Abstract
Purpose
The purpose of this study was to evaluate the effects of mitochondrial supplementation (MS) on early embryonic development and to assess the safety of MS treatments using induced pluripotent stem cells (iPSCs) as the mitochondrial donor.
Methods
In this study, we evaluated the effect of MS on early embryonic development using induced pluripotent stem cells (iPSCs) as the donor. Mouse zygotes were injected with either mitochondria from iPSCs or a vehicle solution. Several parameters were evaluated, including the rates of blastocyst formation and implantation, the weight of E13.5 embryos and placentas, the distribution of the donor mitochondrial DNA (mtDNA), and the pattern of methylation in the differentially methylated regions (DMRs) of the H19 and Snrpn genes.
Results
We found that neither the rates of blastocyst formation and implantation nor the weights of E13.5 embryos and placentas were significantly different between the MS and control groups. Additionally, the mtDNA from the iPSC donors could be detected in the muscle tissue of four fetuses and all placentas in the MS group. Finally, the methylation patterns of H19 and Snrpn DMRs remained unchanged by MS.
Conclusions
iPSC-derived mtDNA was directly involved in the process of embryonic development after MS. No adverse effects were seen when using iPSCs as a mitochondrial donor, but it remains to be seen whether this method can improve embryonic development, especially in older mice.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0948-9) contains supplementary material, which is available to authorized users.
Keywords: Mitochondrial supplementation, Embryonic development, DNA methylation, iPSCs
Introduction
Mitochondria are cellular organelles that perform oxidative phosphorylation to generate adenosine triphosphate (ATP) in eukaryotic cells. As a result, they play an important role in energy production, calcium homeostasis, cellular signal transduction, and apoptosis [1]. Previous studies have suggested that mitochondrial function declines with age [2], which affects the vitality of oocytes [3] and can eventually result in infertility [4, 5].
Previous studies have indicated that providing mitochondrial supplementation (MS) to oocytes prevents the oocytes from undergoing apoptosis [6] and enhances the embryo development [7]. However, other studies have reported conflicting evidence which found that MS of somatic cell did not rescue blastocyst rate in in vivo-aged mouse oocytes [8]. The recent birth of the first “Augment baby” along with a corresponding report by Oktay et al. [9] have suggested that autologous mitochondrial injection (AMI) can improve clinical results in women who have previously experienced failed IVF cycles, although this study lacked a prospective control group. However, few studies have addressed the safety of MS treatments [7]. And the potential risks such as epigenetic aspects should be further evaluated before MS is applied clinically.
H19, which differentially methylated region (DMR) is paternally methylated, encodes a noncoding ribonucleic acid (RNA) and lies at the end of a cluster of imprinted genes [10]. Snrpn is a maternally imprinted gene, encoding a brain-enriched small nuclear ribonucleoprotein (snRNP)-associated polypeptide SmN. Both genes are extensively studied because of their important biological functions and the characteristic phenotypes associated with the absence of their gene products, such as Silver–Russell syndrome [11], Prader–Willi syndrome, and Angelman syndrome [12].
Induced pluripotent stem cells (iPSCs) have extensive self-renewal capacity and represent an unlimited source of research and therapy material [13]. Indeed, iPSCs can be induced from many types of cells of the patients themselves. Moreover, the mitochondrial function can be recovered [14] and a cell line containing an exclusively wild-type mitochondrial DNA (mtDNA) can be derived from the reprogramming process [15]. However, few studies reported the results of iPSCs as the donor for MS. Therefore, in this study, we evaluated whether iPSCs could be used as a donor for MS by analyzing the early embryonic development and the methylation status of DMRs of the H19 and Snrpn genes after MS treatment.
Materials and methods
All chemicals and media used in this study were purchased from Sigma-Aldrich unless otherwise indicated. All animal protocols were approved by and performed in accordance with the requirements of the Institutional Animal Care and Use Committee at the authors’ university.
