Abstract
Purpose
To study the role of Toll-like receptor 4 (TLR4) in human spermatozoa and to assess sperm parameters, oxidative stress markers, and acrosome reaction in response to the stimulation of TLR4 by its ligand, the lipopolysaccharide (LPS), as a major endotoxin of Gram-negative bacteria.
Methods
Our study was carried out in 73 sperm samples from patients undergoing semen analysis for couple infertility investigations. The studied patients were divided into three groups: normozoospermic fertile patients (n = 13), patients with abnormal and leukospermic semen (n = 13), and patients with abnormal and non-leukospermic semen (n = 47). TLR4 expression in human spermatozoa was initially analyzed by western blot. Sperm samples were incubated in the presence of LPS (200 ng/ml) for 18 h. Then, sperm motility and vitality were evaluated by microscopic observation and oxidative stress markers as malondialdehyde (MDA) and carbonyl groups (CG) were spectrophotometrically assessed in neat and selected sperm. A triple-stain technique was also performed to evaluate acrosome reaction in 15 sperm samples from infertile patients.
Results
TLR4 expression was confirmed in human spermatozoa with a molecular weight of 69 kDa. In the normozoospermic group, no significant differences in sperm parameters and oxidative stress markers were shown after incubation with LPS in neat and selected sperms. Regarding samples from the non-leukospermic group, LPS reduced spermatozoa motility and vitality rates in selected sperm (P = 0.003; P = 0.004, respectively). A significant increase of MDA and CG levels was also detected (P = 0.01; P = 0.02, respectively). However, only the MDA levels were significantly increased (P = 0.01) in neat LPS-stimulated sperm. The same results were shown within the leukospermic group. The comparison between the two groups, leukospermic and non-leukospermic, in selected sperms showed a more important LPS effect in the leukospermic group significantly on motility and MDA rates (P = 0.006; P = 0.009, respectively). Furthermore, a significant decrease in reacted spermatozoa rate was detected in response to LPS in selected sperm samples from infertile men (P = 0.03).
Conclusions
These findings indicate that human spermatozoa express TLR4 and respond to LPS stimulation with alterations in viability, motility, and the acrosome reaction implicating reactive oxygen species (ROS) production in sperm samples from infertile patients.
Keywords: Spermatozoa, Toll-like receptor 4, Lipopolysaccharide, Oxidative stress
Introduction
Infection and inflammation within the male reproductive tract could induce detrimental effects on reproduction, which usually manifest as impaired sperm quality, seminal tract obstruction, reduced androgen production, disturbance of spermatogenesis, and temporarily loss of fertility [1, 2]. Gram-negative bacteria can especially reduce the sperm quality, and several studies have revealed that the lipopolysaccharide (LPS), a major component of Gram-negative bacteria cell wall, has male reproductive toxicity [3–7]. The number of leukocytes is often increased in bacteria-infected sperm concomitantly with increased ROS production, which is incriminated in the alteration of sperm quality [8]. In fact, increasing evidences support that the presence of high levels of ROS in the ejaculate plays a critical role in the genesis of defective sperm functions and male infertility resulting in diminished motility, sperm DNA damages, and impaired fertilization [9, 10].
Some previous reports implicate microbial pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), as well as their signaling pathways on ROS generation in phagocytic cells via the enzymatic complex nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [11, 12] or via the expression of inducible nitric oxide synthase (iNOS) gene promoting nitric oxide (NO·) synthesis [13, 14]. TLRs trigger signals responsible for the activation of innate and adaptive immune responses by recognizing pathogen-associated molecular patterns (PAMPs). To date, 13 TLRs have been identified in mammals [15]. According to their localization and ligands, TLRs are divided into two subgroups: cell surface TLRs and cytosolic TLRs. Most classes of TLRs are found in innate immune cells such as monocytes/macrophages, polymorphonuclear neutrophils (PMNs), dendritic cells, natural killer (NK) cells, and mast cells. However, recent studies indicate that TLRs are widely expressed in endothelial cells, epithelial cells, fibroblasts, and cancer cells [16]. According to some literature data, the existence of TLRs 1–10 was also reported in the male reproductive tract as human testis, vas deferens, prostate, and epididymis [17, 18]. Furthermore, the distribution of TLRs was shown on sections obtained from the male rat reproductive tract and spermatozoa [19, 20]. RNAs encoding TLR3, TLR4, and TLR5 were also detected in rat and mouse Sertoli cells [21]. Two recent studies showed the expression of TLR2 and TLR4 in human spermatozoa; however, the role and the operating mode of TLRs in human spermatozoa are still not well understood [22, 23]. It seems that these receptors could play a critical role in spermatozoa especially in the presence of a putative NADPH oxidase (NOx) activity [24–27] and (iNOS) enzyme in the human spermatozoa [28] responsible of ROS production and oxidative stress genesis. Thus, several questions obviously arise including whether these TLRs may act as those in leukocytes concerning ROS production and whether it has an effect on sperm parameters. In this study, we propose to check the expression of TLR4 in human spermatozoa and to evaluate sperm parameters, acrosome reaction, and oxidative stress markers as malondialdehyde (MDA) and carbonyl groups (CG) in human ejaculates before and after sperm selection in response to TLR4 stimulation by bacterial LPS.
Materials and methods
Patients
This study was approved by the Institutional Review Board (IRB) of Medicine Faculty of Sfax, Tunisia. Semen samples were obtained from 73 male partners of infertile couples attending the Histology-Embryology Laboratory of Medicine Faculty of Sfax, Tunisia, for semen investigations. The studied patients were divided into three groups: normozoospermic fertile patients (n = 13), patients with abnormal and leukospermic semen (n = 13), and patients with abnormal and non-leukospermic semen (n = 47). Fertile patients considered as the control group have fathered in the past, and the medical investigations of the couple confirmed a female infertility factor (ovulatory disorders, tubal obstruction, intrauterine adhesions). Informed written consent was obtained from all patients for being included in the study. The patients were aged between 28 and 57 years with a mean age ± standard error (SE) of 39.5 ± 6.4 years. Men with sperm concentrations <10 × 106 ml−1and/or presenting confirmed sperm infection were excluded from the study.
