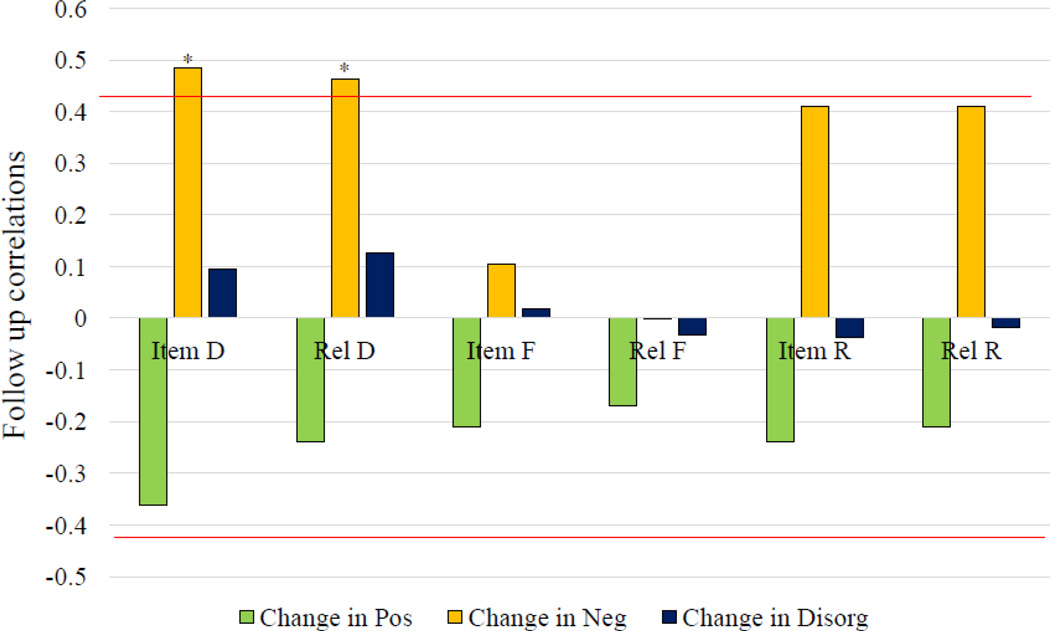
For FE participants with longitudinal data, better memory performance at baseline predicted less severe negative symptoms at 1-year follow-up. Improvement in negative symptoms was associated with better discriminability following item encoding [r(30)=49, p<01] and relational encoding [r(30)=46, p<01] (Bonferroni corrected; critical p value =.0125). Better recollection at baseline showed trend-level associations with improved negative symptoms at 1-year follow-up [item-encoded r(30)=.41, p=.02, relationally-encoded r(30)=.41, p=.02] (Figure 5). SEM revealed that better discriminability strongly predicted better negative symptom outcome [regression itself: β=496, p<001, model with regression (model 4b): CFI=953, SRMR=.082, improved from model without this regression (model 4a, CFI=.906, SRMR=.168), p<01]. Baseline familiarity or recollection performance did not predict any change in negative symptoms.
Figure 5.
Follow-up FE data, n=32. Correlations between memory measures and follow-up changes in positive symptom severity, negative symptom severity, and disorganized symptom severity. *=p<.0125
Discussion
To our knowledge, this is the first episodic memory study to examine relational versus item encoding and recollection versus familiarity retrieval processes in CHR and FE individuals. Based on previous neuropsychological studies showing similar patterns of cognitive impairment in FE and more chronically ill patients (Lewandowski et al., 2011, Bozikas and Andreou 2011), we expected the FE group to show impairments in relational and recollective memory compared to the TD group. We also hypothesized that CHR participants would show attenuated deficits, with memory performance falling between that of TD and FE groups. Study results, however, revealed a more complicated and interesting pattern than expected.
In many ways, the memory performance of the FE group resembled that of patients with chronic schizophrenia. As in previous studies of chronic patients (Ragland et al. 2012a, 2012b, 2015), discriminability following both item and relational encoding was impaired for FE participants. The FE group also showed pronounced recollection deficits following item as well as relational encoding, the same pattern previously noted for patients with long-term illness (Ragland et al. 2012a, 2012b, 2015). One area of difference was familiarity-based retrieval. Although this was previously shown to be less impaired than recollection in chronic patients (see Libby et al., 2013 for review), FE patients in the current study did not show any familiarity deficits, suggesting that familiarity is an area of strength, with deficits occurring only in patients with long-term illness.