Animals
Male B6D2-Tg (CAG/Su9-DsRed2, Acr3-EGFP) RBGS002Osb (no. 03743) mice [16], which express red fluorescent protein (RFP) in their mitochondria and green fluorescent protein (GFP) in their acrosomes, were bred with female C57/6j mice. Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 embryos and used to generate iPSCs with RFP-labeled mitochondria. Zygotes were obtained from BALB/c mice between 8 and 9 weeks of age, and 8- to 10-week-old CD1 mice were used as surrogates. All animals used in this study were purchased from Charles River Inc. (Beijing, China). One week after purchase, superovulartion was induced with 10 IU of pregnant mare serum gonadotropin (PMSG; Ningbo Hormone Products Co.) followed by 10 IU of human chorionic gonadotropin 48 h later (hCG; Ningbo Hormone Products Co.). They were mated with male mice (8 to 10 weeks old). Successfully mated female mice checked by the presence of vaginal plug were sacrificed by CO2 inhalation followed by cervical dislocation for the zygote retrieval.
Preparation of iPSCs
MEFs with RFP-labeled mitochondria were reprogrammed to iPSCs by introducing four factors, Oct3/4, Sox2, c-Myc, and Klf4, and were grown under ES cell culture conditions as described by Takahashi and Yamanaka [17]. The iPSCs were cultured in mES medium supplemented with leukemia inhibitory factor (LIF) and 2i (PD0325901 + CHIR99021). Pluripotency was verified in multiple ways. First, an Alkaline Phosphatase Detection Kit (Millipore) was used according to the manufacturer’s instructions to identify undifferentiated cells. Second, iPSCs (2 × 107) were injected subcutaneously into BALB/c nude mice to assess their ability to form teratomas. Four weeks later, the mice were euthanized by carbon dioxide inhalation, and any teratomas that had developed were excised, fixed in 4% PFA, and embedded in paraffin before being sectioned and stained with hematoxylin and eosin. Finally, immunostaining for OCT4 and SOX2 was performed as previously described [18].
Preparation of mitochondria
Mitochondria were isolated from iPSCs by differential centrifugation using a mitochondria isolation kit (Mitochondria Isolation Kit for Cultured Cells, Thermo Scientific™, 89874) according to the manufacturer’s instructions. The mitochondrial pellet was resuspended in a respiration buffer (225 mM mannitol, 75 mM sucrose, 10 mM KCl, 10 mM Tris–HCl, and 5 mM KH2PO4; pH 7.2) at a concentration of 1–4 mg/mL (total protein concentration) as described by Takeda et al. [19]. Approximately 1–2 pL of the mitochondrial suspension was injected into oocytes using the Piezo micromanipulator system (Prime-Tech) as described by Nagai et al. [20]. The number of mitochondria transferred per zygote was at least 1000 according to the previous report [21]. The mitochondria that had been injected into the zygotes were tracked by fluorescence microscopy (TE300; Nikon, Tokyo, Japan) during early developmental stages.
Embryo culture, embryo transfer, and embryo weight of the D13.5 embryos
Zygotes were retrieved from the ampulla of the oviducts 20 h after the hCG injection. Cumulus cells were removed from the zygotes with hyaluronidase (300 IU/mL) for 1 min in M2 medium (Millipore). Only zygotes with normal morphology were used. Embryos were cultured in 20 μL of KSOM medium (Millipore), covered with paraffin oil, and incubated in a 37 °C incubator with saturated humidity containing 5% CO2.
One hour after the mitochondria injection, at the pronuclear stage, an equal number of embryos in both the control and MS groups were transferred into each of the two sides of the oviducts of the same surrogate mouse. At E13.5, the embryos were excised, and the fetuses and the placentas were weighed using a precision electronic balance. The remaining embryos were used to assess the blastocyst rate and embryo quality.
