Abstract
Purpose
Recently, we established a noninvasive system for selecting human blastocysts with a high pre-transfer implantation potential based on first and second division patterns. The present study was carried out to improve the selection system.
Methods
Embryos that completed first and second divisions within 25.90 and 37.88 h after culture, respectively, were selected using a time-lapse incubator. We examined the effects of compaction and blastocyst formation times on pregnancy rates after transferring these embryos at the blastocyst stage.
Results
The completion of compaction and blastocyst formation times (79.93 and 97.47 h after culture, respectively) of embryos resulting in pregnancies after transfer were significantly (P < 0.01) shorter than those (86.46 and 100.34 h after culture, respectively) of embryos that failed to induce pregnancies. Embryo selection based on completion of compaction time improved pregnancy rates (40.9 vs. 74.6%, P < 0.01).
Conclusions
Of the embryos that formed two cells during the first division within 25.90 h after culture and four cells during the second division within 37.88 h after culture, those that completed compaction within 79.93 h after culture before reaching the blastocyst stage had a high implantation potential.
Keywords: Compaction, Morula, Single blastocyst transfer, Time-lapse incubator
Introduction
The time-lapse incubator, EmbryoScope (VitroLife), which includes a built-in microscope and charge-coupled device camera, has attracted considerable interest in recent years [1–4]. This incubator allows monitoring of embryonic development at all times without removing the embryos. This advantage may enhance the development of embryos into blastocysts by lessening the risks of stress from temperature changes, light exposure, exposure to high oxygen concentrations, and pH changes in the culture medium. In addition, more information can be obtained by analyzing the embryonic growth at various time points rather than morphological observations at single time points.
In assisted reproductive technologies (ARTs), multiple pregnancies have been a serious problem [5, 6]. This problem is related to the number of embryos transferred. Accordingly, the Japan Society of Obstetrics and Gynecology recommended single embryo transfer (ET) in 2008 to reduce fetal and maternal risks. Therefore, avoiding multiple pregnancies without decreasing pregnancy rates is important, and the transfer of a single blastocyst can resolve this issue. To obtain blastocysts for transfer, embryos need to be cultured in vitro for 5–6 days. High-quality embryos can be selected during in vitro culture because embryos that are unable to develop into blastocysts are eliminated. However, when no embryos develop to the blastocyst stage in vitro, blastocyst transfer is ineffective. In the early stage of ET (day 2 or 3), high-quality embryos must be selected without affecting their developmental potential into blastocysts. Therefore, various studies have attempted to establish methods for selecting high-quality embryos with a focus on the division speed and morphology of early stage embryos [7–10]. The time-lapse incubator has played an important part in the detailed observation of embryonic development. Specifically, it has enabled the clarification of the relationships between early cleavage patterns and the developmental potential of embryos. Studies have found correlations between the time from fertilization to the first division and number of blastomeres, and the implantation potential of embryos on day 2 [11]. In addition, correlations have been observed between the first and second division times and the implantation potential of embryos on day 3 [12], as well as between the synchrony of second and third divisions and the formation rate of high-quality blastocysts [13]. Furthermore, the synchrony of the second division has been shown to correlate with the formation rate of blastocysts [14]. In addition, many studies have reported that the first division time of embryos affects pregnancy rates after transfer [15–20], although some studies have shown the opposite result [21–23].
In a previous study [24], embryos that formed two cells during the first division and four cells during the second division, regardless of the presence or absence of fragmentation, and completed first and second divisions within 25.90 and 37.88 h after culture, respectively, were selected as high-quality embryos. Interestingly, the transfer of these embryos on day 2 or 3 did not affect the clinical pregnancy rates. However, when these embryos were cultured in vitro and transferred at the blastocyst stage, clinical pregnancy rates improved. These results clearly indicate that in vitro culture of the selected embryos and transfer of the resultant blastocysts on day 5 is necessary to significantly elevate pregnancy rates. However, many studies in which embryos with a high developmental ability were selected based on early cleavage patterns have suggested that the transfer goal should be on day 2 or 3 ET [8, 9, 12, 16, 17, 19, 20]. However, the clinical pregnancy rate was limited to 55.8% even when high-quality embryos were transferred at the blastocyst stage, indicating that additional criteria for the selection of embryos with a high implantation potential are necessary.