Semen analysis
Semen samples were collected into a sterile container by masturbation after 3–5 days of sexual abstinence and allowed to liquefy for 30 min at 37 °C. The sperm parameters were evaluated according to the current World Health Organization (WHO) criteria [29] including the semen volume, sperm concentration, motility, vitality, and morphology. Sperm samples were also assessed for leukocyte concentration using a peroxidase method [29]. Semen samples with a sperm count ≥15 × 106 ml−1 and total motility “a + b + c” ≥40% were considered normal [29]. Leukocytospermia is defined as the presence of ≥1 × 106 leukocytes/ml in semen [29].
Sperm selection
To eliminate interference with semen cells that express TLR4 including leukocytes, we analyzed the effect of TLR4 stimulation by LPS on selected spermatozoa. Semen samples were proceeded by Sil-Select® (FertiPro NV Belgium) density gradient centrifugation. A two-layer gradient was prepared in a conical 15-ml centrifuge tube using respectively 90 and 45% Sil-Select® solutions. One milliliter of liquefied semen sample was placed carefully at the top of the gradient. The tube was centrifuged at 300×g for 20 min. The supernatant was then removed, and the sperm pellet was suspended in 2 ml of Roswell Park Memorial Institute medium (RPMI 1640; Sigma-Aldrich) supplemented with 10% of fetal bovine serum (FBS; Biowest). After centrifugation at 500×g for 5 min, the final sperm pellet was suspended in 0.4 ml of RPMI medium and we analyzed sperm concentration, motility, and vitality of selected spermatozoa. The remainder of the samples (30 ejaculates) was aliquoted and stored at −80 °C for MDA and CG analyses.
Sperm stimulation by the lipopolysaccharide
For each sample, we added 200 ng/ml of LPS (Sigma-Aldrich) to 200 μl of respectively neat semen and selected semen and we mixed gently the final solutions. The two mixtures were incubated for 18 h at room temperature. After incubation, sperm motility and vitality were assessed.
Western blot analysis
Protein extraction
Sperm protein extraction was performed following a previously described method [30]. Briefly, for each sperm sample, 2 × 106 of selected human spermatozoa were twice washed with 500 μl of phosphate-buffered saline (PBS; pH 7.4) and centrifuged at 10,000×g for 10 min. Then, spermatozoa were suspended in 300 μl of lysis buffer [187 mM Tris–Hcl (pH 6, 8), 2% SDS, 1% NP40, 10% glycerol, 1 mM PMSF, protease inhibitor mixture (Sigma-Aldrich), 1 mM EDTA] and sonicated briefly for 3 cycles of 10 s each at 37%. After centrifugation for 5 min at 14,000×g, 25 μl of the supernatant was mixed with 25 μl of 2× loading buffer (Bio-Rad, France) and heat-denatured for 5 min at 100 °C.
Protein electrophoresis and revelation
Thirty microliters of sperm extract was loaded in each lane (2 × 106 spermatozoa/lane) of an 8–12% SDS–polyacrylamide gradient gel at 100 V using a vertical electrophoresis system (Bio-Rad) and transferred to polyvinylidene fluoride (PVDF) membrane (Bio-Rad). Human leukocytes were used as positive control cells for TLRs, and HeLa cells (immortal cell line derived from cervical cancer cells) obtained from the Cell Culture Laboratory (Biotechnology Institute of Sfax, Tunisia) were used as negative control. The PVDF membrane was saturated at room temperature with phosphate-buffered saline (PBS)–milk (5%) for 30 min. Then, it was incubated with anti-TLR4 antibodies (1/500) (Santa Cruz Biotechnology) overnight in a solution of PBS-Tween (0.1%)–milk (3%). The membrane was washed three times in PBS-Tween (0.5%) for 5 min, and a second antibody (anti-rabbit IgG coupled to peroxidase; 1/1000) (Promega) was added. The final solution was incubated for 2 h at room temperature. Protein revelation was carried out by diaminobenzidine (DAB) (Bio-Rad) substrate (0.05%) in the presence of Tris-buffered saline (TBS) H2O2 (0.1%).
Protein quantification
The protein levels were determined by using the Bio-Rad protein assay based on the Bradford dye procedure [31], and bovine serum albumin is used as standard.
Measurement of lipid peroxidation: malondialdehyde assay
The MDA level was evaluated in semen samples using thiobarbituric acid-reactive species (TBARS) assay previously described by Buege et al. [32]. Briefly, 107 sperm cells were resuspended in 500 μl of distilled water and two volumes of thiobarbituric acid (TBA) reagent (15% trichloroacetic acid and 0.8% thiobarbituric acid in 0.25 HCl) were added. The final mixture was then heated for 15 min at 95 °C. After cooling, the mixture was centrifuged at 3000×g for 10 min. The content of MDA was measured spectrophotometrically by the determination of the optical density of the supernatant at 532 nm. The results were expressed as nanomoles of MDA per milliliter. Values were reported to a calibration curve of 1,1,3,3-tetraethoxypropane.
Measurement of protein oxidation: carbonyl group assay
From each semen sample, 1 mg of seminal plasma proteins and 1 × 106 spermatozoa (from neat sperm and selected sperm) were used. The method was based on the reaction of the carbonyl group with 2,4-dinitrophenylhydrazine (DNPH) to give the corresponding hydrazone. The latter can be quantified spectrophotometrically at 370 nm. The protocol used here is similar to that described previously by Reznick et al. [33]. The results were expressed as nanomoles of CG per 106 spermatozoa in neat and selected sperm and nanomoles of CG per milligram of proteins in seminal plasma.
Evaluation of acrosome reaction
Acrosome reaction was assessed in response to LPS in 15 sperm samples from infertile patients. It was induced using heparin as previously described by Kitiyanant et al. [34] with little modifications. It was assessed in neat sperm and in selected spermatozoa respectively before and after incubation with 200 ng/ml LPS during 18 h.
