Abstract
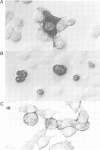
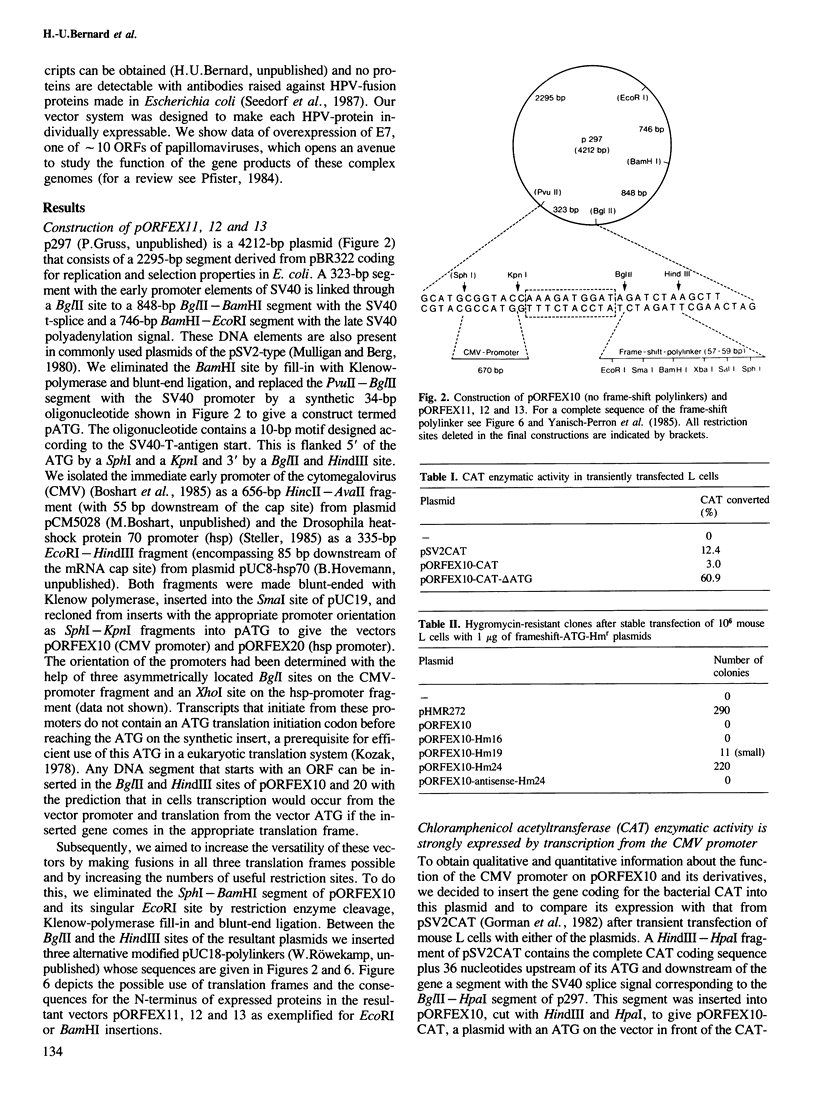
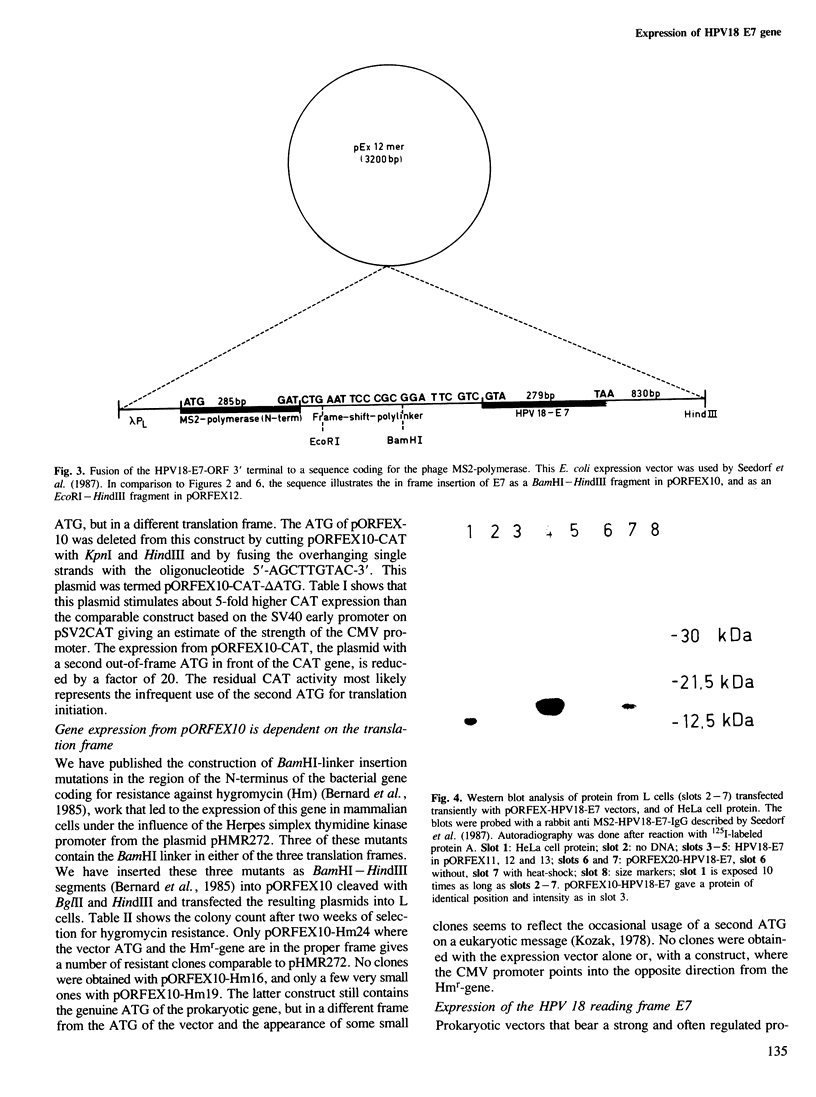
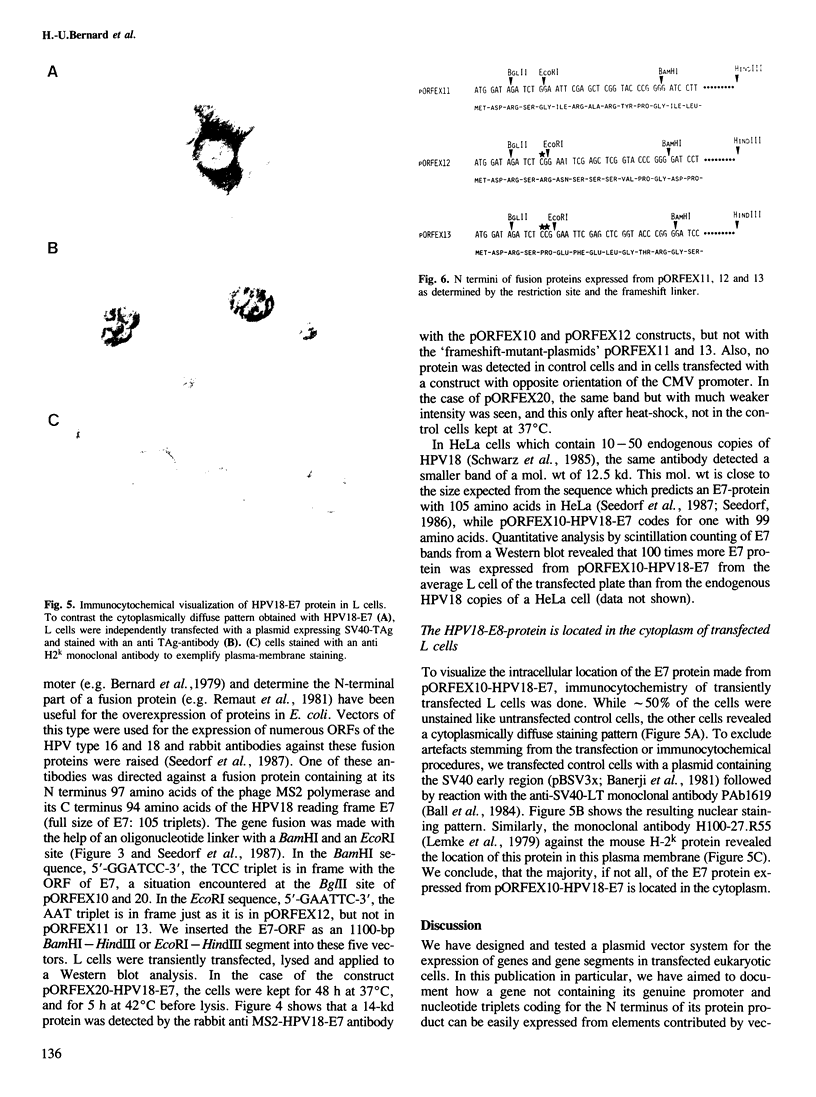
We have constructed and functionally tested a cassette-vector-system for the transcription and translation of open reading frames (ORFs) in cells of higher eukaryotes. The vectors are derived from the plasmid pBR322 and can be selected and amplified in Escherichia coli. Alternative eukaryotic promoters can be inserted between the restriction sites SphI and KpnI, translation initiation motifs between KpnI and BglII, linkers for the adjustment of the translation reading frame and the insertion of genes or gene segments between BglII and HindIII, followed by a HindIII-EcoRI segment with splicing and polyadenylation signals derived from SV40. A prototype vector system, pORFEX11, 12 and 13, contains the strong cytomegalovirus immediately early promoter and a 10-bp motif of the SV40 T-antigen translation start. Polylinkers derived from pUC18 permit the insertion of ATG-less ORFs downstream from the ATG of the vector. Either of the three alternative polylinkers adjusts the appropriate translation