In the present study, we sought to improve the noninvasive selection system of human embryos with a high implantation potential before transfer established in the previous study. Therefore, we evaluated the relationship between the first and second divisions and the compaction and blastocyst formation times of the selected embryos and pregnancy rates after transfer.
Materials and methods
Patients
All study participants provided written informed consent, and the study design was approved by the Ethics Committee of the Hospital. We examined the outcome of 299 (243 patients) of the embryos produced using ART between May 2014 and June 2016 that had formed two and four cells during the first and second divisions, respectively, regardless of the presence or absence of fragmentation, and had been transferred at the blastocyst stage based on a previous study [24]. The mean age ± standard deviation of patients at the retrieval cycles was 37.0 ± 4.0 years.
Production, culture, and transfer of embryos
The ovaries were stimulated using the gonadotropin-releasing hormone analog buserelin acetate (Fuji Pharmaceutical) and the short or long protocol together with follicle-stimulating hormone and human menopausal gonadotrophin (ASUKA Pharmaceutical) stimulation. Human chorionic gonadotropin (Fuji Pharmaceutical) or leuprolide was administered when the maximum diameter of two or more follicles reached 18 mm. The cumulus-oocyte complexes were retrieved using ultrasound-guided transvaginal follicle aspiration approximately 36 h after the human chorionic gonadotropin injection. The oocytes were fertilized using conventional in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), or the split cycle using standard techniques. For the split cycle, the oocytes were randomized to conventional IVF or ICSI. Conventional IVF embryos were loaded into an EmbryoScope 4–5 h after insemination, while the ICSI embryos were cultured in the EmbryoScope immediately after microinjection. The microinjection and insemination times were defined as the start of culture for ICSI and conventional IVF embryos, respectively. Embryos were cultured in One Step Medium (NAKA Medical) covered with sterile mineral oil at 37 °C exposed to 5% CO2, 5% O2, and 90% N2 for 5 or 6 days. After culturing, the blastocysts were cryopreserved using a Cryotop® (Kitazato) [25, 26]. After thawing, single cryopreserved blastocyst transfer (299 retrieval cycles) was performed during the hormone replacement therapy cycles. Clinical pregnancy rates were determined according to the presence of a gestational sac, as visualized using ultrasound at week 3.
Experimental design
Experiment 1
To confirm the high implantation potential of embryos cleaving early to the two- and four-cell stages, 299 embryos were divided into two groups, consisting of embryos that completed the first and second divisions within 25.90 and 37.88 h after culture, respectively (early cleaved embryos), and the remaining embryos. As described in a previous study, the time point when one of the two cells divided was regarded as the second division time. Only when two cells had divided almost simultaneously (within 1 h), they were classified as a four-cell formation. For example, embryos were excluded when one of the two cells had divided to form three cells and, a few hours later, the other original cells had divided to form four cells [24]. We compared the clinical pregnancy rates after transfer of blastocysts derived from the embryos between the groups.
Experiment 2
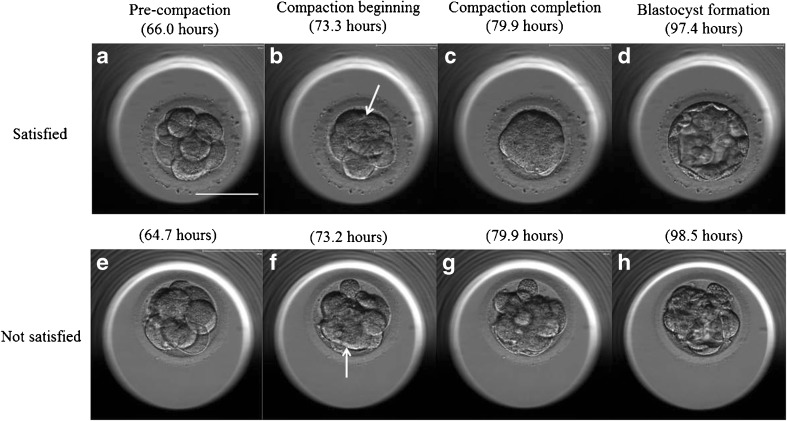
One-hundred and eighty-two early cleaved embryos were divided into two groups, consisting of embryos that resulted in pregnancies after transfer and the other embryos. We compared the first and second division times, the beginning and completion of compaction, and blastocyst formation between the groups. After several cell divisions (Fig. 1a, e), the blastomeres became flattened. The time point when the intercellular boundaries became obscured somewhere in the embryo (i.e., blastomeres are arranged tightly, and some or all of them start to “merge,” but individual cells are still easily distinguishable; Fig. 1b, f) was regarded as the beginning of the compaction time. The duration until blastomeres finally unified into one cluster (i.e., blastomeres are completely “merged” together, and the individual cell boundaries cannot be easily identified, but the nuclei are visible and intercellular space disappears) was regarded as the compaction completion time (Fig. 1c, g). The time point when the embryo developed into a blastocyst was scored as 3 in the Gardner classification [27] (i.e., a formed blastocoele increases in volume and fills the embryo completely) and was regarded as the blastocyst formation time (Fig. 1d, h).