For each semen sample, two tubes were prepared containing respectively 200 μl of neat sperm and 200 μl of selected spermatozoa. The content of every tube was diluted with 1 ml of PBS and centrifuged at 2000×g for 5 min. The supernatants were discarded, each pellet was resuspended with 200 μl of fresh PBS, and samples were then incubated with 10 μg/ml heparin for 2 h at 37 °C. After incubation, the acrosome reaction was analyzed using the triple-stain technique as previously described by Talbot et al. [35]. Respectively, 200 μl of sperm suspensions from neat and selected spermatozoa was diluted with 200 μl of 2% Trypan Blue and incubated for 15 min at 37 °C. Smears of 10 μl of each sample were made and air-dried. After 1 min of brief rinsing in water, smears were fixed in 3% glutaraldehyde solution for 45 min at room temperature, rinsed in water, and air-dried. Subsequently, smears were stained in 0.5% Bismark Brown solution for 10 min at 40 °C, rinsed in water, and air-dried. Finally, the smears were stained with 0.8% Rose Bengal solution. Washed and dried slides were examined with a light microscope at ×1000 magnification. The percentage of live acrosome-reacted spermatozoa was evaluated in at least 200 spermatozoa.
Statistical analyses
A statistical analysis including paired t test was performed to compare differences between unstimulated and stimulated sperms using SPSS 20.0 software. All data were expressed as means ± standard error (SD). The statistical significance was considered for 푃 values <0.05.
Results
Sperm parameter analyses before and after sperm selection
The mean values ± SD of different sperm parameters before and after sperm selection are summarized in Table 1.
Table 1.
Description of sperm parameters in neat sperm and selected sperm in leukospermic, non-leukospermic, and normozoospermic control groups
| Sperm parameters | Normozoospermic control group (n = 13) | Non-leukospermic group (n = 47) | Leukospermic group (n = 13) |
|---|---|---|---|
| Sperm volume (ml) | 3.55 ± 1.33 | 3.41 ± 1.23 | 3.86 ± 1.42 |
| Sperm concentration (×106 ml−1) | 109.67 ± 46.48 | 58.61 ± 53.56 | 76.88 ± 60.49 |
| Total motility in neat sperm “a + b + c” (%) | 54.23 ± 4.49 | 46.17 ± 8.09 | 44.61 ± 6.60 |
| Progressive motility in neat sperm “a + b” (%) | 47.30 ± 5.63 | 39.04 ± 9.47 | 37.69 ± 7.25 |
| Sperm vitality (%) | 86.46 ± 5.99 | 72.95 ± 11.47 | 73.53 ± 8.1 |
| Normal sperm morphology (%) | 17 ± 2.64 | 7.67 ± 5.69 | 8.61 ± 3.47 |
| Leucocytes (×106 ml−1) | 0.16 ± 0.2 | 0.07 ± 0.09 | 2.08 ± 2.29 |
| Total motility in selected sperm “a + b + c” (%) | 81.15 ± 7.4 | 66.8 ± 18.89 | 67.3 ± 14.08 |
Values are means ± standard deviation (SD). Grade of sperm motility according to WHO criteria [29]: “a” rapid progressive motility; “b” slow progressive motility; “c” non-progressive motility
Expression of TLR4 in human sperm
TLR4 was detected by western blots in selected spermatozoa as well as in leukocyte control. After revelation, one band of 69 kDa was shown (Fig. 1).
Fig. 1.
Western blot analyses of TLR4 in human spermatozoa. The expression of TLR4 protein was detected in selected spermatozoa (S). Human leukocytes were used as positive control cells (C+) for TLRs. HeLa cells were used as negative control (C−)
Effect of lipopolysaccharide on sperm parameters before and after sperm selection
Sperm motility
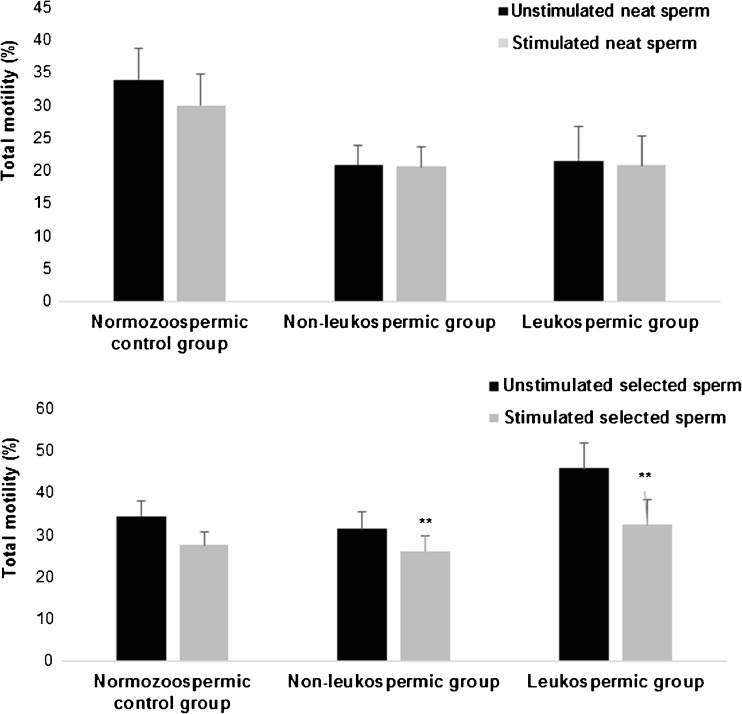
In the normozoospermic group, no significant effect of LPS was detected on sperm motility both in neat and selected sperm (Fig. 2). In non-leukospermic and leukospermic groups and after exposure of selected sperm to LPS, we noted a significant decrease in total motility in comparison with those observed on unstimulated selected sperm (31.38 ± 4.12 vs 26.17 ± 3.51%, P = 0.003, and 45.76 ± 6.81 vs 32.3 ± 6.08%, P = 0.004, respectively) (Fig. 2b). However, no significant results were shown in neat sperm in the two groups (P > 0.05) (Fig. 2a). As compared with the non-leukospermic group, the LPS had a more important and significant effect on sperm motility in the leukospermic group (P = 0.006) as evidenced by the differences between unstimulated and stimulated sperm within the two groups.
Fig. 2.