Detection of exogenous mtDNA in embryos
According to the NCBI database, three bases vary between the mtDNA of C57BL/6j and BALB/c mice. The base pair variation at position 9347 allowed mtDNA from C57/6j mice but not from BALB/c mice to be digested by the PsyI restriction enzyme (Thermo Scientific). To identify exogenous mtDNA in early embryos, both allele-specific PCR (AS-PCR) and restriction enzyme analysis were used in this study as previously described by Luo et al. [22]. Briefly, three primers [22] were used for 2-cycle allele-specific PCR (AS-PCR), which could detect one copy of exogenous mtDNA even when it was mixed with the oocyte’s mtDNA. Using the 2× TaqMasterMix PCR Kit (CoWin Biotech), an 880-bp DNA product was amplified by the first AS-PCR reaction and was subsequently used as a template for the second AS-PCR reaction. The 520-bp DNA product obtained in the second AS-PCR product was digested to fragments of 383 and 157 bp DNA using the PsyI restriction enzyme, and the fragments were examined by agarose gel electrophoresis.
Bisulfite sequencing
For the methylation analysis, 20 D4.5 blastocysts were collected from each group. The bisulfite conversion was completed using the EZ DNA Methylation-Direct Kit (Zymo Research), and the bisulfite sequencing was carried out as previously described [23]. The sequencing data were uploaded and analyzed using the online tool QUMA.
Mitochondria ultrastructure and morphology of iPSCs
The mitochondrial ultrastructure and morphology of iPSCs was observed by transmission electron microscopy (TEM). IPSCs were processed for TEM for ultrastructural analysis as previously described [24].
Results
Detection of iPSC-derived mitochondria in early stage embryos
The iPSCs used in this study were positive for AP, could form teratomas containing derivatives of all three germ layer cells, and expressed pluripotency-specific genes (OCT4 and SOX2), all of which confirmed that the iPSCs were pluripotent (Supplemental Fig. 1). We also observed the morphology of mitochondria in iPSCs by TEM and found that the mitochondria were round or oval, with few cristae and an electron dense matrix (Supplemental Fig. 2).
Mitochondria were isolated from these iPSCs, and approximately 1–2 pL of either a mitochondrial suspension or the suspension buffer alone was injected into the zygotes using a Piezo micromanipulator. RFP-labeled mitochondria were detected using fluorescence microscopy (Fig. 1a).
Fig. 1.
a Mitochondria observed after extraction and microinjection into receptor zygotes. a Mitochondria extracted from iPSCs (scale bar = 100 μm). b–d Mitochondria injection procedure (scale bar = 50 μm). b Live cell fluorescence imaging of the injected mitochondria (red) in early embryos. The injected mitochondria were distributed unevenly during early developmental stages (scale bar = 20 μm)
Fluorescently labeled mitochondria were observed in both zygotes and early-stage embryos that underwent MS (Fig. 1b). In most cases, the injected mitochondria were unevenly distributed in blastomeres during cleavage and throughout the blastocyst stages.
Both the rate of blastocyst formation and the quality of those blastocysts were evaluated 4.5 days after fertilization. No significant differences were observed in terms of survival rate or developmental ability of zygotes that had been injected with a vehicle or the MS. Embryo transfer (ET) was performed in seven cases, and four of those mice ended up pregnant. The embryo implantation rate was similar between the two groups (Table 1).
Table 1.
The effect of iPSC-derived MS on embryonic development
| Control | MS | P | |
|---|---|---|---|
| Number of oocytes | 190 | 215 | – |
| Survival rate (%) | 64.74 (123/190) | 60.00 (129/215) | 0.326 |
| Cleavage rate (%) | 67.48 (83/123) | 62.02 (80/129) | 0.365 |
| Blastocyst rate (%) | 61.45 (51/83) | 65.00 (52/80) | 0.638 |
| High-quality blastocyst rate (%) | 59.04 (49/83) | 57.50 (46/80) | 0.842 |
| Implantation rate | 31.13 (22/72) | 37.88 (25/66) | 0.335 |
The effect of MS on the weight of fetuses and placentas at E13.5
E13.5 fetuses and placentas were weighed separately. Of the four pregnant mice, one was excluded because only three implantations occurred on the side where MS embryos were transferred. Additionally, there were one and two empty bursae in the control and MS groups, respectively, which were also excluded from the final statistics. A total of 41 embryos were included in the analysis, including 21 control embryos and 20 embryos in the MS group. The average weight of both the fetuses and placentas between the two groups was similar (Fig. 2).
Fig. 2.