Fig. 1.
In vitro development of a–d “satisfied” and e–h “not satisfied” embryos. a, e Pre-compaction, b, f compaction beginning, c compaction completion, d blastocyst, g incomplete compaction, and h incomplete blastocyst. Culture times are shown in each picture. The arrows show the obscured intercellular boundaries by the merging of blastomeres. Scale bar 100 μm
Experiment 3
We examined the effects of compaction completion, blastocyst formation times, or both on the clinical pregnancy of early cleaved embryos. The average compaction completion time and blastocyst formation of embryos that resulted in pregnancies after the transfer was 79.93 and 97.47 h, respectively, in experiment 2. Therefore, embryos that completed compaction within 79.93 h after culture or developed into blastocysts within 97.47 h after culture or both were transferred, and pregnancy rates were compared with those obtained by transferring the other embryos.
Statistical analysis
The statistical analysis was performed using the chi-squared (χ 2) test with continuity correction (experiments 1 and 3) or Kruskal-Wallis analysis of variance (experiment 2). A P < 0.05 was considered statistically significant.
Results
Experiment 1
As shown in Table 1, the pregnancy rate after transfer of early cleaved embryos (53.3%) was significantly (P < 0.05) higher than that of the other embryos (39.3%) was. There was no significant difference in the rates of deliveries or ongoing pregnancies (69.6 vs. 77.3%) between the groups.
Table 1.
Effects of embryo selection based on first and second division times on clinical pregnancy after single blastocyst transfer
| Criteriona | Embryos transferred, n | Pregnancies, n (%) | Deliveries or ongoing pregnancies, n b (%)c |
|---|---|---|---|
| Satisfied | 182 | 97 (53.3)d | 75 [68 + 7] (77.3) |
| Not satisfied | 117 | 46 (39.3)e | 32 [29 + 3] (69.6) |
aEmbryos that formed two and four cells during the first and second divisions within 25.90 and 37.88 h, respectively, were regarded as satisfied embryos
bThe numbers of deliveries (first) and ongoing pregnancies (second) are indicated in brackets
cPercentage per pregnancy
dValues with different superscript letters are significantly different (P < 0.05)
eValues with different superscript letters are significantly different (P < 0.05)
Experiment 2
As shown in Table 2, there were no significant differences in the first division (23.53 vs. 23.62 h), second division (35.07 vs. 35.15 h), and the beginning of compaction (73.36 vs. 74.10 h) times between the groups. However, the compaction completion and blastocyst formation times (79.93 and 97.47 h, respectively) of embryos resulting in pregnancies after transfer were significantly (P < 0.01) shorter than those (86.46 and 100.34 h, respectively) of embryos that failed to induce pregnancies.
Table 2.
