Abstract
This study is aimed at assessing the biobleaching activity of fungal xylanase on paper pulp isolated from Tirumala forest, Eastern Ghats of India. Of the 98 fungal isolates obtained after initial screening, eight isolates were selected and one potential strain was further cultivated under submerged fermentation for production of xylanase. The biobleaching efficiency on waste paper pulp and paper industry effluent was tested with crude enzyme. Xylanolytic activity by the chosen organism in submerged fermentation reached the maximum (981.1 U ml−1) on the 5th day of incubation. Molecular characterisation of the isolate led to its identification as Trichoderma asperellum which exhibited the production of enzyme even at alkaline pH of the culture medium. Xylanase pretreatment of paper pulp had shown reduction in the Kappa number by 4.2 points and increased brightness by 4.0 points. FTIR and SEM studies revealed loosening of pulp fibres after enzyme treatment. In conclusion, xylanase of Trichoderma asperellum was effective as a pulp biobleaching agent and the process is economical as well as eco-friendly.
Keywords: Biobleaching, Deinking, Fermentation, Paper/pulp industry, Trichoderma asperellum, Xylanase
Introduction
Due to the expanded usage of enzymes industrially throughout the world, demand for microbial enzymes has increased replacing the traditional plant and animal enzymes. The most significant enzymes are hydrolases, which catalyse the hydrolysis of larger compounds into simple substrates and highly effective in biorefinery processes because of efficient surface binding, low production cost and high stability. Enzyme-catalysed reactions rarely form wasteful side products, generally pose no threat to the environment and can mediate reactions under moderate conditions of temperature, pH, and pressure. Microbial enzymes, of late, play a vital role in various biotechnological applications and in particular xylanases while cellulases remain dominant among them.
Total annual requirement of paper and paper products in India is estimated to be around 5 MT. In view of this high demand, paper and pulp industries depend on wood pulp, paper waste and on paper recycling (Sadhasivam et al. 2010; Pathak et al. 2011). Deinking, an important step in the recycling process, involves the dislodgement of ink particles from fibre surface and removal of the detached ink particles by flotation, washing, etc. It is difficult to remove ink or toners in recycling and reuse of waste paper. In addition, to obtain high-quality paper, embedded lignin that causes the dark colour of the pulp should be eliminated. Bleaching is generally used to remove this lignin present in the pulp with chlorine-based chemical agents such as Cl2, ClO2, hypochlorite and NaOCl after cooking (Tezel et al. 2001). Chloro-organic derivatives, produced in chemical bleaching dumped into the surrounding water bodies showed cytotoxic and cytomutagenic effects on various living organisms (Walden and Howard 1997). In order to overcome these adverse environmental impacts and economic concerns associated with conventional methods in recent years, paper makers have evinced interest to find ecofriendly processes such as biodeinking (use of enzymes for deinking) (Jeffries et al. 1994; Bajpai and Bajpai 1998; Vyas and Lachke 2003; Pala et al. 2004; Bajpai 2010a, b; Pathak et al. 2010, 2011; Maity et al. 2012; Singh et al. 2012). Besides, huge volumes of effluent generated from paper mills after pulp washing are rich in COD, BOD, colour, pH, etc. Although there are many existing biological and chemical treatment processes for paper and board mill effluents such as aerobic, anaerobic, fungal, ozonation, electrochemical, photocatalysis, coagulation–flocculation treatments (Belmonte et al. 2006; Buzzini and Pires 2007), they are not effective enough to meet the increasingly stringent discharge requirements of paper/pulp industries (Thompson et al. 2001). So there is need for a single and combined treatment process in laboratory scale for effective treatment of biobleaching and effluents discharged from paper industry (Sadhasivam et al. 2009). Cellulases and xylanases are the predominant enzymes for several biotechnological applications in the paper industry due to their high specificity and cost-effectiveness (Bhat 2000). Besides, laccase has also been the most actively investigated enzyme for biobleaching of kraft pulp (Li et al. 1999).
In view of the above, the present study was aimed at production and evaluation of an enzyme treatment produced by a potential fungus Trichoderma asperellum isolated from Seshachala forest soils of Tirumala, Eastern Ghats, AP, India. To our knowledge, this is the first report on biobleaching ability of crude xylanases by Trichoderma asperellum and its bleaching ability on wastepaper pulp and in treatment of paper industry effluent.
Materials and methods
Isolation of microorganisms
The organism used in the present study was isolated from the soil samples of Tirumala forest with huge amount of decaying forest litter. This strain was identified by morphological and molecular characterisation and deposited in our laboratory fungal collection. Stock cultures were maintained at 30 °C on slants of potato dextrose agar (PDA) medium and stored at 4 °C.
Identification and phylogenetic analysis
The phylogeny of the test fungal strain was identified by ITS (Internal Transcribed Spacer) gene sequencing. Genomic DNA of fungal strain was extracted from mycelia using the GENEI Mini Kit (Bangalore, India) according to the manufacturer’s instructions, and PCR was performed. The ITS1-5.8S-ITS2 region was amplified with the primer pair ITS-4(5_-TCCTCCGCTTATTGATATGC-3_) and ITS-6 (5_-GAAGGTGAAGTCGTAACAAGG-3_). DNA sequencing was performed using an ABI Prism 3130x1 Genetic Analyzer System (Applied Biosystems, USA). The complete sequence of the amplified ITS region was compared with those of the GenBank database using BLAST, while multiple alignments were conducted using CLUSTAL W.