A weight comparison of the fetuses and placentas between the control and MS groups (P > 0.05)
The distribution of donor mitochondria in E13.5 embryos
Donor mtDNA was identified in early embryos by 2-cycle AS-PCR and restriction enzyme analysis. Besides the 20 embryos used for weighting, three embryos implanted only in the MS side in one of the four pregnant mice were also tested. Both placental and fetal muscle tissue from E13.5 embryos were tested (Fig. 3), and donor mtDNA could be detected in the placentas of all 23 embryos. In contrast, donor mtDNA was only detected in the muscle tissue of four fetuses (Table 2).
Fig. 3.
AS-PCR and PsyI restriction enzyme digestion of MS embryos. Lanes 1, 3, 5, 7, and 9 contain PCR products from fetal muscle tissue mtDNA; lanes 2, 4, 6, 8, and 10 contain PCR products from placental mtDNA
Table 2.
Donor mtDNA detected in E13.5 placental and fetal tissue
| Fetus | placenta | |
|---|---|---|
| Donor mtDNA positive | 4 | 23 |
No purpose DNA bands were amplified in lane 7 (Fig. 3), which was similar to the results of Luo et al. [22]. It might be the results of base mismatches between the templates and primers, since there were two mismatched bases at the 3′ ends of the two antisense primers used in 2-cycle AS-PCR, respectively.
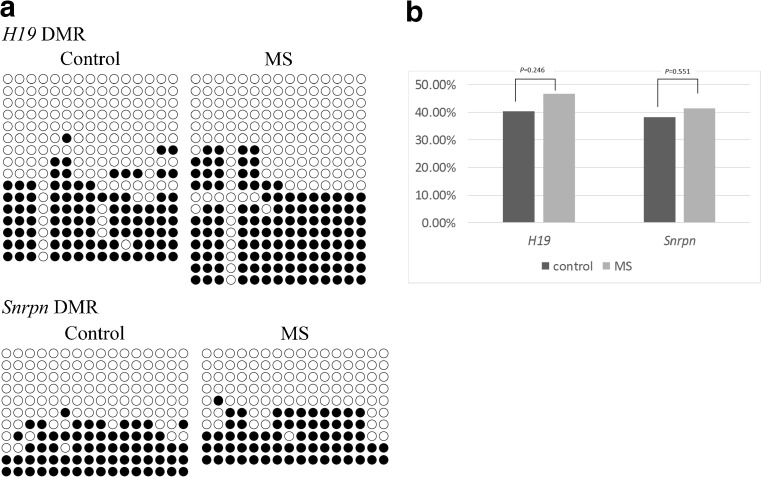
Methylation patterns in H19 and Snrpn DMRs
To identify whether MS of these embryos affected the DNA methylation pattern of imprinted genes, 15 CpG (cytosine and guanine connected by a phosphodiester bond) islands located in the DMR of the H19 gene along with 16 CpG regions located in the DMR of the Snrpn gene were analyzed (Fig. 4a). The results showed that the DNA methylation patterns of the H19 and Snrpn DMRs were similar between the MS and control groups (Fig. 4b).
Fig. 4.
DNA methylation patterns of H19 and Snrpn DMRs in blastocysts derived from the control and MS groups. a Methylation pattern of H19 and Snrpn DMRs. Each row represents a unique methylation status within the pool of clones sequenced. Filled and open circles represent methylated and unmethylated CpGs, respectively. b Analyzed results of the DMRs of H19 and Snrpn
Discussion
Our results indicate no significant differences between the MS group and the control group in terms of survival rate and embryonic development potential. E13.5 fetuses and placentas also showed no difference in weight between the twos groups. Additionally, the methylation status of H19 and Snrpn DMRs was unchanged by iPSC-derived MS, which preliminarily demonstrates the safety of using iPSCs as a mitochondrial donor for MS.