Effect of lipopolysaccharide on sperm total motility. Values are means ± SD. a Effect of lipopolysaccharide on sperm motility in neat sperm within the three groups: normozoospermic control group (n = 13), non-leukospermic group (n = 47), and leukospermic group (n = 13). b Effect of lipopolysaccharide on sperm motility in selected sperm within the three groups. ** P < 0.01: significant difference between LPS-stimulated sperms and unstimulated sperms
Sperm vitality
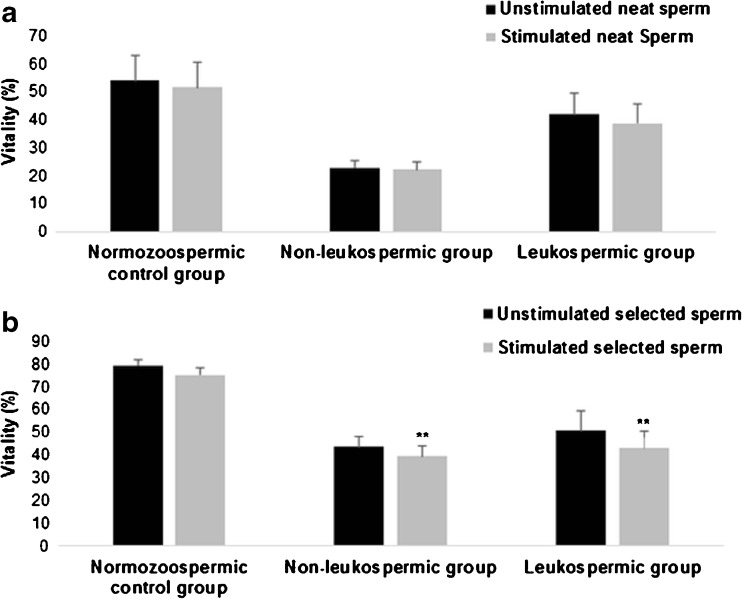
In the normozoospermic group, no significant effect of LPS was detected on sperm vitality both in neat and selected sperms (Fig. 3). After stimulation of selected sperm by LPS, a significant decrease of vitality was noted in non-leukospermic and leukospermic groups (43.51 ± 4.65 vs 39.51 ± 4.34%, P = 0.004, and 50.76 ± 8.59 vs 42.92 ± 7.65%, P = 0.01, respectively) (Fig. 3b). Otherwise, no significant differences in vitality rates were shown in the two groups after exposure to LPS in neat sperm (P > 0.05) (Fig. 3a).
Fig. 3.
Effect of lipopolysaccharide on sperm vitality. Values are means ± SD. a Effect of lipopolysaccharide on sperm vitality in neat sperm within the three groups: normozoospermic control group (n = 13), non-leukospermic group (n = 47), and leukospermic group (n = 13). b Effect of lipopolysaccharide on sperm vitality in selected sperm within the three groups.* P < 0.05: significant difference between LPS-stimulated sperms and unstimulated sperms.** P < 0.01: significant difference between LPS-stimulated sperms and unstimulated sperms
Summary statistics results concerning total motility and vitality (in neat and selected sperm) before and after LPS stimulation are given in Table 2.
Table 2.
Summary statistics of comparisons between sperm parameters in neat and selected sperm before and after LPS stimulation in leukospermic, non-leukospermic, and normozoospermic control groups
| Mean values (± SD) | Normozoospermic control group (n = 13) | Non-leukospermic group (n = 47) | Leukospermic group (n = 13) |
|---|---|---|---|
| Sperm total motility | |||
| Neat sperm | P = 0.254 | P = 0.8 | P = 0.7 |
| Selected sperm | P = 0.318 | P = 0.003 | P = 0.004 |
| Sperm vitality | |||
| Neat sperm | P = 0.121 | P = 0.091 | P = 0.2 |
| Selected sperm | P = 0.168 | P = 0.004 | P = 0.01 |
Significant differences (P values): bold characters
Evaluation of oxidative stress markers in the sperm samples in response to the stimulation by lipopolysaccharide
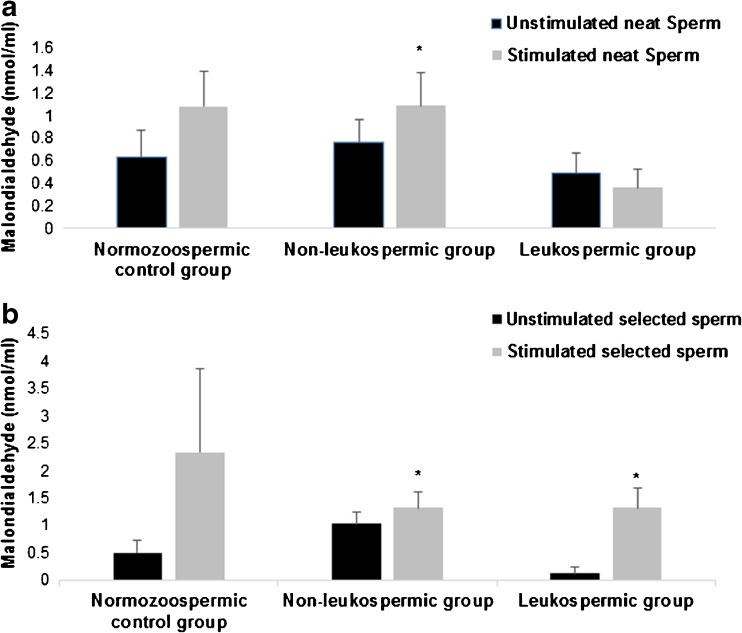
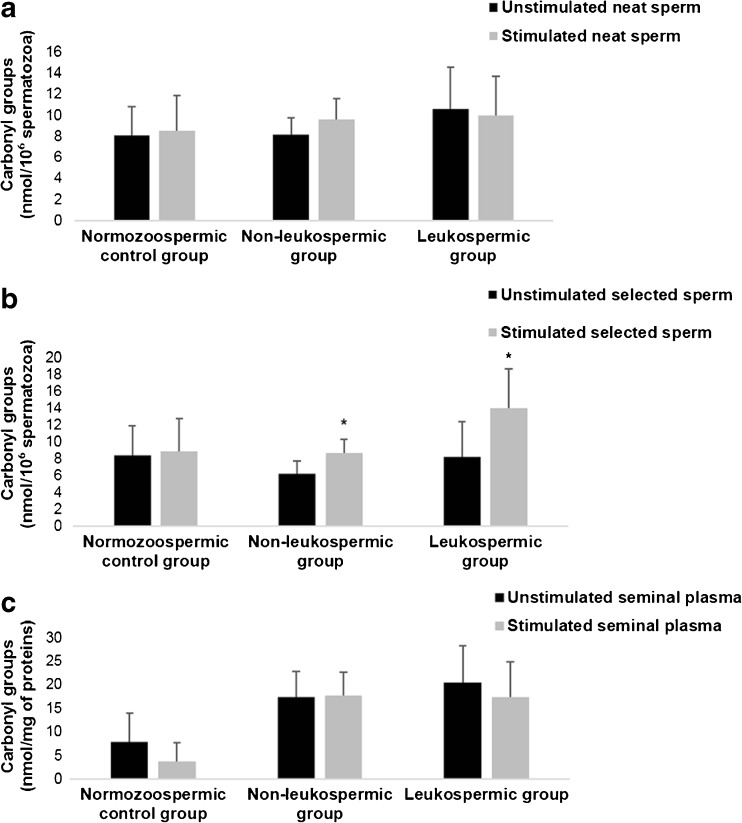
In normozoospermic control samples, the LPS stimulation had no significant effect on MDA and CG levels before and after sperm selection (Figs. 4 and 5).
