Abstract
Endometriosis is an inflammatory condition that is associated with progesterone resistance and cell proliferation, resulting in pain, infertility and pregnancy loss. We previously demonstrated phosphorylation of STAT3 in eutopic endometrium of infertile women with this disorder leading to over-expression of the oncogene BCL6 and stabilization of hypoxia-induced factor 1 alpha (HIF-1α). Here we report coordinated activation of KRAS and over-expression of Sirtuin 1 (SIRT1), a histone deacetylase and gene silencer, in the eutopic endometrium from women with endometriosis throughout the menstrual cycle. The mice with conditional activation of KRAS in the PGR positive cells reveal an increase of SIRT1 expression in the endometrium compared to control mice. The expression of progesterone receptor target genes including the Indian Hedgehog pathway genes are significantly down-regulated in the mutant mice. SIRT1 co-localizes with BCL6 in the nuclei of affected individuals and both proteins bind to and suppress the promoter of GLI1, a critical mediator of progesterone action in the Indian Hedgehog pathway, by ChIP analysis. In eutopic endometrium, GLI1 expression is reduced in women with endometriosis. Together, these data suggest that KRAS, SIRT1 and BCL6 are coordinately over-expressed in eutopic endometrium of women with endometriosis and likely participate in the pathogenesis of endometriosis.
Introduction
Endometriosis is a gynecologic disorder defined by the presence of endometrial cells outside of the uterine cavity. Endometriosis adds significantly to health care costs, upwards of $22 billion dollars per year in the US1. Symptoms of endometriosis vary widely and include dysmenorrhea, dyspareunia, noncyclic chronic pelvic pain, and infertility, with a considerable negative impact on quality of life1, 2. Endometriosis is associated with both infertility and pelvic pain and affects about 5% of reproductive-age women and up to 50% of these are infertile3, 4. Surgical removal of ectopic lesions and/or hormonal suppression focused on reducing estrogen, such as progestins, androgens, gonadotropin-releasing hormone (GnRH) agonists, and aromatase inhibitors are the current gold standards of therapy. However, both approaches are associated with various side effects and a high incidence of relapse5. Therefore, identification of mechanisms involved in the pathogenesis of endometriosis and strategic therapies for treatment remain critical.
The eutopic endometrium of women with endometriosis exhibits inflammation, aberrant estrogen activity, cellular proliferation and a resistance to progesterone (P4)5. The biological mechanisms linking endometriotic lesions to these endometrial alterations remains uncertain and controversial, while P4 resistance and estrogen dominance likely contributes to the pathophysiology and survival of ectopic lesion and contributes to infertility6, 7. KRAS has been proposed as a strong candidate gene in the pathophysiology of endometriosis. Activation of KRAS in mice was associated with endometriosis-like lesions on the peritoneum and ovaries8 and lesions derived from mice with activated KRAS mutation survived longer in wild type mice9. While there is no direct link between KRAS mutations and the risk for endometriosis in humans10, inflammation associated events including changes in miRNA expression in endometriosis11, may play a role in its activation12. We previously showed that miRNA34b was dramatically decreased in eutopic endometrium of women with endometriosis13. This miRNA has been shown to have benefit in KRAS induced mouse models of other carcinomas14. Both let-7b and miRNA 34 have been shown to target KRAS15, and both miR34 and p53 can act synergistically to suppress tumor growth16.
BCL6 (B Cell Lymphoma 6) is a transcriptional gene repressor and is necessary for B cell development and oncogenesis17. BCL6 has six Krüppel-type zinc finger domains and a BTB/POZ (bric-á-brac, tramtrack, broad complex/pox virus zinc finger) domain, which can bind to transcriptional factors, including Interferon Regulatory Factor (IRF) 4 and BCL6-associated zinc finger (BAZF)18. BCL6 is one of the human proto-oncogenes and is associated with an increase in cell proliferation through the repression of genes such as p53 and p30019. BCL6 DNA binding site (TTCCT(A/C)GAA) is similar with Signal Transduction and Activators of Transcription (STAT) factors and BCL6 can repress transcription via STAT factor binding sites and thus inhibit cytokine-induced transcription20. Furthermore, BCL6 is up-regulated by STAT 321. STAT3 signaling is aberrantly activated in eutopic endometrium from women with endometriosis compared to those without this disease22. Recently, we reported that BCL6 is highly over-expressed in endometrium from women with endometriosis during the secretory phase of the menstrual cycle compared to women without endometriosis23.