Effects of cell division, compaction, and blastocyst formation times on clinical pregnancy after single blastocyst transfer
| Pregnancy | Embryos transferreda, n | Time (h), mean ± SD | ||||
|---|---|---|---|---|---|---|
| First division | Second division | Compaction beginning | Compaction completion | Blastocyst formation | ||
| + | 97 | 23.62 ± 1.59 | 35.15 ± 2.24 | 73.36 ± 6.83 | 79.93 ± 7.19b | 97.47 ± 6.02b |
| − | 85 | 23.53 ± 1.67 | 35.07 ± 1.95 | 74.10 ± 6.44 | 86.46 ± 7.30c | 100.34 ± 7.94c |
aEmbryos that formed two and four cells during the first and second divisions within 25.90 and 37.88 h, respectively, were selected
bValues with different superscript letters in each column are significantly different (P < 0.01)
cValues with different superscript letters in each column are significantly different (P < 0.01)
Experiment 3
As shown in Table 3, the pregnancy rate after transfer of embryos that completed compaction within 79.93 h after culture (74.6%) was significantly (P < 0.01) higher than that of the other embryos (40.9%). Similarly, the pregnancy rate after transfer of embryos that completed compaction within 79.93 h after culture and developed into blastocysts within 97.47 h after culture (73.5%) was significantly (P < 0.01) higher than that of the other embryos (45.9%) was. In contrast, the pregnancy rate after transfer of embryos that developed into blastocysts within 97.47 h after culture (60.2%) was not different from that of the other embryos (47.5%). Embryo selection based on compaction completion, blastocyst formation times, or both did not affect the rates of deliveries or ongoing pregnancies (74.0 vs. 80.9, 76.6 vs. 78.0, and 75.0 vs. 78.7%, respectively).
Table 3.
Effects of embryo selection based on compaction completion, blastocyst formation times, or both on clinical pregnancy after single blastocyst transfer
| Criterion | Outcome | Satisfied | Not satisfied |
|---|---|---|---|
| Compaction completed within 79.93 h | Embryos transferred, n | 67 | 115 |
| Pregnancies, n (%) | 50 (74.6)c | 47 (40.9)d | |
| Deliveries or ongoing pregnancies, n a (%)b | 37 [34 + 3] (74.0) | 38 [34 + 4] (80.9) | |
| Blastocyst formed within 97.47 h | Embryos transferred, n | 83 | 99 |
| Pregnancies, n (%) | 50 (60.2) | 47 (47.5) | |
| Deliveries or ongoing pregnancies, n a (%)b | 39 [36 + 3] (78.0) | 36 [32 + 4] (76.6) | |
| Compaction completed and blastocyst formed within 79.93 and 97.47 h, respectively | Embryos transferred, n | 49 | 133 |
| Pregnancies, n (%) | 36 (73.5)c | 61 (45.9)d | |
| Deliveries or ongoing pregnancies, n a (%)b | 27 [25 + 2] (75.0) | 48 [43 + 5] (78.7) |
aThe number of deliveries (first) and ongoing pregnancies (second) are indicated in brackets
bPercentage per pregnancy
cValues with different superscript letters within each row are significantly different (P < 0.01)
dValues with different superscript letters within each row are significantly different (P < 0.01)
Discussion
In the present study, embryos that formed two and four cells during the first and second divisions within 25.90 and 37.88 h, respectively, were selected. After reaching the blastocyst stage, the embryos were cryopreserved and then thawed for single blastocyst transfer during hormone replacement therapy cycles. The present study showed that selecting embryos that completed compaction within 79.93 h after culture from early cleaved embryos and transferring them at the blastocyst stage led to high pregnancy rates.
The results of the present study confirm previous findings showing that embryos that cleaved early to the two- and four-cell stages had a high implantation potential, but the clinical pregnancy rate was limited to approximately 50% even when these embryos were transferred [24]. Initially, we expected that selecting embryos that cleave earlier would improve pregnancy rates. However, the first and second division times of embryos resulting in pregnancies were not different from those of embryos that failed to induce pregnancies, indicating that other criteria for embryo selection are necessary to improve pregnancy rates.