Fig. 4.
Effect of lipopolysaccharide on sperm malondialdehyde levels. Values are means ± SD. a Effect of lipopolysaccharide on sperm malondialdehyde levels in neat sperm within the three groups: normozoospermic control group, non-leukospermic group, and leukospermic group. b Effect of lipopolysaccharide on sperm malondialdehyde levels in selected sperm within the three groups.* P < 0.05: significant difference between LPS-stimulated sperms and unstimulated sperms
Fig. 5.
Effect of lipopolysaccharide on carbonyl groups levels. Values are means ± SD a Effect of lipopolysaccharide on sperm carbonyl group levels in neat sperm within the three groups: normozoospermic control group, non-leukospermic group, and leukospermic group. b Effect of lipopolysaccharide on sperm carbonyl group levels in selected sperm within the three groups. c Effect of lipopolysaccharide on sperm carbonyl group levels in seminal plasma within the three groups.* P < 0.05: significant difference between LPS-stimulated sperms and unstimulated sperms
Regarding the non-leukospermic samples, we noted a significant increase of MDA mean values in sperm after LPS stimulation compared to those in unstimulated sperms. This effect was observed in both neat sperm (Fig. 4a) and selected sperm (Fig. 4b) (0.76 ± 0.21 vs 1.09 ± 0.29 nmol MDA/ml, P = 0.01, and 1.03 ± 0.32 vs 1.33 ± 0.38 nmol MDA/ml, P = 0.01, respectively). Moreover, we detected a significant increase of CG levels after LPS stimulation in selected sperm (6.21 ± 1.4 vs 8.63 ± 1.65 nmol CG/106 spermatozoa, P = 0.02) (Fig. 5a) but not in neat sperm and seminal plasma (P < 0.05).
Concerning the leukospermic group, a significant increase in MDA and CG levels was noted only in selected sperm after stimulation with LPS (0.13 ± 0.11 vs 1.32 ± 0.37 nmol MDA/ml, P = 0.03 and 8.18 ± 4.19 vs 13.94 ± 4.67 nmol MDA/ml, P = 0.04, respectively) (Figs. 4b and 5b). In addition, the LPS had a more important and significant effect on MDA levels in the leukospermic group than in the non-leukospermic group (P = 0.009).
Summary statistics results concerning oxidative stress markers (in neat and selected sperm) before and after LPS stimulation are shown in Table 3.
Table 3.
Summary statistics of comparisons between oxidative stress markers in neat and selected sperm before and after LPS stimulation in leukospermic, non-leukospermic, and normozoospermic control groups
| Mean values (± SD) | Normozoospermic control group (n = 13) | Non-leukospermic group (n = 47) | Leukospermic group (n = 13) |
|---|---|---|---|
| Malondialdehyde (MDA) | |||
| Neat sperm | P = 0.2 | P = 0.01 | P = 0.3 |
| Selected sperm | P = 0.2 | P = 0.01 | P = 0.03 |
| Carbonyl groups (CG) | |||
| Neat sperm | P = 0.6 | P = 0.18 | P = 0.7 |
| Selected sperm | P = 0.88 | P = 0.02 | P = 0.04 |
| Seminal plasma | P = 0.37 | P = 0.19 | P = 0.1 |
Significant differences (P values): bold characters
Effect of lipopolysaccharide on acrosome reaction in vitro
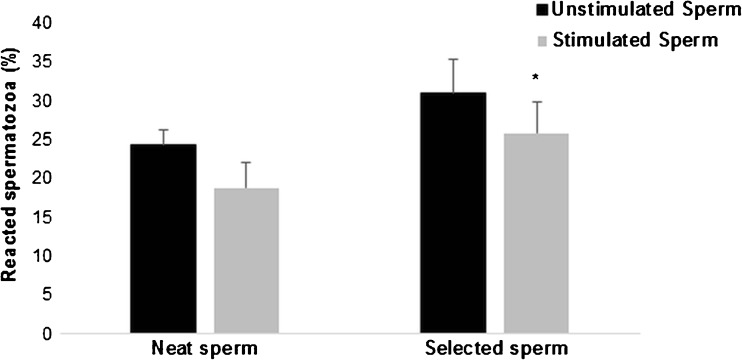
The deleterious effect of LPS on sperms from infertile men was confirmed on acrosome reaction by a significant decrease in the rates of reacted spermatozoa after LPS stimulation compared to unstimulated samples (28.8 ± 4.09 vs 31 ± 4.27%, P = 0.03, respectively) always in selected sperm (Fig. 6).
Fig. 6.