Screening of fungal culture for xylanase, cellulase and laccase production
The fungal isolate was tested for xylanase production on xylan-agar medium with 1% w/v of birch wood xylan. Four-day-old fungal culture was taken and 5 mm disc from these was placed on the plates and incubated at 30 °C. After 6 days of incubation period, the plates were flooded with congo red solution for 15 min followed by destaining with 1 M NaCl. Formation of clear zones against the red background around the fungal colonies was scored as positive reaction.
Screening of isolated fungal strain for cellulase activity was repeated with 1% CMC as substrate in place of 1% xylan. Zone of clearance around the fungal colony indicates positive result. The test fungus was also examined for its ability to produce laccase by laccase plate assay, in which the fungal strain was grown on guaiacol-agar medium with 0.02% w/v guaiacol. 5 mm disc of 4-day-old culture of the test fungus was inoculated in this medium, and the plates were incubated at 30 °C and checked for the appearance of brown colour in the medium.
Xylanase production
Fermentation for xylanase production was performed by growing T. asperellum on MYG (Malt extract, Yeast extract and Glucose) broth containing malt extract; 0.5 g L−1, yeast extract; 0.25 g L−1, glucose; 1.0 g L−1, and 1% birchwood xylan and incubated at 30 °C for 6 days. After incubating the conical flasks for 6 days at 30 °C the content of each flask was filtered through Whatmann filter paper no. 1. The supernatant solution was stored at 4 °C for subsequent use as crude enzyme preparations. Duplicates were used in all the experiments.
Enzyme assay
The activity of xylanase was determined by the method of Bailey et al. (1992) by estimating the amount of reducing sugar released in the filtrate. Enzyme activity was expressed as 1 μmol of xylose released per min per millilitre.
Biomass determination
Mycelial mat from culture filtrate was filtered through pre-weighed Whatmann no. 1 filter paper and was dried at 70 °C in an oven for overnight. Difference between the weight of the filter paper having mycelial mat and weight of only filter paper represents biomass of fungal mat. Fungal growth was expressed as mg flask−1.
Estimation of total protein
The amount of protein content in culture broth was determined according to Lowry et al. (1951).
Determination of reducing sugars
Reducing sugars in the culture filtrate were determined by DNS method (Miller 1959).
Effect of xylanase on waste paper pulp
Paper pulp was prepared by dispensing finely ground oven-dried waste paper in distilled water. Enzyme-treated pulp was prepared by taking 5% (W/V) of pulp and treated with an enzyme dose of 20–100 U ml−1 per gramme of pulp for a time period of 1 h at 50 °C with gentle shaking. The treated pulp was filtered and the residual pulp was washed thoroughly with tap water and pressed flat between two stainless steel plates to form hard sheets which were dried at 50 °C for 3 h. A control was maintained without the enzyme under the same conditions with 1 ml of distilled water. The efficiency of treatment process was measured in terms of reduction in kappa number (TAPPI 1990). The brightness of the pulp was determined according to the method of Jordan and Popson (1994). The reducing sugar in the pulp-free filtrate was measured by the DNS method and its absorption maxima (λ max) was read at 237 and 465 nm for measuring chromophores and hydrophobic compounds (Manimaran and Vatsala 2007).
Characterisation of pulp
The control and enzyme-treated pulp were subjected to potassium bromide pelleting and subjected to FT-IR analysis using FTIR—Perkinelmer, USA at room temperature. The peaks obtained after analysis were compared with standard FT-IR spectra.
Scanning electron microscopy
The fractured surfaces of xylanase-treated and -untreated samples were observed with FE-SEM. The samples were dehydrated in an ascending grade of acetone, critical point dried (Critical point dryer, Polaron), and mounted on sample stubs. They were sputter coated with colloidal gold and observed under a field emission scanning electron microscope (JSM-6701F, JEOL, JAPAN) at an operating voltage of 3 kV. Images were digitally acquired using a CCD camera attached to the microscope. The magnification for observing the changes on fibre surfaces was at 100×.
Effluent characterisation
The effluent, before and after enzymatic treatment, was characterised before disposal by following standard method. The parameters like colour, pH, TSS, TDS, BOD, COD, residual chlorine content and reducing sugar were estimated.