After some cellular divisions in the initial stages of embryonic development, the intercellular boundaries are obscured in a process called compaction, which maximizes the intercellular contact and forms the morula. The cell-cell adhesion protein E-cadherin is first expressed during compaction, enabling the cells to adhere more tightly [28–32]. Although the compaction of embryos has not received sufficient attention in ART, some studies have focused on the relationships between the compaction patterns of embryos and their developmental potential. Embryos that begin compaction before the eight-cell stage exhibit aberrant in vitro development [33]. Conversely, embryos that complete compaction on day 5 have a lower ability to develop into high-quality blastocysts than those that compact on day 4 do [34]. In addition, the compaction patterns of embryos affect pregnancy rates after transfer [35, 36]. These studies suggest that the compaction patterns of embryos can facilitate the prediction of their developmental ability in vitro and in vivo. Therefore, we hypothesized that the compaction time is a useful criterion for selection of embryos with a high implantation potential. The results of the present study showed that the beginning of compaction time of embryos resulting in pregnancies was not different from that of embryos that failed to induce pregnancies. This observation indicates that embryos with a high implantation potential cannot be selected based on this criterion. In contrast, the compaction completion time of the former is significantly shorter than that of the latter. In addition, the selection of embryos that completed compaction within 79.93 h after culture and transferring them at the blastocyst stage improved the pregnancy rate. These results indicate that there is a correlation between the time from the beginning to completion of compaction and the implantation potential of embryos. This finding is in agreement with the results of previous studies where the compaction patterns of embryos affected the rates of good quality blastocyst formation and implantation [33, 35, 36]. In contrast, some studies have reported that the compaction time of embryos does not affect clinical pregnancy rates [37, 38].
Skiadas et al. [36] reported that IVF or ICSI embryos that completed compaction at 68–72 h after insemination and exhibited <10% fragmentation result in a high pregnancy rate (47%) after transfer on day 3. However, this criterion may not be practical because the percentage of satisfied embryos was only 4.5% of the transferred embryos. In the present study, 36.8% of transferred embryos satisfied the criterion. Nevertheless, the pregnancy rate after transferring the satisfied embryos was also remarkably high (74.6%). This improvement may be attributed to three factors. First, we selected embryos that completed compaction within 79.93 h after culture. At 68–72 h after insemination, 53.5–60.1% of the transferred embryos were at the partial compaction stage in which some membrane fusion is evident, but the number of blastomeres is easily countable [36]. Most likely, some of these embryos completed compaction by 79.93 h after culture. Secondly, we selected embryos that not only completed compaction early but also cleaved early to the two- and four-cell stages. According to Skiadas et al. [36], 20–33% of embryos in the partial compaction stage at 68–72 h after insemination implant after transfer. The additional criterion might facilitate the selection of embryos with a high implantation potential from slowly compacting embryos. Finally, we transferred selected embryos at the blastocyst stage on day 5 or 6. This procedure significantly improved pregnancy rates after transferring the selected embryos based on early cleavage patterns compared with transfers on day 2 or 3 in our previous study [24]. Our embryo selection system would be useful for increasing the number of successful pregnancies from IVF or ICSI embryos.
It is unclear why the transfer of embryos that complete compaction early results in high pregnancy rates. Compaction is accompanied by functional changes resulting from the shift in the gene transcription profile from the maternal to the zygotic genome. These functional changes lead to differentiation of the cells into the inner cell mass and trophectoderm, which is essential for blastocyst formation [33]. A previous study showed the association between delayed compaction of embryos and a smaller inner cell mass volume in developed blastocysts [34]. This finding suggests that delayed compaction reduces the quality of embryos, thereby resulting in lower pregnancy rates. The selection of embryos that complete compaction early may prevent the transfer of low-quality embryos. The reason for delays in the compaction process is also unclear. A normal cell cycle plays an important role in normal embryonic development [13]. External factors due to in vitro culturing may have altered the cell cycle, resulting in delayed embryonic development. Most embryos in which compaction occurred during the four- to seven-cell stage contained multinucleated blastomeres and failed to undergo cytokinesis [33]. Good quality blastocysts were not obtained from these embryos. Therefore, abnormal cell cycle, multinucleated blastomeres, and cytokinetic failure may be also involved in the delayed compaction completion of embryos.
In the present study, the blastocyst formation time of embryos resulting in pregnancies was also shorter than that of embryos that failed to induce pregnancies, but the selection of embryos based on blastocyst formation time did not improve the pregnancy rate. Although the compaction completion time of embryos that result in pregnancies was 6.53 h shorter than that of embryos that failed to induce pregnancies, the difference in the blastocyst formation times between the groups was 2.87 h. These results indicate that the blastocyst formation of embryos that completed compaction early was delayed or that embryos completing compaction tardily develop early into blastocysts or both. Therefore, low-quality embryos may have also been transferred when embryos were selected based on blastocyst formation time.