SIRT1 is a member of the sirtuin family of proteins and homologs to the yeast Sir2 protein. Sirtuin family proteins are Class III HDACs24. SIRT1 can deacetylate both histones and non-histone proteins such as p5325. Its deacetylation activity enables it to regulate gene transcription and implicates the influence of a variety of cellular processes such as aging, apoptosis, inflammation, stress resistance, and metabolism26. SIRT1 has a dual role as oncogenic function as well as tumor suppressor27. According to previous reports, SIRT1 plays a role as a tumor promoter in endometrial cancer by targeting sterol regulatory element binding protein 1 (SREBP1) and lipogenesis28. Additionally, SIRT1 has an important role in the regulation of inflammatory cytokines expression in endometriotic stromal cells29 and SIRT1 has been associated with poor prognosis ovarian cancers30. However, the role of SIRT1 in endometriosis and uterine biology has not been examined.
In this study, we investigated the levels of KRAS and SIRT1 proteins in eutopic endometrium from women with endometriosis. The levels of SIRT1 and KRAS were compared in endometrium of women with and without endometriosis. Using a mouse model, we investigated the potential link between KRAS activation and SIRT1 expression. We report here for the first time in endometrium, direct protein-protein interactions between SIRT1 and BCL6 in human endometrial tissue, co-localizing in the nuclei of endometriosis cases. Further, we show that GLI1, a promoter target for both BCL6 and SIRT1, is reduced in eutopic endometrium of women with this disease. Based on these results we suggest that aberrant overexpression of SIRT1 is driven by KRAS activation, and co-localizes with BCL6 contributing to the phenomenon of P4 resistance through gene inactivation of the GLI1 promoter.
Results
Endometriosis and Inflammation
Endometriosis is the presence of glands and stroma outside the uterus. It is often found on the ovary and peritoneum. Endometriosis is a chronic inflammation disease. To better understand the systemic inflammation status of endometriosis patients, we measured inflammatory cytokines in plasma of women with and without endometriosis using a multi-plex array from Eve Technologies. Our results revealed significant elevations in IL-1a, IL-6 and IL-17, among others (Fig. 1).
Figure 1.
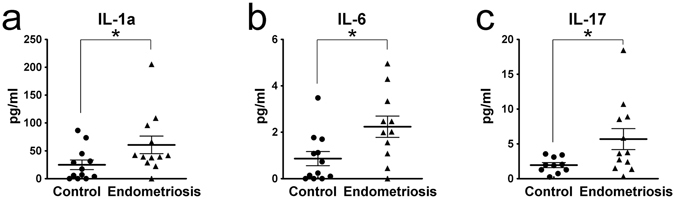
Endometriosis is associated with elevated serum inflammatory cytokines including (a) IL-1a, (b) IL-6 and (c) IL-17, compared to normal controls.
Overexpression of KRAS and SIRT1 in eutopic endometrial tissue from women with endometriosis
IL-6 activates JAK kinases and Ras-mediated signaling. Activation of KRAS, the key regulator of Ras/ERK pathway, in the endometrium of mice causes ectopic lesion establishment9. KRAS appears to be dysregulated in endometriosis. To document this, we first examined the expression of KRAS in endometrium using Western blot, using women with and without the disease. While the expression levels of KRAS did not differ between proliferative (n = 21) and secretory phase (n = 44), the levels of KRAS were significantly higher in the endometrium that originated in subjects with endometriosis (n = 54) as compared to controls (n = 11) (Fig. 2a and b). KRAS activation is associated with overexpression of SIRT131. KRAS activation regulates epithelial-mesenchymal transition and cell migration through SIRT132. To better understand the finding of increased KRAS in the endometrium of women with endometriosis, we investigated the association between KRAS activation and SIRT1 expression. The levels of SIRT1 protein were significantly increased in the samples from women with endometriosis (n = 54) compared with controls (n = 11) (Fig. 2a and b). However, SIRT1 expression was low and unchanged during the menstrual cycle in the control group. Figure 2c showed a significant positive correlation between KRAS and SIRT1 proteins in the endometrium of the control and endometriosis group (Spearman correlation coefficient r = 0.6155, p < 0.0001).