Effect of lipopolysaccharide on sperm acrosome reaction. Values are means ± SD. The effect of lipopolysaccharide on sperm acrosome reaction was assessed in neat and selected sperms from infertile patients (n = 15).* P < 0.05: significant difference between LPS-stimulated sperms and unstimulated sperms
Discussion
To date, the expression of TLR4 has not been widely studied in human sperm. In the present study, we firstly confirmed the expression of TLR4 in human spermatozoa using the western blot technique. Our results are in consistence with the studies of Fujita et al. [22] and Hagan et al. [23] which revealed the expression of this receptor in the flagella and the acrosome of human and mouse spermatozoa. In our study, sperm protein electrophoresis and revelation showed the presence of one band of 69 kDa on selected human spermatozoa. This molecular weight is lower than that reported in regular TLR4, which is 95 kDa. The 69-kDa band could result from the presence of the variant form of TLR4 protein. Indeed, there are previous reports on the possibility of alternative splicing of TLR4 mRNA, which could result in lower molecular weight [36]. Janardhan et al. [37] showed also the expression of TLR4 protein with two different molecular weights 69 and 87 kDa in lungs from the control and LPS-treated rats.
In a second step, we focused on studying the effects of LPS on sperm parameters including sperm motility and vitality. In the infertile men group (from leukospermic and non-leukospermic patients), we noted a non-significant decrease in neat sperm motility and vitality after exposure to LPS compared to those in the non-exposed samples. In contrast, a significant decrease in the motility and vitality was noted after sperm selection. These deleterious effects induced by LPS on selected spermatozoa have been confirmed in other studies showing that the incubation of sperms from infertile patients with the LPS reduced sperm motility, induced sperm apoptosis, and impaired significantly the sperm fertilization potential [22, 38]. In a recent study, Zhongyuan et al. [39] reported that LPS inhibits human sperm motility by decreasing intracellular cAMP involved in LPS-mediated signaling pathways. On the other hand, the intraperitoneal administration of this endotoxin to rats inhibits steroidogenesis via inflammatory mediators such as cytokines and ROS [40]. Furthermore, our results showed that LPS had in the leukospermic group a more important effect on sperm parameters and oxidative stress markers significantly shown in total motility and MDA rates in comparison with the non-leukospermic group. In corroboration with our data, Hagan et al. [23] mentioned that TLR4 expression in spermatozoa and some pro-inflammatory cytokines which have been associated with male infertility were upregulated in sperm samples from leukospermic patients as compared with sperms from non-leukospermic patients. Therefore, the upregulation of TLR4 expression can be associated with the accentuated effect of LPS detected in the leukospermic group.
The difference between results found before and after sperm selection can be explained by the high enzymatic and non-enzymatic antioxidant contents of seminal plasma that would probably mask oxidative stress in neat sperm. In fact, it has been reported that glutathione, ascorbate, and α-tocopherol, in association with other antioxidant enzymes, constitute a protective antioxidant system in the semen and preserve the functional competence of spermatozoa exposed to an oxidative attack [41]. Subtracting these different molecules after sperm selection would have accentuated the oxidative stress effect of LPS on spermatozoa.
To verify this hypothesis, we were interested in a third step of our study to analyze markers of oxidative stress in sperm including lipid peroxidation and oxidative modification of proteins. Thus, we realized MDA and CG assays after sperm exposure to the bacterial LPS. Our results showed that LPS addition induces a significant increase in the MDA levels both in neat and selected sperm. Thereby, this TLR4 ligand appears to play an important role in the activation of some pathways responsible for ROS production leading to the increase of oxidative damage in sperm membrane lipids. The increase in sperm MDA levels is favored by the high polyunsaturated fatty acid content of spermatozoa membrane making them a preferred target of the radical attack [42]. Endotoxin-induced ROS may also result in oxidative DNA damage that could affect sperm quality and increase the risk of genetic effects [43]. Moreover, protein oxidation observed in selected spermatozoa corroborates the hypothesis of ROS production by spermatozoa, which would be responsible for spermatozoa alterations. The presence of high but not significant rates of CG in native sperm and seminal plasma correlates with studies of sperm parameters and confirms the crucial role of seminal plasma in the antioxidant defense against the deleterious effects of ROS.
The deleterious effects of LPS on sperm parameters and oxidative damages detected in sperms from infertile patients but not in the normozoospermic fertile group noted in the current study can be associated with the poor sperm quality of infertile patients which made it more vulnerable than sperms from fertile men since it was already under stress and damaged. In fact, it has been reported that TLRs can also be activated by endogenous “danger signals” called danger-associated molecular patterns (DAMPs), which are released from injured or stressed cells under situations of sterile inflammation [44]. Herein our findings are in discordance with a previous data claiming that sperm motility was decreased in the LPS-treated sperm samples from normozoospermic men and ROS level was significantly higher in the LPS-treated group than in the control group but using doses and kinetic different from ours [45].
Altogether, our data suggests that TLR4 activation by LPS affects negatively sperm quality implicating ROS production especially in sperms from infertile patients. Hence, various sources of ROS production in this study are possible. Firstly, we can mention leukocytes expressing TLR4. Indeed, the lipopolysaccharide can activate the transcription of the iNOS gene in macrophage allowing the production of (NO·) [46, 47]. In addition, activation of macrophage TLR4 can activate the mitochondrial production of ROS and this is via the tumor necrosis receptor-associated factor 6 (TRAF6) protein [48]. Some literature studies have also shown that LPS recognized by TLR4 in monocytes/macrophages plays a role in the production of ROS via activation of the enzyme NADPH oxidase complex [11, 12, 48]. The second source of ROS can be spermatozoa themselves which can also play a role in the production of ROS and that is confirmed by the oxidative damage observed in selected spermatozoa. Indeed, we have demonstrated the presence of oxidative stress damages in human selected spermatozoa in response to stimulation by LPS suggesting ROS production by itself. This production may be effected via the NADPH oxidase enzyme expressed on spermatozoa as the presence of NADPH oxidase in spermatozoa membrane has been reported in many studies [24, 26], but its function is still unknown. Mitochondrial production of ROS via activation of the protein evolutionarily conserved signaling intermediate in Toll pathways (ECSIT) by TLR remains as a very possible hypothesis [49].