Pradeep et al. [25] first used a technique involving mitochondria-targeted restriction endonucleases, or TALENs, to eliminate mutations in the mtDNA of mouse oocytes and embryos, which suggested the possibility of preventing human mitochondrial diseases. However, because mtDNA replication does not occur in oocytes and preimplantation embryos, eliminating their mutated mtDNA can lead to a dramatic reduction in mtDNA copy number, which may result in a failure to implant [25]. Shimizu et al. [26] has reported that the mitochondrial disease only occurs when the mtDNA mutation rate in oocytes exceeds 65%. Recent study suggested that the function of mitochondria that carried dysfunctional mtDNAs could be restored by fusion with normal mitochondria [27]. This study provided a favorable theoretical support for improving the developmental potential of oocytes by MS.
The recent advance of Augment technology has drawn attention to the use of MS in human-assisted reproduction. However, this technology requires that mitochondria be extracted from the patient’s own oocyte stem cells before they can be injected into the patient’s zygote [9], which limits the availability of mitochondria for the procedure. IPSCs have extensive self-renewal capacity and represent an unlimited source of therapy material [13]. And our results showed that the mitochondrial morphology of iPSCs (Supplemental Fig. 2) was similar to that of the mitochondria of the oocytes reported by Pietro et al. [28], which meant that their function and metabolism may be more similar. Additionally, the ethical problems of introducing a third genetic material to the next generation could be avoided if the iPSCs induced from the patients themselves.
Yi and his colleague [7] reported that injecting mitochondria extracted from hepatocytes into zygotes improved the rate of blastocyst formation in both young and old mice. However, Hideki et al. [8] demonstrated that MS with somatic cell-derived mitochondria was not sufficient to rescue in vivo-aged mouse oocytes. Our data suggest that the rates of blastocyst formation and implantation are similar between the MS and control groups in 8–10-week mice. These differences may result from the different mitochondrial source or the different amount of mitochondria that were injected. Another reason may be that most zygotes for MS from young mice maintain the ability of normal development. So zygotes with worse embryo development may be better model to access the physiological role of MS in embryo development [29]. And it should be noted that iPSCs used in this study were not derived from the oocyte donor itself. This leads to mitochondrial heterogeneity inevitably, which may exhibit incompatibilities between the mitochondrial and nuclear genomes [30]. But our results, at least, suggested that MS with iPSC-derived mitochondria was not adverse to the embryo development.
Luo et al. [22] observed that sperm mtDNA was distributed unevenly in both preimplantation embryos and fetal tissues, most of which could be detected in the placenta. Similarly, our study demonstrated that the injected mitochondria were unevenly distributed in blastomeres during cleavage and the blastocyst stages. And the donor mtDNA could be detected in the placentas of all MS embryos at E13.5, but it could only be detected in four of the MS fetuses. The uneven distribution of the donor mitochondria among different tissues may be a normal phenomenon of the mitochondrial maternal inheritance mechanism [22], which leads to an uneven distribution of exogenous mtDNA in blastomeres and subsequent fetal tissues [31]. So the reason for the low detection rate of iPSC-derived mtDNA may be that the injected mitochondria may also exist in other tissues besides muscle tissue, such as heart, brain, and intestine [22], which was not investigated here. Another reason might be that the blastomeres containing injected mitochondrial tend to develop into trophectoderm cells. Anyway, our results suggested at least that iPSC-derived mitochondria could be detected in fetal tissues and may also be involved in late embryonic development.
H19 and Snrpn are paternally and maternally imprinted genes, respectively, and both are important for embryonic development [23]. Previous studies have reported that approximately 35–65% of Silver–Russell syndrome cases are caused by hypomethylation of the H19 DMR [11]. Additionally, Snrpn has been identified as a candidate gene involved in Prader–Willi and Angelman syndromes [12]. Our results indicate that the methylation patterns of the H19 and Snrpn DMRs are similar between the MS and control groups, which preliminarily demonstrates the safety of using iPSC-derived mitochondria for MS.
Finally, our results indicate that iPSC-derived mitochondria can directly participate in the developmental process of early embryos. Injecting iPSC-derived mitochondria into mouse zygotes neither affected their development nor changed the methylation status of the H19 and Snrpn DMRs. However, additional studies are needed to determine whether the iPSC-derived mitochondria can improve embryonic developmental potency, especially for embryos taken from older mice.