Results
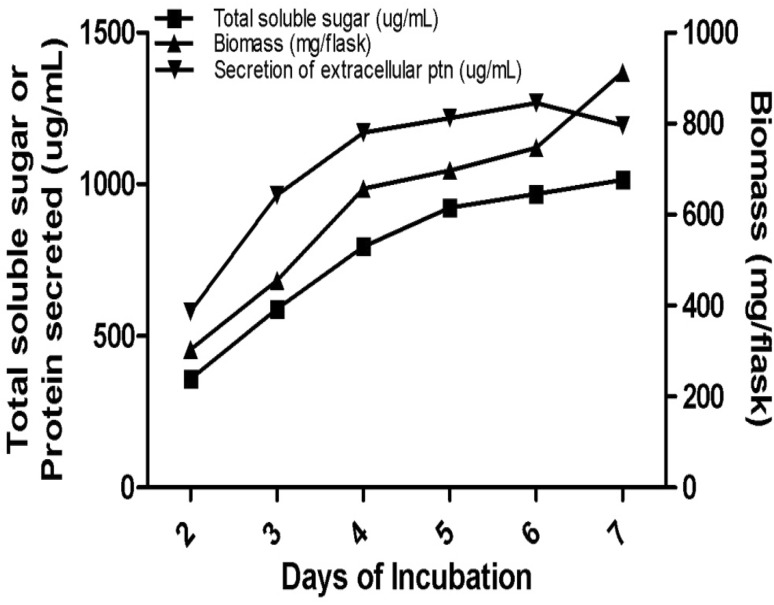
The study carried out on screening for lignocellulosic enzymes resulted in the isolation of 98 isolates with varying ability to produce xylanase and cellulase enzymes and also laccase by 8 isolates. Isolate 75, designated as G2, was identified and used for xylanase production as it was the highest producer of xylanase with little of cellulase and laccase activities also. After initial plate screening, the selected fungal strain, when grown in liquid medium under shaking conditions, exhibited xylanase activity (Fig. 1). Enzyme production touched peak (981.1 U ml−1), on 5th day of incubation and declined later (Table 1). There was an increase in pH of culture broth that shifted to alkalinity during the course of incubation time from initial set pH (Table 1). It is interesting to note that the production of xylanase continued even when the pH of culture broth turned to alkaline also.
Fig. 1.
Production of extracellular protein, release of soluble sugar, and biomass of Trichoderma asperellum grown on MYG medium
Table 1.
Activity of xylanase in submerged culture at different time intervals of incubation
| Days of incubation | Xylanase activity (U mL−1) | Change in pH of the medium |
|---|---|---|
| 2 | 161.6 | 3.1 |
| 3 | 529.2 | 4.3 |
| 4 | 637.7 | 5.2 |
| 5 | 981.1 | 6.1 |
| 6 | 680.7 | 7.3 |
| 7 | 484.8 | 7.9 |
Growth of fungal strain was initially slow for 4 days and increased gradually from 5th day of incubation and reached maximum biomass production (1.369 g flask−1) on 7th day of incubation. The secretion of extracellular protein increased with increase in incubation time and reached maximum on 6th day of incubation (845 μg ml−1) and then declined, whereas the maximum soluble sugar (1014 μg ml−1) was produced on the 7th day of incubation which clearly indicated that the organism grew well in MYG liquid medium used in this study under shaking conditions as reflected by large biomass, high protein secretion and release of total soluble sugar.
Molecular identification of the selected fungal strain was determined by sequencing of the internal transcribed spacer (ITS) region. Based on phylogenetic analyses and nucleotide homology the isolated strain was identified as T. asperellum and sequence data have been deposited in the GenBank databases under accession number KM386096.
Effect of xylanase on waste paper pulp
Paper pulp was pretreated with crude xylanase of T. asperellum at variable dose for a time course of 1 h at 50 °C and pH 5.0. After biobleaching, enzyme-treated and -untreated pulp samples were analysed for Kappa number and brightness. With enzyme treatment, reduction in kappa number and increase in brightness were observed indicating the release of lignin. Further, with different enzyme doses, there was decrease in kappa number and brightness improvement compared to untreated sample (Table 2) confirming better efficiency of the enzyme treatment. During the study maximum removal of chromophores and hydrophobic compounds were observed in xylanase-treated pulp samples (Table 2). The breakdown of pulp fibers were observed upon treatment with T. asperellum xylanase which assist in discharge of more reducing sugars. In present study, the treated samples had shown maximum free reducing sugars released from pulp when compared to untreated sample. This study also revealed that biobleaching efficiency of T. asperellum xylanase was found to be effective, but still the optimum enzyme dose remains to be established.
Table 2.
Comparison of kappa number and brightness (different parameters) of paper pulp with control before and after treatment with variable doses of crude xylanase (20–100 U g−1)
| Parameter | Kappa number | Brightness (ISO units) | Chromophoric compounds (λ 237) | Hydrophobic compounds (λ 465) | Reducing sugar (mg g−1) |
|---|---|---|---|---|---|
| Untreated | 23.1 | 39.2 | 0.189 | 0.091 | 1.23 |
| Xylanase (20 U g−1) | 21.8 | 40.6 | 0.708 | 0.209 | 2.45 |
| Xylanase (40 U g−1) | 20.2 | 41.5 | 1.09 | 0.984 | 6.89 |
| Xylanase (80 U g−1) | 19.4 | 42.8 | 2.43 | 1.566 | 9.67 |
| Xylanase (100 U g−1) | 18.9 | 43.2 | 2.56 | 1.842 | 10.34 |
Pulp characterisation after enzyme treatment
Crude xylanase at a dose of 100 U g−1 treated pulp samples was analysed by SEM and FTIR. SEM analysis revealed noticeable changes in fibre disintegration on paper surface in enzyme-treated samples were observed when compared to untreated samples. After enzymatic treatment, the fibre network in paper was broken.