Surprisingly, CHR results did not support predictions of intermediate-level performance deficits. Instead, the CHR group was either unimpaired, or showed equivalent deficits to FE patients depending upon memory domain. Equivalent deficits were observed for discriminability and for recollection following relational encoding. However, while FE participants showed recollection impairments following both item and relational encoding, CHR participants only showed recollection impairments following relational encoding.
Evidence of recognition accuracy deficits in the CHR group suggests that overall discriminability may be compromised before formal onset of an Axis 1 psychotic disorder and may reflect neurodevelopmental abnormalities that contribute to early signs and symptoms of psychosis, even if these symptoms never reach the threshold for diagnosing an Axis I disorder. Several studies show abnormalities in the structure or function of the prefrontal cortex (PFC) and hippocampus in CHR individuals (Nenadic et al., 2015, Niendam et al., 2014 Allen et al., 2011 Falkenberg et al., 2015). These structures are important to healthy episodic memory functioning (Francis et al., 2016, Weiss et al., 2003, and Blumenfeld et al., 2007, Eichenbaum et al., 2007 for reviews), and are potential mechanisms of CHR memory dysfunction. In addition to being an early marker of psychosis risk, discriminability performance also appeared to influence 1-year clinical outcomes in patients who were in their first episode of a psychotic disorder. Better discriminability performance at baseline predicted less severe negative symptoms at clinical follow-up. This finding converges with research suggesting that episodic memory may mediate clinical outcomes through a lessening of negative symptom severity, which can facilitate increased engagement in educational, occupational, and social activities that promote recovery (LePage et al., 2014 for review). Of course, while better memory may help individuals to remember the steps needed to engage in the world, it is possible that correlations between negative symptoms and memory might result from other brain processes affecting both domains. The unimpaired performance by patients on the familiarity portion of the task suggests that memory deficits are not the result of a failure of attention, or of a lack of motivation to try to do the task. However, other known areas of difficulty in CHR and FE, including cognitive control (Hou et al. 2016) and meta-cognition (Trauelsen et al 2016, Cotter et al. 2016) cannot be ruled out as a source of memory deficits, and could be mediating the relationship between memory and negative symptoms. Familiarity was unimpaired in both FE and CHR relative to TD groups. Intact familiarity following item encoding was expected. However, lack of familiarity impairments following relational encoding was surprising and is the one area of difference from previously published results in chronic patients (Ragland et al. 2012a, 2012b, 2015). This suggests that there may be clinical state-related factors that lead to an additional impairment of relational encoding and/or familiarity processes that occurs in chronic psychosis.
Finally, consistent with our previous RiSE research (Ragland et al. 2012a, 2012b, 2015), recollection was impaired in both clinical groups. Recollection impairments were observed in the FE group following both item and relational encoding. This is a pattern that was also seen in chronic patients. Moreover, the CHR group also showed a recollection impairment following relational encoding, suggesting that relational episodic encoding and retrieval processes may represent an early marker of psychosis risk. Interestingly however, CHR individuals did not show a deficit in recollection following item encoding.
In sum, this pattern of results suggest both an early neurodevelopmental insult in brain systems that support recollection of relational memory representations, with further illness related changes in recollection following item encoding related to severity of negative symptoms. Because these were cross-sectional data we were not able to determine if these illness-related changes also reflected neurodegeneration, and a longitudinal study is clearly warranted. Nevertheless, we speculate that relational encoding in support of subsequent recollection appears to be a core deficit in the psychosis spectrum, occurring before the onset of a first episode. Because the ability to encode item features appears to be a relative strength, these CHR individuals can recollect information following item encoding. However, this ability to encode item features also becomes disrupted, leading to additional recollection impairments when one is in the first episode of a psychotic disorder. Finally, because discriminability reflects both recollection and familiarity retrieval processes, it can appear impaired very early in the risk state even when more process-pure familiarity estimates are found to be intact.