The deleterious effect of LPS in sperms from infertile men was also confirmed on acrosome reaction. Our results showed a significant decrease of acrosome-reacted spermatozoa in LPS-treated sperm after spermatozoa selection but not in neat sperm. In contrast, Zhongyuan et al. [39] reported that LPS has no effect on the intracellular [Ca2+]-dependent functions, such as sperm capacitation and acrosome reaction. Capacitation and acrosome reaction involve sperm membrane changes and require perfect membrane integrity. These physiological phenomena are also considered as part of an oxidative process involving superoxide, hydrogen peroxide, and nitric oxide (NO·) [50, 51]. Besides, improving sperm motility at low concentrations, NO· is also known to enhance capacitation and acrosome reaction in mouse and human spermatozoa [52]. However, higher concentrations of l-arginine, a substrate of nitric oxide synthase (NOS) producing NO·, can have adverse effects on motility and fertility of human spermatozoa [53]. Thus, the fact that LPS has a negative effect on acrosome reaction in selected human spermatozoa suggests a higher amount of ROS generated relevant to the action of LPS via TLR4.
On the other hand, increasing data have shown that TLR4 can play an important role in contributing to acute inflammatory processes and organ dysfunction in settings related to critical illness, including sepsis, trauma, hyperoxia, and ischemia reperfusion injury. The results from our study are novel and provide important new insights into signaling pathways involved in the activation of spermatozoa TLRs implicating oxidative stress generation. It is definitely interesting that there is a mechanism related to genital infection and/or inflammation that induces TLR4 sperm activation. This signaling pathway could be responsible for reduction of motility, vitality, acrosome reaction, fertilization potential, and increased ROS production. Nonetheless, fundamental questions remain to be addressed not only in order to explore the pathophysiologic mechanisms that contribute to spermatozoa dysfunction and outcome in critical male infertility but also to search direct therapeutic approaches to ameliorate such TLR4-mediated responses that may potentially be of clinical benefit in critically infertile patients.
In conclusion, our study has made an attempt to explore the effect of LPS on human sperm. Spermatozoa respond to the bacterial endotoxin LPS by affecting negatively the motility, the vitality, and the acrosome reaction notably in sperms from infertile patients. Our study provides additional data on the alteration of these sperm parameters involving oxidative damage, and this could explain some male infertility factors associated with genital infections. Thus, to enhance sperm quality, it seems that TLR4 could serve as target for further development therapeutics using TLR antagonists. Our findings encourage continuing and deepening the study of signaling pathways involved in the activation of spermatozoa TLRs, especially those responsible for oxidative stress generation.
Acknowledgements
The authors thank all the members of the Higher Institute of Biotechnology, Sfax, and Mrs. Olfa Abida, a member of Autoimmunity and Immunogenetics Research Unit, Habib Bourguiba Hospital, Sfax, who generously contributed to this study. We thank also Mr. Mourad Ben Hmida for his helpful review of the manuscript.
Compliance with ethical standards
This study was approved by the Institutional Review Board (IRB) of Medicine Faculty of Sfax, Tunisia. Informed written consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE. History of febrile illness and variation in semen quality. Hum Reprod. 2003;18:2089–2082. doi: 10.1093/humrep/deg412. [DOI] [PubMed] [Google Scholar]
- 2.Nariculam J, Minhas S, Adeniyi A, Ralph DJ, Freeman A. Review of the efficacity of surgical treatment for and pathological changes in patients with chronic scrotal pain. BJU Int. 2007;99:1091–1093. doi: 10.1111/j.1464-410X.2006.06733.x. [DOI] [PubMed] [Google Scholar]
- 3.Brecchia G, Cardinali R, Mourvaki E, Collodel G, Moretti E, Dal Bosco A, et al. Short- and long-term effects of lipopolysaccharide induced inflammation on rabbit sperm quality. Anim Reprod Sci. 2010;118:310–316. doi: 10.1016/j.anireprosci.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Metukuri MR, Reddy CM, Reddy PR, Reddanna P. Bacterial LPS-mediated acute inflammation-induced spermatogenic failure in rats: role of stress response proteins and mitochondrial dysfunction. Inflammation. 2010;33:235–233. doi: 10.1007/s10753-009-9177-4. [DOI] [PubMed] [Google Scholar]
- 5.Winnal WR, Hedger MP. Differential responses of epithelial Sertoli cells of the rat testis to toll-like receptor 2 and 4 ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. J Innate Immun. 2011;17:123–136. doi: 10.1177/1753425909354764. [DOI] [PubMed] [Google Scholar]
- 6.Aly HA, Lightfoot DA, El-Shemy HA. Bacterial lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Toxicol in Vitro. 2010;24:1266–1272. doi: 10.1016/j.tiv.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Nii T, Isobe N, Yoshimura Y. Expression of Toll-like receptors and effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokine in the testis and epididymis of roosters. Poult Sci. 2012;91:1997–2003. doi: 10.3382/ps.2012-02236. [DOI] [PubMed] [Google Scholar]
- 8.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–642. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun SB, Jee HL, Soo HC, Sunah K, Felicidad A, Joseph LW, et al. Macrophages generate reactive oxygen species in response to minimally oxidized LDL: TLR4- and Syk dependent activation of Nox2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NADPH oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 13.Mark P. Toll-like receptors and signaling in spermatogenesis and responses to inflammation-aperspective. J Reprod Immunol. 2011;88:130–141. doi: 10.1016/j.jri.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoulong D, Kun Y, Baolu Z, Yuchang Y, Zhixian W, Jinlong Z, et al. Toll-like receptor 4 promotes NO synthesis by upregulating GCHI expression under oxidative stress conditions in sheep monocytes/macrophages. Oxidative Med Cell Longev. 2015;2015:359315. doi: 10.1155/2015/359315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791–808. doi: 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura M, Naito S. Tissue-specific Mrna expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 18.Saeidi S, Shapouri F, Amirchaghmaghi E, Hoseinifar H, Sabbaghian M, SadighiGilani MA, et al. Sperm protection in the male reproductive tract by Toll-like. Recept Androl. 2014;46:784–790. doi: 10.1111/and.12149. [DOI] [PubMed] [Google Scholar]
- 19.Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod. 2007;76:958–964. doi: 10.1095/biolreprod.106.059410. [DOI] [PubMed] [Google Scholar]