Electronic supplementary material
The pluripotency detection of iPSCs. A) AP test; B) three germ layersof the teratoma; C) immunostaining of OCT4 and SOX2. (JPEG 8614 kb)
Mitochondria ultrastructure and morphology of iPSCs. IPSCs mitochondria were immature, with round or oval shapes, possess few cristae and have an electron dense matrix. Mitochondria (arrow), nucleus (*) (12000×).. (JPEG 15112 kb)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81370680, 81402168, and 31571497), the Specialized Research Fund for the Doctoral Program of the Chinese Ministry of Education (Grant No. 20130171130009), and the Natural Science Foundation’s Key Research Project of Guangdong Province (Grant No. 2013020012660).
Authors’ roles
Ruiqi Li analyzed the data and drafted the manuscript. Bingqiang Wen induced iPSCs and evaluated the pluripotency of iPSCs. Haijing Zhao also drafted the manuscript. Nengyong Ouyang, Songbang O, and Meiqi Mai collected the data. Wenjun Wang revised the manuscript. Jianyong Han and Dongzi Yang conceived and designed the study. All authors interpreted the data.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All animal protocols were approved by and performed in accordance with the requirements of the Institutional Animal Care and Use Committee at the authors’ university.
Footnotes
Ruiqi Li and Bingqiang Wen contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0948-9) contains supplementary material, which is available to authorized users.
Contributor Information
Jianyong Han, Phone: +86010-62734382, Email: hanjy@cau.edu.cn.
Dongzi Yang, Phone: +86020-81332230, Email: yangdz@mail.sysu.edu.cn.
References
- 1.Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science. 2004;304:253–257. doi: 10.1126/science.1094884. [DOI] [PubMed] [Google Scholar]
- 2.de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, Te VE. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod. 2004;70:419–424. doi: 10.1095/biolreprod.103.015784. [DOI] [PubMed] [Google Scholar]
- 3.Bentov Y, Casper RF. The aging oocyte—can mitochondrial function be improved? Fertil Steril. 2013;99:18–22. doi: 10.1016/j.fertnstert.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 4.St John J. Ooplasm donation in humans: the need to investigate the transmission of mitochondrial DNA following cytoplasmic transfer. Hum Reprod. 2002;17:1954–8. [DOI] [PubMed]
- 5.Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78:1046–1048. doi: 10.1016/S0015-0282(02)04214-0. [DOI] [PubMed] [Google Scholar]
- 6.Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403:500–501. doi: 10.1038/35000651. [DOI] [PubMed] [Google Scholar]
- 7.Yi YC, Chen MJ, Ho JY, Guu HF, Ho ES. Mitochondria transfer can enhance the murine embryo development. J Assist Reprod Genet. 2007;24:445–449. doi: 10.1007/s10815-007-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igarashi H, Takahashi T, Abe H, Nakano H, Nakajima O, Nagase S. Poor embryo development in post-ovulatoryin vivo-aged mouse oocytes is associated with mitochondrial dysfunction, but mitochondrial transfer from somatic cells is not sufficient for rejuvenation. Hum Reprod. 2016;31:2331–2338. doi: 10.1093/humrep/dew203. [DOI] [PubMed] [Google Scholar]
- 9.Oktay K, Baltaci V, Sonmezer M, Turan V, Unsal E, Baltaci A, et al. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22:1612–1617. doi: 10.1177/1933719115612137. [DOI] [PubMed] [Google Scholar]
- 10.Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Amero S, Monk D, Frost J, Preece M, Stanier P, Moore GE. The genetic aetiology of Silver-Russell syndrome. J Med Genet. 2008;45:193–199. doi: 10.1136/jmg.2007.053017. [DOI] [PubMed] [Google Scholar]