Structural equation modeling also revealed disruption in the structure of the associations between memory processes. Recollection and familiarity were less orthogonal to each other in FE patients, suggesting that dissociations between retrieval processes (i.e. recollection and familiarity) commonly observed in typically developing individuals are less pronounced in FE patients. Furthermore, the relationship of recollection with discriminability was disrupted in CHR participants, indicating an additional departure from the pattern of memory processes’ interactions seen in typically developing individuals. FE and CHR groups also showed strong correlations between familiarity and discriminability that were not present in the TD group, suggesting that individuals with impaired recollection may show an over-reliance on familiarity processes to guide discriminability judgments, whereas those with intact recollection are less likely to use a compensatory familiarity process.
Limited by the small sample size and lack of follow-up data from the CHR group, we were unable to investigate if specific memory impairments could be used to predict conversion from CHR to FE. Similarly, we had insufficient functional outcome data to examine the effects of memory performance on functional outcomes in CHR. We hope that future studies addressing these limitations could use specific patterns of memory impairments to identify those CHR individuals most likely to convert to psychosis, and identify FE individuals most likely to experience persistent functional deficits and most in need of intervention.
Supplementary Material
Acknowledgments
We thank Neli Mihov for assistance with data collection; and our participants and their families for their time and effort.
Funding body agreements and policies
Research was supported by NIMH grants R01MH105411 (PI: Ragland) and RO1MH059883 (PI: Carter).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure
No disclosures.
Portions of this work were presented at the 5th Biennial Schizophrenia International Research Society Conference, Florence, Italy, 2016.
Contributors
JDR designed the study. SGW took primary responsibility for data analysis. SGW and JDR worked together on manuscript preparation. TN and JDR provided clinical expertise with TN especially contributing to the clinical high-risk discussion. EF provided statistical expertise with SEM theory and implementation. All authors contributed to and have approved the final manuscript.
Conflicts of interest
All authors declare that they have no conflicts of interest.
References
- Albus M, Hubmann W, Mohr F, Hecht S, Hinterberger-Weber P, Seitz NN, Kuchenhoff H. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2006;256:442–451. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- Allen P, Seal ML, Valli I, Fusar-Poli P, Perlini C, Day F, Wood SJ, Williams SC, McGuire PK. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37:746–756. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderasen NC. Scale for the assessment of positive symptoms (SAPS) Iowa City: The University of Iowa; 1983a. [Google Scholar]
- Anderasen NC. Scale for the assessment of negative symptoms (SANS) Iowa City: The University of Iowa; 1983b. [Google Scholar]
- Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW, 3rd, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Andreou C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust NZJ Psychiatry. 2011;45:93–108. doi: 10.3109/00048674.2010.541418. [DOI] [PubMed] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, et al. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci. 2014;14:443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res. 1997;24:289–298. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- de Paula AL, Hallak JE, Maia-de-Oliveira JP, Bressan RA, Machado-de-Sousa JP. Cognition in at-risk mental states for psychosis. Neurosci Biobehav Rev. 2015;57:199–208. doi: 10.1016/j.neubiorev.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenacher S, Rausch F, Ainser F, Mier D, Veckenstedt R, Schirmbeck F, Lewien A, Englisch S, Andreou C, Moritz S, Meyer-Lindenberg A, Kirsch P, Zink M. Investigation of metamemory functioning in the at-risk mental state for psychosis. Psychol Med. 2015;45:3329–3340. doi: 10.1017/S0033291715001373. [DOI] [PubMed] [Google Scholar]