In conclusion, we established a noninvasive selection system for human blastocysts with a high implantation potential before transfer based on early cleavage and compaction patterns.
Acknowledgements
The authors thank their fellow embryologist’s doctors and nursing staff at Aiiku Ladies Clinic (Kagoshima, Japan) for their technical assistance and support.
Compliance with ethical standards
Funding declaration
The studied received no external funding.
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants
This study was approved by the Institutional Review Board of AIIKUKAI Medical Corporation.
Informed consent
Written informed consent for their treatment and for their outcomes to be described was obtained from all patients.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and National Research Committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical considerations
All study participants provided informed consent, and the study design was approved by the appropriate ethics review boards. Written informed consent for their treatment and outcomes to be described was obtained from all patients.
References
- 1.Kirkegaard K, Juhl Hindkjaer J, Ingerslev HJ. Human embryonic development after blastomere removal: a time-lapse analysis. Hum Reprod. 2012;27:97–105. doi: 10.1093/humrep/der382. [DOI] [PubMed] [Google Scholar]
- 2.Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–1285. doi: 10.1093/humrep/des079. [DOI] [PubMed] [Google Scholar]
- 3.Cruz M, Gadea B, Garrido N, Pedersen KS, Martínez M, Pérez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28:569–573. doi: 10.1007/s10815-011-9549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio I, Galán A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102:1287–1294. doi: 10.1016/j.fertnstert.2014.07.738. [DOI] [PubMed] [Google Scholar]
- 5.Pinborg A, Loft A, Schmidt L, Andersen AN. Morbidity in a Danish national cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Hum Reprod. 2003;18:1234–1243. doi: 10.1093/humrep/deg257. [DOI] [PubMed] [Google Scholar]
- 6.Pinborg A, Loft A, Rasmussen S, Schmidt L, Langhoff-Roos J, Greisen G, Andersen AN. Neonatal outcome in a Danish national cohort of 3438 IVF/ICSI and 10,362 non-IVF/ICSI twins born between 1995 and 2000. Hum Reprod. 2004;19:435–441. doi: 10.1093/humrep/deh063. [DOI] [PubMed] [Google Scholar]
- 7.Milki AA, Hinckley MD, Fisch JD, Dasig D, Behr B. Comparison of blastocyst transfer with day 3 embryo transfer in similar patient populations. Fertil Steril. 2000;73:126–129. doi: 10.1016/S0015-0282(99)00485-9. [DOI] [PubMed] [Google Scholar]
- 8.Dennis SJ, Thomas MA, Williams DB, Robins JC. Embryo morphology score on day 3 is predictive of implantation and live birth rates. J Assist Reprod Genet. 2006;23:171–175. doi: 10.1007/s10815-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MJ, Lee RKK, Lin MH, Hwu YM. Cleavage speed and implantation potential of early-cleavage embryos in IVF or ICSI cycles. J Assist Reprod Genet. 2012;29:745–750. doi: 10.1007/s10815-012-9777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–419. doi: 10.1016/j.fertnstert.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod BioMed Online. 2008;17:385–391. doi: 10.1016/S1472-6483(10)60222-2. [DOI] [PubMed] [Google Scholar]
- 12.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remoh J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly(dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–337. doi: 10.1016/j.fertnstert.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Cruz M, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod BioMed Online. 2012;25:371–381. doi: 10.1016/j.rbmo.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Kirkegaard K, Sundvall L, Erlandsen M, Hindkjær JJ, Knudsen UB, Ingerslev HJ. Timing of human preimplantation embryonic development is confounded by embryo origin. Hum Reprod. 2016;31:324–331. doi: 10.1093/humrep/dev296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoukir Y, Campana A, Farley T, Sakkas D. Early cleavage of in-vitro fertilized human embryos to the 2-cell stage: a novel indicator of embryo quality and viability. Hum Reprod. 1997;12:1531–1536. doi: 10.1093/humrep/12.7.1531. [DOI] [PubMed] [Google Scholar]