frame. A similar construct contains the regulatable promoter of the Drosophila heat shock gene 70. We inserted genes or gene segments, that code for the bacterial chloramphenicol acetyltransferase, the bacterial gene conferring resistance against hygromycin, and the ORF E7 of the human papillomavirus type 18 into these vectors. After transfection of mouse L fibroblasts, all proteins and functions were expressed in accordance with the prediction. In transiently transfected L cells, the E7 protein expressed from pORFEX12 constitutes approximately 2.0% of total cell protein. This E7 protein could be localized by immunocytochemistry as a cytoplasmic component.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Krämmer G., Röwekamp W. G. Construction of a fusion gene that confers resistance against hygromycin B to mammalian cells in culture. Exp Cell Res. 1985 May;158(1):237–243. doi: 10.1016/0014-4827(85)90446-x. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung N., Fitts R., Wilson S., Milne A., Hamer D. Efficient production of hepatitis B surface antigen using a bovine papilloma virus-metallothionein vector. J Mol Appl Genet. 1984;2(5):497–506. [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Wolfe S. A., Bartlett G. L., Zaino R. J., Sedlacek T., Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985 Oct 17;317(6038):639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- LaPorta R. F., Taichman L. B. Human papilloma viral DNA replicates as a stable episome in cultured epidermal keratinocytes. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3393–3397. doi: 10.1073/pnas.79.11.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Matthias P., Boeger U., Danesch U., Schütz G., Bernard H. U. Physical state, expression and regulation of two glucocorticoid-controlled genes on bovine papilloma virus vectors. J Mol Biol. 1986 Feb 20;187(4):557–568. doi: 10.1016/0022-2836(86)90334-7. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nakabayashi Y., Chattopadhyay S. K., Lowy D. R. The transforming function of bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5832–5836. doi: 10.1073/pnas.80.19.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Oltersdorf T., Krämmer G., Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987 Jan;6(1):139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]