- 12.Leff SE, Brannan CI, Reed ML, Ozcelik T, Francke U, Copeland NG, Jenkins NA. Maternal imprinting of the mouse Snrpn gene and conserved linkage homology with the human Prader-Willi syndrome region. Nat Genet. 1992;2:259–264. doi: 10.1038/ng1292-259. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Yuan P, Yang H, Zhang J, Soh BS, Li P, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume O, Ohnishi S, Mito T, Shimizu A, Iashikawa K, Nakada K, et al. Epigenetic regulation of the nuclear-coded GCAT and SHMT2 genes confers human age-associated mitochondrial respiration defects. Sci Rep-UK. 2015;5:10434. doi: 10.1038/srep10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma H, Folmes CD, Wu J, Morey R, Mora-Castilla S, Ocampo A, et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature. 2015;524(7564):234–238. doi: 10.1038/nature14546. [DOI] [PubMed] [Google Scholar]
- 16.Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59:105–107. doi: 10.1538/expanim.59.105. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Pei Y, Yue L, Zhang W, Wang Y, Wen B, Zhong L, et al. Improvement in mouse iPSC induction by Rab32 reveals the importance of lipid metabolism during reprogramming. Sci Rep. 2015;5:16539. doi: 10.1038/srep16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K. Microinjection of cytoplasm or mitochondria derived from somatic cells affects parthenogenetic development of murine oocytes. Biol Reprod. 2005;72:1397–1404. doi: 10.1095/biolreprod.104.036129. [DOI] [PubMed] [Google Scholar]
- 20.Nagai S, Kasai T, Hirata S, Hoshi K, Yanagimachi R, Huang T. Cytoplasmic transfer in the mouse in conjunction with intracytoplasmic sperm injection. Reprod BioMed Online. 2004;8:75–80. doi: 10.1016/S1472-6483(10)60500-7. [DOI] [PubMed] [Google Scholar]
- 21.Yi Y, Chen M, Ho JY, Guu H, Ho ES. Mitochondria transfer can enhance the murine embryo development. J Assist Reprod Gen. 2007;24:445–449. doi: 10.1007/s10815-007-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo SM, Ge ZJ, Wang ZW, Jiang ZZ, Wang ZB, Ouyang YC, et al. Unique insights into maternal mitochondrial inheritance in mice. Proc Natl Acad Sci. 2013;110:13038–13043. doi: 10.1073/pnas.1303231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng K, Fu X, Zhang R, Jia G, Hou Y, Zhu S. Effect of oocyte vitrification on deoxyribonucleic acid methylation of H19, Peg3, and Snrpn differentially methylated regions in mouse blastocysts. Fertil Steril. 2014;102:1183–1190. doi: 10.1016/j.fertnstert.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Hao Y, Wax D, Zhong Z, Murphy C, Ross JW, Rieke A, et al. Porcine skin-derived stem cells can serve as donor cells for nuclear transfer. Cloning Stem Cells. 2009;11:101–110. doi: 10.1089/clo.2008.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161:459–469. doi: 10.1016/j.cell.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu A, Mito T, Hayashi C, Ogasawara E, Koba R, Negishi I, et al. Transmitochondrial mice as models for primary prevention of diseases caused by mutation in the tRNA(Lys) gene. Proc Natl Acad Sci U S A. 2014;111:3104–3109. doi: 10.1073/pnas.1318109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Long Q, Liu J, Tang H, Li Y, Bao F, et al. Mitochondrial fusion provides an ‘initial metabolic complementation’ controlled by mtDNA. Cell Mol Life Sci. 2015;72:2585–2598. doi: 10.1007/s00018-015-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15(Suppl 2):129–147. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- 29.Cagnone GL, Tsai TS, Makanji Y, Matthews P, Gould J, Bonkowski MS, et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci Rep. 2016;6:23229. doi: 10.1038/srep23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow EH, Reinhardt K, Wolff JN, Dowling DK. Risks inherent to mitochondrial replacement. EMBO Rep. 2015;16:541–544. doi: 10.15252/embr.201439110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HS, Ma H, Juanes RC, Tachibana M, Sparman M, Woodward J, et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012;1:506–515. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pluripotency detection of iPSCs. A) AP test; B) three germ layersof the teratoma; C) immunostaining of OCT4 and SOX2. (JPEG 8614 kb)
Mitochondria ultrastructure and morphology of iPSCs. IPSCs mitochondria were immature, with round or oval shapes, possess few cristae and have an electron dense matrix. Mitochondria (arrow), nucleus (*) (12000×).. (JPEG 15112 kb)