FT-IR spectrum of enzyme-treated and -untreated paper pulp was compared. As the functional groups levels were modified in treated and untreated samples, there were changes in the peaks also (Fig. 2A, B). The stretches in FT-IR spectrum are listed in Table 3.
Fig. 2.
FTIR spectra of untreated (A) and enzyme-treated (B) paper pulp at regions 4000–400 cm
Table 3.
FTIR spectra showing functional group stretches of paper pulp before and after enzyme treatment obtained at different wave numbers (cm−1)
| Untreated | Enzyme-treated | ||
|---|---|---|---|
| Absorption ranges Ranges (cm−1) |
Type of vibration | Absorption ranges Ranges (cm−1) |
Type of vibration |
| 3357.00 | –OH stretching of hydrogen-bonding | 3357.00 | –OH stretching of hydrogen-bonding |
| 2925.80 | =C–H stretch | 2925 | – |
| 2359.91 | OH asymmetrical stretching vibration in carboxylic acids | 2360.63 | OH asymmetrical stretching vibration in carboxylic acids |
| 2138 | – | 2138 | CH asymmetrical stretching vibration in CH3, CH2 |
| 1636.37 | C=O stretch | 1636.92 | C=O Stretch |
| 1312.60 | – | 1312.60 | C=O stretch vibration in syringyl ring |
| 1048.06 | C–H | 1048 | – |
| 1183.74 | – | 1183.74 | –COO– (carboxylate ion) groups |
| 668.2 | N–O stretch | 670.99 | N–O stretch |
| 647.76 | – | 647.76 | C–O |
| 616 | – | 616 | C=O Stretch |
Characterisation of effluent
Effluents discharged from paper industries contain high concentration of polluting materials. An effective treatment is necessary prior to discharge of the effluent (Robertson 1996). The present study made a focus on treatment of effluent by biological means for effective removal of colour, COD and BOD. The physico-chemical properties of the treated and untreated effluents are shown in Table 4.
Table 4.
Comparison of characteristics of the effluent load with BIS standards before and after enzyme treatment
| Parameter | Untreated | Enzyme treated | BIS limits |
|---|---|---|---|
| Colour | Light brown | Pale white | Absent |
| pH | 6.6 | 7.2 | 5.5–9.0 |
| BOD (mg L−1) | 718 | 73 | 115 |
| COD (mg L−1) | 4426 | 769 | 1490 |
| TSS (mg L−1) | 2746 | 765 | 1340 |
| TDS (mg L−1) | 2138 | 998 | 1630 |
| Residual chlorine (mg L−1) | 59.1 | 13.12 | Nil |
The results revealed that after treatment, colour of the effluent turned from light brown to pale white, reduction in total suspended solids and total dissolved solids from 2746 to 765 and 2138 to 998 mg L−1, respectively. There was reduction in BOD, COD and residual chlorine content of effluent also. Treated effluent analysis revealed that BOD was reduced from 718 to 73 mg L−1, COD from 4426 to 769 mg L−1 and residual chlorine content from 59.1 to 13.12, respectively (Table 4) showing that the results are within irrigational standards as per BIS limits.
Discussion
Lignocellulolytic enzymes are now in focus at different industrial levels throughout the world. To meet the increasing demand, potential producers of extracellular enzymes are essential. Although there are diverse groups of microorganisms as enzyme producers, fungi are considered to be the major producers of lignocellulolytic enzymes and in particular largely terrestrial isolates of Aspergillus and Trichoderma species on industrial scale (Fengxia et al. 2008).
A potential isolate T. asperellum, isolated from forest soils of Eastern Ghats and after screening was identified as the highest xylanase producer with minimal cellulase and laccase activities. Isolation of fungi from other forest sources exhibiting hydrolytic enzyme activities were studied by some other researchers. A high percentage (93%) of the mangrove fungal (MF) isolates exhibited xylanolytic activities (Vinodkumar et al. 2014). In comparison, Bucher et al. (2004) reported that 84% of the 45 mangrove fungi that they tested were xylanolytic. Moreover, 82% (37 of 45) of their test fungi exhibited both cellulolytic and xylanolytic activities. Likewise, 526 isolates were obtained from the different degrading wood chips among which 4 were selected because of their relatively high potential for production of xylanase (Adesina and Onilude 2013). According to Singh et al. (2013), among 14 strains of white rot basidiomycetous fungi, 50% showed maximum xylanase activity on wheat bran agar medium. The fungal strain A. niger F7, isolated from soil was selected among 11 strains of fungi, due to higher production of xylanase (Sharma et al. 2012). All these studies support high incidence of fungal isolates producing hydrolytic enzymes from forest litter, decaying wood chips and other iomasses.