- 20.Palladino MA, Savarese MA, Chapman JL, Dughi MK, Plaska D. Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol. 2008;60:541–555. doi: 10.1111/j.1600-0897.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhushan S, Tchatalbachev S, Klug J, Fijak M, Pineau C, Chakraborty T, et al. Uropathogenic Escherichia coli block MyD88- dependent and activate MyD88 independent signaling pathways in rat testicular cells. J Immunol. 2008;180:5537–5547. doi: 10.4049/jimmunol.180.8.5537. [DOI] [PubMed] [Google Scholar]
- 22.Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C, et al. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod. 2011;26:2799–2806. doi: 10.1093/humrep/der234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagan S, Khurana N, Chandra S, Abdel-Mageed AB, Mondal D, Hellstrom WJG, et al. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology. 2015;3:848–855. doi: 10.1111/andr.12074. [DOI] [PubMed] [Google Scholar]
- 24.Aitken RJ, Fisher HM, Fulton N, Gomez E, Knox W, Lewis B, et al. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenyleneiodonium and quinacrine. Mol Reprod Dev. 1997;47:468–482. doi: 10.1002/(SICI)1098-2795(199708)47:4<468::AID-MRD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, et al. A Ca (2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 26.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, et al. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem. 2012;287:9376–9388. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez E, Buckingham D, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress and sperm function. J Androl. 1996;17:276–287. [PubMed] [Google Scholar]
- 28.Lewis SE, Donnelly ET, Sterling ES, Kennedy MS, Thompson W, et al. Nitric oxide synthase and nitrite production in human spermatozoa: evidence that endogenous nitric oxide is beneficial to sperm motility. Mol Hum Reprod. 1996;2:873–878. doi: 10.1093/molehr/2.11.873. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization . Laboratory manual for the examination and processing of human semen. 5. Geneva: WHO; 2010. [Google Scholar]
- 30.Chandrasekhar A, Laloraya M, Kumar PG. Modulation of nicotinamide adenine dinucleotide phosphate oxidase activity through sequential post translational modifications of p22 phagocytic oxidase during capacitation and acrosome reaction in goat spermatozoa. J Anim Sci. 2011;89:2995–3007. doi: 10.2527/jas.2010-3731. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Buege J, Aust S. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 33.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 34.Kitiyanant Y, Chaisalee B, Pavasuthipaisit K. Evaluation of the acrosome reaction and viability in buffalo spermatozoa using two staining methods: the effects of heparin and calcium ionophore A23187. Int J Androl. 2002;25:215–222. doi: 10.1046/j.1365-2605.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 35.Talbot P, Chacon RS. A triple-stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool. 1981;215:201–208. doi: 10.1002/jez.1402150210. [DOI] [PubMed] [Google Scholar]
- 36.Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: naturally occurring soluble form of mouse toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165:6682–6686. doi: 10.4049/jimmunol.165.12.6682. [DOI] [PubMed] [Google Scholar]
- 37.Janardhan KS, McIsaac M, Fowlie J, Shrivastav A, Caldwell S, Sharma RK, et al. Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation. Histol Histopathol. 2006;21:687–696. doi: 10.14670/HH-21.687. [DOI] [PubMed] [Google Scholar]
- 38.Hosseinzadeh S, Pacey AA, Eley A. Chlamydia trachomatis-induced death of human spermatozoa is caused primarily by lipopolysaccharide. J Med Microbiol. 2003;52:193–200. doi: 10.1099/jmm.0.04836-0. [DOI] [PubMed] [Google Scholar]
- 39.Zhongyuan L, Dahu Z, Yuanqiao H, Zhiyong D, Fei M, Tao L, et al. Lipopolysaccharide compromises human sperm function by reducing intracellular cAMP. Tohoku J Exp Med. 2016;238:105–112. doi: 10.1620/tjem.238.105. [DOI] [PubMed] [Google Scholar]
- 40.Gow RM, O’Bryan MK, Canny BJ, Ooi GT, Hedger MP. Differential effects of dexamethasone treatment on lipopolysaccharide induced testicular inflammation and reproductive hormone inhibition in adult rats. J Endocrinol. 2001;168:193–201. doi: 10.1677/joe.0.1680193. [DOI] [PubMed] [Google Scholar]
- 41.Ochsendorf FR, Buhl R, Bastlein A, Beschmann H. Glutathione in spermatozoa and seminal plasma of infertile men. Hum Reprod. 1998;13:353–359. doi: 10.1093/humrep/13.2.353. [DOI] [PubMed] [Google Scholar]
- 42.Jones R, Mann T, Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979;31:531–537. doi: 10.1016/S0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 43.Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urata K, Narahara H, Tanaka Y, Egashira T, Takayama F, Miyakawa Effect of endotoxin-induced reactive oxygen species on sperm motility. Fertil Steril. 2001;76:163–166. doi: 10.1016/S0015-0282(01)01850-7. [DOI] [PubMed] [Google Scholar]
- 45.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christopher A, Anthony R, Tricia A, Scott H, Joseph E N. LPS-stimulated RAW 264.7 macrophage inducible nitric oxide synthase (iNOS) and nitric oxide production is decreased by an omega-3 fatty acid lipid emulsion. J Surg Res. 2008;149:296–302. doi: 10.1016/j.jss.2007.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sa T, Cheryl LS, Elebeoba EM. Investigating the role of TNF-α and IFN-γ activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS One. 2016;11(6):e0153289. doi: 10.1371/journal.pone.0153289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signaling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cloonan SM, Choi AM. Mitochondria: sensors and mediators of innate immune receptor signaling. Curr Opin Microbiol. 2013;16:327–338. doi: 10.1016/j.mib.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Lamirande E, Lamothe G, Villemure M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic Biol Med. 2009;46:1420–1427. doi: 10.1016/j.freeradbiomed.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Griveau JF, Renard P, Le Lannou D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int J Androl. 1994;17:300–307. doi: 10.1111/j.1365-2605.1994.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 52.Revelli A, Costamagna C, Moffa F, Aldieri E, Ochetti S, Bosia A, et al. Signaling pathway of nitric oxide-induced acrosome reaction in human spermatozoa. Biol Reprod. 2001;64:1708–1712. doi: 10.1095/biolreprod64.6.1708. [DOI] [PubMed] [Google Scholar]
- 53.Rosselli M, Dubey RK, Imthurn B, Macas E, Keller PJ. Effects of nitric oxide on human spermatozoa: evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum Reprod. 1995;10:1786–1790. doi: 10.1093/oxfordjournals.humrep.a136174. [DOI] [PubMed] [Google Scholar]