- 17.Sakkas D, Shoukir Y, Chardonnens D, Bianchi PG, Campana A. Early cleavage of human embryos to the two-cell stage after intracytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod. 1998;13:182–187. doi: 10.1093/humrep/13.1.182. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick J, Platteau P, Murdoch AP, Herbert M. Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Hum Reprod. 2002;17:407–412. doi: 10.1093/humrep/17.2.407. [DOI] [PubMed] [Google Scholar]
- 19.Salumets A, Hydén-Granskog C, Mäkinen S, Suikkari AM, Tiitinen A, Tuuri T. Early cleavage predicts the viability of human embryos in elective single embryo transfer procedures. Hum Reprod. 2003;18:821–825. doi: 10.1093/humrep/deg184. [DOI] [PubMed] [Google Scholar]
- 20.Giorgetti C, Hans E, Terriou P, Salzmann J, Barry B, Chabert-Orsini V, et al. Early cleavage: an additional predictor of high implantation rate following elective single embryo transfer. Reprod BioMed Online. 2007;14:85–91. doi: 10.1016/S1472-6483(10)60768-7. [DOI] [PubMed] [Google Scholar]
- 21.Emiliani S, Fasano G, Vandamme B, Vannin AS, Verdoodt M, Biramane J, et al. Impact of the assessment of early cleavage in a single embryo transfer policy. Reprod BioMed Online. 2006;13:255–260. doi: 10.1016/S1472-6483(10)60623-2. [DOI] [PubMed] [Google Scholar]
- 22.Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;12:54. doi: 10.1186/1477-7827-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman RH, Kaser DJ, Missmer SA, Srouji SS, Farland LV, Racowsky C. Building a model to increase live birth rate through patient-specific optimization of embryo transfer day. J Assist Reprod Genet. 2016;33:1525–1532. doi: 10.1007/s10815-016-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizobe Y, Oya N, Iwakiri R, Yoshida N, Sato Y, Miyoshi K, et al. Effects of early cleavage patterns of human embryos on subsequent in vitro development and implantation. Fertil Steril. 2016;106:348–353. doi: 10.1016/j.fertnstert.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod BioMed Online. 2005;11:300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 26.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 28.Nikas G, Ao A, Winston RM, Handyside AH. Compaction and surface polarity in the human embryo in vitro. Biol Reprod. 1996;55:32–37. doi: 10.1095/biolreprod55.1.32. [DOI] [PubMed] [Google Scholar]
- 29.Fleming TP, Sheth B, Fesenko I. Cell adhesion in the preimplantation mammalian embryo and its role in trophectoderm differentiation and blastocyst morphogenesis. Front Biosci. 2001;6:D1000–D1007. doi: 10.2741/A662. [DOI] [PubMed] [Google Scholar]
- 30.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell CE, Calder MD, Watson AJ. Genomic RNA profiling and the programme controlling preimplantation mammalian development. Mol Hum Reprod. 2008;14:691–701. doi: 10.1093/molehr/gan063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alikani M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum Reprod. 2005;20:3369–3375. doi: 10.1093/humrep/dei242. [DOI] [PubMed] [Google Scholar]
- 33.Iwata K, Yumoto K, Sugishima M, Mizoguchi C, Kai Y, Iba Y, et al. Analysis of compaction initiation in human embryos by using time-lapse cinematography. J Assist Reprod Genet. 2014;31:421–426. doi: 10.1007/s10815-014-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivec M, Kovacic B, Vlaisavljevic V. Prediction of human blastocyst development from morulas with delayed and/or incomplete compaction. Fertil Steril. 2011;96:1473–1478. doi: 10.1016/j.fertnstert.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Tao J, Tamis R, Fink K, Williams B, Nelson-White T, Craig R. The neglected morula/compact stage embryo transfer. Hum Reprod. 2002;17:1513–1518. doi: 10.1093/humrep/17.6.1513. [DOI] [PubMed] [Google Scholar]
- 36.Skiadas CC, Jackson KV, Racowsky C. Early compaction on day 3 may be associated with increased implantation potential. Fertil Steril. 2006;86:1386–1391. doi: 10.1016/j.fertnstert.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 37.Chamayou S, Patrizio P, Storaci G, Tomaselli V, Alecci C, Ragolia C, et al. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet. 2013;30:703–710. doi: 10.1007/s10815-013-9992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkegaard K, Kesmodel US, Hindkjær JJ, Ingerslev HJ. Time-lapse parameters as preductors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–2651. doi: 10.1093/humrep/det300. [DOI] [PubMed] [Google Scholar]