After 5 days of growth in submerged fermentation, T. asperellum had yielded 981 U ml−1. The synthesis of xylan-degrading enzymes by xylanolytic fungi cultured with xylan as carbon source has been reported by many researchers. T. harzianum strain P49P11 produced 8000 IU g−1 (Delabona et al. 2012) while Trichoderma inhamatum yielded 244.02 U ml−1 of xylanase when grown with xylan as carbon source (de Oliveira da Silva and Carmona 2008). Similarly, xylanase activity of Aureobasidium pullulans Y-2311- 1 exhibited xylanase activity of 27.28 and 25.54 U ml−1 at a concentration of 1% Birch-wood xylan and oat bran, respectively (Li and Ljungdahl 1994). The synthesis of highest xylanase after incubation period of 7 days was documented by Singh et al. (2013). Xylanase activity was found to be 90–126 U ml−1 after 90 h of induction in Thermomyces lanuginosus IOC-4145 (Damaso et al. 2003), 850 U ml−1 in Thermomyces lanuginosus IOC-4145 (Damaso et al. 2004) and 8000 IU g−1 in T. harzianum strain P49P11 (Delabona et al. 2012). However, some fungi produced high levels of xylanase on the 6th day of incubation and beyond (Jiang et al. 2010) which was attributed to the temporal expression of xylanase in a highly efficient xylan-degrading microorganism in the early to mid-log phase (Emami et al. 2002). Numerous studies have also reported high xylanase production from fungi using corn-derived substrates as the carbon source (Bakir et al. 2001; Li et al. 2006; Jiang et al. 2010). Similarly, time course analyses from other studies showed that, regardless of substrate and fermentation setup, maximal xylanase production by filamentous fungi generally reached after 5 days of incubation (Bakir et al. 2001; Li et al. 2006; Murthy and Naidu 2012). The present study is also in agreement and comparable with other studies.
Application of biotechnology to pulp and paper industry operations is a potentially useful alternative proposition for the future. In the present study also we investigated the bleaching efficiency of a crude xylanase for the recycling of wastepaper pulp as crude culture filtrate to develop an inexpensive process. Reduction in kappa number and increase in brightness in enzyme-treated paper pulp than -untreated pulp were observed in the present study. Several other reports also support xylanase pretreatment with varying activities in pulp biotechnology (Beg et al. 2000). The ability of T. harzianum WL1 crude laccase in the biobleaching of wastepaper pulp and treatment of paper industry effluent investigation revealed kappa number reduction of 18.4 and brightness of 43.9 ISO units (Sadhasivam et al. 2010). The maximum efficiency of the enzyme in biobleaching was obtained after 120 min of incubation which caused reduction in mean Kappa number from 18.7 to 18.2 (Nagar et al. 2013). Several other researchers had also reported increase in brightness and decline in Kappa number at different magnitudes after the xylanase pretreatment (Sadhasivam et al. 2010; Garg et al. 1998; Das et al. 2013; Sridevi et al. 2016). The increase in brightness after enzyme pretreatment might be due two reasons. First, the xylanase would act on the xylan precipitated on the lignin (Viikari et al. 1986). This xylan was precipitated due to lowering of the pH at the end of cooking stage. Its removal by the action of xylanase would enhance the accessibility of bleaching chemicals to the pulp fibres. The second reason was based on the ability of lignin to form complexes with polysaccharide such as xylan and some of the bonds being alkali-resistant might not have been hydrolyzed during the kraft process (Buchert et al. 1992). The xylanase acted by cleaving the remaining bonds between lignin and xylan, opening the structure of the cellulose pulp and leading to the fragmentation of xylan and subsequent extraction of the fragments (Paice et al. 1978). Laccase from P. cinnabarinus showed a substantial increase in pulp brightness to 77–79 and 53–56% ISO units after 12 h of bleaching over control in low and high kappa number pulps, respectively.
FTIR spectra of treated and untreated pulp were compared in this study. Similar bands observed in this study were also reported by others (Fantahun et al. 2013; Dhiman et al. 2014; Vinodkumar et al. 2014). Spectra obtained after enzymatic pretreatment in this study had shown as the stretches around the region 3500–3000 cm−1 depict –OH stretching of hydrogen bonding. The increase in their relative intensity after enzymatic treatments is attributed to the increase in cellulosic content of the pulp. The bands at 2350–2360 cm−1 are assigned to –OH stretching of carboxylic acid with more intensity in enzymatic treated pulp. The degradation of aliphatic side chains was observed with intensity of bands at 2200–2100 cm−1 which are assigned to CH asymmetrical stretching vibration in CH3, CH2, and CH in consecutively pretreated pulp. The increased relative intensity around 1617–1652 cm−1 in pretreated pulp is attributed due to the release of free carbonyl groups (C=O) due to the action of the enzyme on lignin’s aromatic ring. New bands that appeared at 1312, 1183 and 1048 cm−1 in pulp pretreated with our fungal xylanase are assigned to CH stretching vibration in CH3, CH2 1and C=O stretch vibration in syringyl ring. Appearance of 670 and 647 and 616 cm−1 bands only indicates that there is change in lignin structure.