- Falkenberg I, Chaddock C, Murray RM, McDonald C, Modinos G, Bramon E, Walshe M, Broome M, McGuire P, Allen P. Failure to deactivate medial prefrontal cortex in people at high risk for psychosis. Eur Psychiatry. 2015;30:633–640. doi: 10.1016/j.eurpsy.2015.03.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Francis MM, Hummer TA, Vohs JL, Yung MG, Liffick E, Mehdiyoun NF, Radnovich AJ, McDonald BC, Saykin AJ, Breier A. Functional neuroanatomical correlates of episodic memory impairment in early phase psychosis. Brain Imaging Behav. 2016;10:1–11. doi: 10.1007/s11682-015-9357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Brett C, Tabraham P, Johns L, Valmaggia L, Broome M, Woolley J, Bramon E, Howes O, Byrne M, McGuire P. Spatial working memory ability in individuals at ultra high risk for psychosis. J Psychiatr Res. 2014;50:100–105. doi: 10.1016/j.jpsychires.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, Xiao C, Zhao J. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep. 2015;5:17275. doi: 10.1038/srep17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J Clin Exp Neuropsychol. 2002;24:765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CL, Xiang YT, Wang ZL, Everall I, Tang Y, Yang C, Xu MZ, Correll CU, Jia FJ. Cognitive functioning in individuals at ultra-high risk for psychosis, first-degree relatives of patients with psychosis and patients with first-episode schizophrenia. Schizophr Res. 2016;174:71–76. doi: 10.1016/j.schres.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Jung W, Lee SH. Memory deficit in patients with schizophrenia and posttraumatic stress disorder: relational vs item-specific memory. Neuropsychiatr Dis Treat. 2016;12:1157–1166. doi: 10.2147/NDT.S104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lepage M, Bodnar M, Bowie CR. Neurocognition: clinical and functional outcomes in schizophrenia. Can J Psychiatry. 2014;59:5–12. doi: 10.1177/070674371405900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, Solomon M, Carter CS. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72:226–234. doi: 10.1001/jamapsychiatry.2014.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Libby LA, Yonelinas AP, Ranganath C, Ragland JD. Recollection and familiarity in schizophrenia: a quantitative review. Biol Psychiatry. 2013;73:944–950. doi: 10.1016/j.biopsych.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF. The symptoms of chronic schizophrenia A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- Trauelsen AM, Gumley A, Jansen JE, Pedersen MB, Nielsen HG, Trier CH, Haahr UH, Simonsen E. Metacognition in first-episode psychosis and its association with positive and negative syptom profiles. Psychiatry Res. 2016;30:14–23. doi: 10.1016/j.psychres.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Cotter J, Yung AR, Carney R, Drake RJ. Metacognitive beliefs in the at-risk mental state: A systematic review and meta-analysis. Behave Res Ther. 2016;8:25–31. doi: 10.1016/j.brat.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27:563–570. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Dietzek M, Schonfeld N, Lorenz C, Gussew A, Reichenbach JR, Sauer H, Gaser C, Smesny S. Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia, and healthy controls: a VBM study. Schizophr Res. 2015;161:169–176. doi: 10.1016/j.schres.2014.10.041. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Lesh TAJ, Yoon AJ, Westphal N, Hutchison Ragland JD, Solomon M, Minzenberg M, Carter CS. Impaired context processing as a potential marker of psychosis risk state. Psychiatry Res. 2014;221:13–20. doi: 10.1016/j.pscychresns.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JR, Gorham DR. The brief psychiatric rating scale. Journal of Operational Psychiatry. 1980;11:48–64. [Google Scholar]
- Raaijmakers JGW, Shiffrin RM. Models for recall and recognition. Annu Rev Psychol. 1992;43:205–234. doi: 10.1146/annurev.ps.43.020192.001225. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Ranganath C, Barch DM, Gold JM, Haley B, MacDonald AW, 3rd, Silverstein SM, Strauss ME, Yonelinas AP, Carter CS. Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophr Bull. 2012a;38:114–124. doi: 10.1093/schbul/sbr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Blumenfeld RS, Ramsay IS, Yonelinas A, Yoon J, Solomon M, Carter CS, Ranganath C. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. Neuroimage. 2012b;59:1719–1726. doi: 10.1016/j.neuroimage.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Ranganath C, Harms MP, Barch DM, Gold JM, Layher E, Lesh TA, MacDonald AW, 3rd, Niendam TA, Phillips J, Silverstein SM, Yonelinas AP, Carter CS. Functional and Neuroanatomic Specificity of Episodic Memory Dysfunction in Schizophrenia: A Functional Magnetic Resonance Imaging Study of the Relational and Item-Specific Encoding Task. JAMA Psychiatry. 2015;72:909–916. doi: 10.1001/jamapsychiatry.2015.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- van Erp TG, Lesh TA, Knowlton BJ, Bearden CE, Hardt M, Karlsgodt KH, Shirinyan D, Rao V, Green MF, Subotnik KL, Nuechterlein K, Cannon TD. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100:181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Williams LE, Must A, Avery S, Woolard A, Woodward ND, Cohen NJ, Heckers S. Eye-movement behavior reveals relational memory impairment in schizophrenia. Biol Psychiatry. 2010;68:617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.