The pulp and paper industries generate large volumes of effluent and cause environmental pollution problems. Paper industries employ treatments to reduce effluent load with respect to cleaner production and to meet government standards and environmental impacts, while improving profitability at the same time (Lindsay and Smith 1995). Biological treatment was proposed to be an inexpensive and effective to reduce the environmental problems and bleaching technologies. An effective treatment system is required which can degrade the polluting materials prior to discharge of the effluent (Robertson 1996). Hence, this study is also focused on the effluent treatment process. The effluent that is let out must be treated properly before mixing with water courses. The anaerobic treatment of paper industry effluent in an activated sludge plant resulted in a BOD reduction of 80–85% (Habets et al. 2002). Combined sequential, biological (T. versicolor) and photocatalytic treatments showed a effective reduction in COD and colour at 82 and 80%, respectively, corresponding to the laccase and manganese peroxidase (MnP) activities of 345 and 78 U l−1 (Pedroza et al. 2007). The use of Paecilomyces sp. resulted in the reduction of colour (95%), lignin (86%), COD (88%) and phenol (63%), whereas M. luteum showed removal of colour (76%), lignin (69%), COD (75%) and phenol (93%) on day 3 (Singh and Thakur 2006). Xylanase treatment had led to the reduction of effluent load (Bajpai 2010a, b; Sadhasivam et al. 2010; Yin et al. 2011; Das et al. 2013). In comparison with the above all, this treatment can be implemented to effectively reduce the organic/inorganic materials and the overall impact of xylanase treatment has been shown to be very effective at the laboratory scale. As this biobleaching process is cost-effective and environmentally beneficial, the optimisation and use of this fungal enzyme could be recommended for scaling up to meet out the industrial needs.
The present investigation was carried out on enzymatic pretreatment of paper pulp with xylanase produced by T. asperellum. Compared to untreated, pulp enzyme treatment showed reduced kappa number, higher brightness and removed chromophores and hydrophobic compounds. FTIR had shown different stretches confirming an increase in relative intensity of some compounds. SEM observations also showed that enzyme treatments may expose cellulose fibres to the surface. The above characteristics of this enzyme make it suitable for use and the present isolate as a promising organism for the production of xylanase. Optimisation of enzyme production together with utilisation of cheap substrates might lead to low-cost enzyme production that can be better useful as a biobleaching agent on waste papers and reduces effluent load so as to minimise the risk of environmental pollution.
Acknowledgements
The first author is thankful to University Grant Commission for the Post Doctoral Fellowship (PDFWM-2011-12-Ge-AND-7691) and financial assistance.
Compliance with ethical standards
Conflict of interest
Authors declare that there are no conflicts of interests among the authors about the publication of the manuscript.
Contributor Information
A. Sridevi, Email: gollasridevi@gmail.com
P. Suvarnalatha Devi, Email: drsuvarna.pallipati@gmail.com
References
- Adesina FC, Onilude AA. Isolation, identification and screening of xylanase and glucanase-producing microfungi from degrading wood in Nigeria. Afr J Agric Res. 2013;8:4414–4421. doi: 10.5897/AJAR2013.6993. [DOI] [Google Scholar]
- Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- Bajpai P. Environmentally friendly for paper and pulp production. New jersey: Wiley; 2010. [Google Scholar]
- Bajpai PK. Solving the problems of recycled fiber processing with enzymes. BioResources. 2010;5:1311–1325. [Google Scholar]
- Bajpai P, Bajpai PK. Deinking with enzymes: a review. Tappi J. 1998;81:111–117. [Google Scholar]
- Bakir U, Yavascaoglu S, Guvenc F, Ersayin A. An endo-b 1,4-xylanase from Rhizopus oryzae: production, partial purification and biochemical characterization. Enzyme Microbiol Technol. 2001;29:328–334. doi: 10.1016/S0141-0229(01)00379-9. [DOI] [Google Scholar]
- Beg QK, Bhushan B, Kapoor M, Hoondal GS (2000) Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in biobleaching of eucalytpus kraft-pulp. Enzyme Microb Technol 27:459–466 [DOI] [PubMed]
- Belmonte M, Xavier C, Decap J, Martinez M, Sierra-Alvarez R, Vidal G. Improved aerobic biodegradation of abietic acid in ECF bleached kraft mill effluent due to biomass adaptation. J Hazard Mater. 2006;135:256–263. doi: 10.1016/j.jhazmat.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Bhat MK. Biotechnol Adv. 2000;18:355–383. doi: 10.1016/S0734-9750(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Bucher VVC, Hyde KD, Pointing SB, Reddy CA. Production of wood decay enzymes, mass loss and lignin solubilization in wood by marine ascomycetes and their anamorphs. Fungal Divers. 2004;15:1–14. [Google Scholar]
- Buchert J, Tenkanen M, Kantelinen A, Viikari L. Application of xylanases in the pulp and paper industry. Biores Technol. 1992;50:65–72. doi: 10.1016/0960-8524(94)90222-4. [DOI] [Google Scholar]
- Buzzini AP, Pires EC. Evaluation of a up flow anaerobic sludge blanket reactor with partial recirculation of effluent used to treat wastewaters from pulp and paper plants. Biores Technol. 2007;98:1838–1848. doi: 10.1016/j.biortech.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Damaso MC, Almeida MS, Kurtenbach E, Martins OB, Pereira N, Andrade CM, Albano RM. Optimized expression of a thermostable xylanase from Thermomyces lanuginosus in Pichia pastoris. Appl Environ Microbiol. 2003;69:6064–6072. doi: 10.1128/AEM.69.10.6064-6072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaso MC, de Castro AM, Castro RM, Andrade CM, Pereira N. Application of xylanase from Thermomyces lanuginosus IOC-4145 for enzymatic hydrolysis of corncob and sugarcane bagasse. Appl Biochem Biotechnol. 2004;113–116:1003–1012. doi: 10.1385/ABAB:115:1-3:1003. [DOI] [PubMed] [Google Scholar]
- Das A, Paul T, Halder SK, Jana A, Maity C, Mohapatra PKD. Production of cellulolytic enzymes by Aspergillus fumigatus ABK9 in wheat bran-rice straw mixed substrate and use of cocktail enzymes for deinking of waste office paper pulp. Biores Technol. 2013;128:290–296. doi: 10.1016/j.biortech.2012.10.080. [DOI] [PubMed] [Google Scholar]
- Delabona PS, Farinas CS, da Silva MR, Azzoni SF, Pradella JG. Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Biores Technol. 2012;107:517–521. doi: 10.1016/j.biortech.2011.12.048. [DOI] [PubMed] [Google Scholar]
- de Oliveira da Silva LA, Carmona EC (2008) Production and characterization of cellulase-free xylanase from Trichoderma inhamatum. Appl Biochem Biotechnol 150(2):117–125 [DOI] [PubMed]
- Dhiman SS, Gaurav G, Sharma J, Kalia VC, Yun CK, Lee JK. Reduction in acute ecotoxicity of paper mill effluent by sequential application of xylanase and laccase. PLoS One. 2014 doi: 10.1371/journal.pone.0102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami K, Nagy T, Fontes CMGA, Ferreira LMA, Gilbert HJ. Evidence for temporal regulation of the two Pseudomonas cellulosa xylanases belonging to glycoside hydrolase family. J Bacteriol. 2002;184:4124–4133. doi: 10.1128/JB.184.15.4124-4133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantahun W, Antar PV, Naveen G, Sharma P. Biobleaching of mixed wood kraft pulp with alkalophilic bacterial xylanase, mannanase and laccase-mediator system. J Microbiol Biotechnol Res. 2013;3:32–41. [Google Scholar]
- Fengxia L, Mei L, Zhaoxin L, Xiaomei B, Haizhen Z. Purification and characterization of xylanase from Aspergillus ficuum AF-98. Bioresour Technol. 2008;99:5938–5941. doi: 10.1016/j.biortech.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Garg AP, Roberts JC, McCarthy AJ. Bleach boosting effect of cellulase-free xylanase of Streptomyces thermoviolaceus and its comparison with two commercial enzyme preparations on birchwood kraft pulp. Enzyme Microbial Technol. 1998;22:594–598. doi: 10.1016/S0141-0229(97)00250-0. [DOI] [Google Scholar]
- Habets L, Zumbragel M, Tielbaard M (2002) The value of anaerobic purification for pulp and paper mill effluents. TAPPI Environ 6–13
- Jeffries TW, Klungness JH, Marguerite S, Cropsey KR. Comparison of enzyme-enhanced with conventional deinking of xerographic and laser printed paper. Tappi J. 1994;77:173–179. [Google Scholar]
- Jiang Z, Cong Q, Yan Q, Kumar N, Du X. Characterisation of a thermostable xylanase from Chaetomium sp. and its application in Chinese steamed bread. Food Chem. 2010;20:457–462. doi: 10.1016/j.foodchem.2009.10.038. [DOI] [Google Scholar]
- Jordan BD, Popson SJ. Measuring the concentration of residual ink in recycled news print. J Pulp Pap Sci. 1994;20:161–167. [Google Scholar]
- Li XL, Ljungdahl LG. Cloning, sequencing and regulation of a xylanase gene from the fungus Aureobasidium pullulans Y-2311-1. J Appl Environ Microbiol. 1994;60:3160. doi: 10.1128/aem.60.9.3160-3166.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Xu F, Eriksson KEL. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol. 1999;65:2654–2660. doi: 10.1128/aem.65.6.2654-2660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Tian H, Cheng Y, Jiang Z, Yang S. Purification and characterization of a thermostable cellulase-free xylanase from the newly isolated Paecilomyces thermophila. Enzyme Microbiol Technol. 2006;38:780–787. doi: 10.1016/j.enzmictec.2005.08.007. [DOI] [Google Scholar]
- Lindsay KM, Smith DW. Factors influencing pulp and mill effluent treatment in Alberta. J Environ Manag. 1995;44:11–27. doi: 10.1006/jema.1995.0027. [DOI] [Google Scholar]
- Lowry OM, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275 [PubMed]
- Maity C, Ghosh K, Halder SK, Jana A, Adak A, Mohapatra PKD, Pati BR, Mondal KC. Xylanase isozymes from the newly isolated Bacillus sp. CKBx1D and optimization of its deinking potentiality. Appl Biochem Biotechnol. 2012;167:1208–1219. doi: 10.1007/s12010-012-9556-4. [DOI] [PubMed] [Google Scholar]
- Manimaran A, Vatsala TM. Biobleaching of banana Wbre pulp using Bacillus subtilis C O1 xylanase produced from wheat bran under solid-state cultivation. J Ind Microbiol Biotechnol. 2007;34:745–749. doi: 10.1007/s10295-007-0248-y. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicilic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–429. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Murthy PS, Naidu MM. Production and application of xylanase from Penicillium sp. utilizing coffee by-products. Food Bioprocess Technol. 2012;5:657–664. doi: 10.1007/s11947-010-0331-7. [DOI] [Google Scholar]
- Nagar S, Jain RK, Thakur VV, Gupta VK (2013) Biobleaching application of cellulase poor and alkali stable xylanase from Bacillus pumilus SV-85S. Biotechnology 3(3):277–285 [DOI] [PMC free article] [PubMed]
- Paice MG, Jurasek L, Carpenter MR, Smillie LB. Production, characterization and partial amino acid sequence of xylanase from Schizophyllumcommune. Appl Environ Microbiol. 1978;36:802–808. doi: 10.1128/aem.36.6.802-808.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala H, Mota M, Gama FM. Enzymaatic versus chemical deinking of non-impact ink printed paper. J Biotechnol. 2004;108:79–89. doi: 10.1016/j.jbiotec.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Pathak P, Bhardwaj NK, Singh AK. Enzymatic deinking of office waste paper: an overview. Tappi J. 2010;22:83–88. [Google Scholar]
- Pathak P, Bhardwaj NK, Singh AK. Optimization of chemical and enzymatic deinking of photocopier waste paper. BioResources. 2011;6:447–463. [Google Scholar]
- Pedroza AM, Mosqueda R, Allonso-Vante N, Rodriguez-Vazquez R. Sequential treatment via Trametes versicolor and UV/TiO2/Rux Sey to reduce contaminants in waste water resulting from the bleaching process during paper production. Chemosphere. 2007;67:793–801. doi: 10.1016/j.chemosphere.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Robertson PKJ. Semiconductor photocatalysis: an environmentally acceptable alternative production technique and effluent treatment process. J Clean Prod. 1996;4:203–212. doi: 10.1016/S0959-6526(96)00044-3. [DOI] [Google Scholar]
- Sadhasivam S, Savitha S, Swaminathan S. Redox-mediated decolorization of recalcitrant textile dyes by Trichoderma harzianum WL1 laccase. World J Microbiol Biotechnol. 2009;25:1733–1741. doi: 10.1007/s11274-009-0069-4. [DOI] [Google Scholar]
- Sadhasivam S, Savitha S, Swaminathan S. Deployment of Trichoderma harzianum WL1 laccase in pulp bleaching and paper industry effluent treatment. J Clean Prod. 2010;18:799–806. doi: 10.1016/j.jclepro.2009.11.014. [DOI] [Google Scholar]
- Sharma N, Kaushal R, Gupta R, Kumar S. A biodegradation study of forest biomass by Aspergillus niger F7: correlation between enzymatic activity, hydrolytic percentage and biodegradation index. Braz J Microbiol. 2012;43:467–475. doi: 10.1590/S1517-83822012000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Thakur IS. Colour removal of anaerobically treated pulp and paper mill effluent by microorganisms in two steps bioreactor. Biores Technol. 2006;97:218–223. doi: 10.1016/j.biortech.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Singh A, Yadav RD, Kaur A, Mahajan R. An ecofriendly cost effective enzymatic methodology for deinking of school waste paper. Biores Technol. 2012;120:322–332. doi: 10.1016/j.biortech.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Singh S, Dutt D, Tyagi CH (2013) Screening of xylanases from indigenously isolated white rot fungal strains for possible application in pulp biobleaching. Open Access Scientific Reports
- Sridevi A, Sandhya A, Ramanjaneyulu G, Narasimha G, Suvarnalatha Devi P. Biocatalytic activity of Aspergillus niger xylanase in paper pulp biobleaching. 3 Biotech. 2016;6:165. doi: 10.1007/s13205-016-0480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPPI . Technical association of the pulp and paper industry, standard T230. Atlanta: TAPPI Press; 1990. [Google Scholar]
- Tezel U, Guven E, Erguer TH, Demirer GN. Sequential (anaerobic/aerobic) biological treatment of Dalaman SEKA pulp and paper industry effluent. Waste Manage. 2001;21:717–724. doi: 10.1016/S0956-053X(01)00013-7. [DOI] [PubMed] [Google Scholar]
- Thompson G, Swain J, Kay M, Froster CF. The treatment of pulp and paper mill effluent: a review. Biores Technol. 2001;77:275–286. doi: 10.1016/S0960-8524(00)00060-2. [DOI] [PubMed] [Google Scholar]
- Viikari L, Ranua M, Kantelinen A, Sundquist J, Linko M (1986) Bleaching with enzymes. In: Proceedings of 3rd International Conference Biotechnology Pulp Paper Industry, pp 67–69
- Vinodkumar N, Estherrani M, Gunaseeli R, Kannan ND (2014) Potential of xylanase from Trichoderma Viride VKF3 in waste paper pulp characteristics modification. International conference on chemical. Environ Biol Sci
- Vyas S, Lachke A. Biodeinking of mixed office waste paper by alkaline active cellulases from alkalotolerant Fusarium sp. Enzyme Microbial Technol. 2003;32:236–245. doi: 10.1016/S0141-0229(02)00273-9. [DOI] [Google Scholar]
- Walden CC, Howard TE. Toxicity of pulp and paper mill effluents. TAPPI. 1997;60:122. [Google Scholar]
- Yin C, Goyal G, Trepow A, Kalka J (2011) Effect of low dose xylanase on pulp in prebeach treatment process. US patent application. 20110108222. Patent