Figure 2.
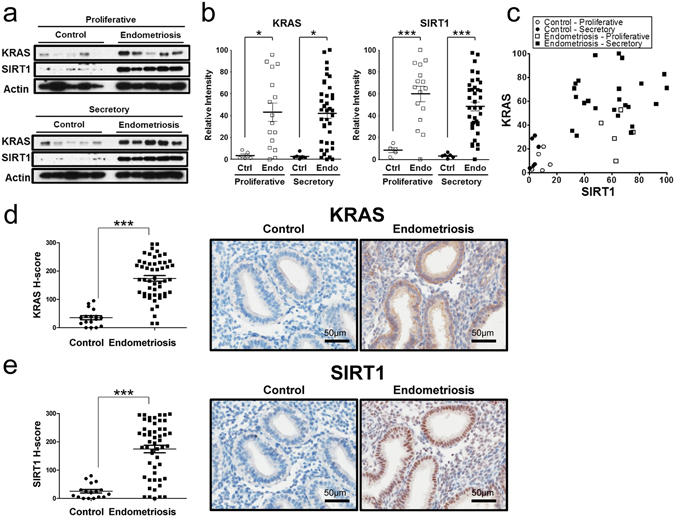
Correlation of between KRAS and SIRT1 in human endometrium with endometriosis. (a) Western blot analysis of SIRT1 and BCL6 proteins in proliferative and secretory phases of human endometrium with endometriosis. β-actin was used as sample-loading control. Representative blots have been cropped to reduce unnecessary area. Full-length blots are presented in Supplementary Fig. S2. (b) Densitometric analysis of KRAS and SIRT1 protein levels by Western blot analysis in eutopic endometrium from proliferative and secretory phase in women with and without endometriosis. (c) Correlation between SIRT1 and KRAS in women with endometriosis throughout the menstrual cycle phases based on Western blot analysis (correlation coefficient = 0.4641, p = 0.0009). (d and e) H-score of KRAS (d) and SIRT1 (e) expression in endometrium from women with and without endometriosis and representative photomicrograph of immunohistochemical staining of KRAS in endometrium from women without and with endometriosis. The results represent the mean ± SEM. ***p < 0.001.
To determine the cell-specific expression of KRAS and SIRT1, we performed immunohistochemical analysis in endometrium from women with and without endometriosis (Fig. 2d and e). In control women KRAS and SIRT1 proteins were weakly detected in the stromal and epithelial cells of endometrium from the proliferative phase and early, mid, and late secretory phases in women without endometriosis (n ≥ 4 per phase) (Supplementary Fig. S1). Interestingly, the levels of KRAS protein were significantly increased in the stromal and epithelial cells of endometrium from proliferative and secretory phase endometriosis patients (n = 52) as compared to control patients (n = 17) (Fig. 2d). The levels of SIRT1 were also significantly higher in both the stromal and epithelial cells of endometriosis patients compared to women without endometriosis (Fig. 2e). These results argue that aberrant activation of KRAS and SIRT1 is integral to pathogenesis of endometriosis. In addition, like BCL6, they appear to be specific endometrial biomarkers for the diagnosis of endometriosis.
Correlation between SIRT1 and BCL6 in endometriosis
BCL6 is a transcriptional repressor involved in B cell development and oncogenesis and known to be involved in the recruitment of SIRT1 deacetylase33. We previously reported over-expression of BCL6 in eutopic endometrium of infertile women with endometriosis23. Since both proteins appear to be elevated, we analyzed the relationship between SIRT1 and BCL6 proteins in eutopic endometrium of endometriosis patients. The levels of SIRT1 and BCL6 were examined and compared in eutopic endometrium using Western blot analysis. Our results showed a strong positive correlation between SIRT1 and BCL6 levels in women with endometriosis throughout the menstrual cycle phases (n = 44). Based on the Western blot band intensity, we show a scattergram with a correlation coefficient = 0.5659, p < 0.0001, between BCL6 and SIRT1 expression (Fig. 3a).
Figure 3.
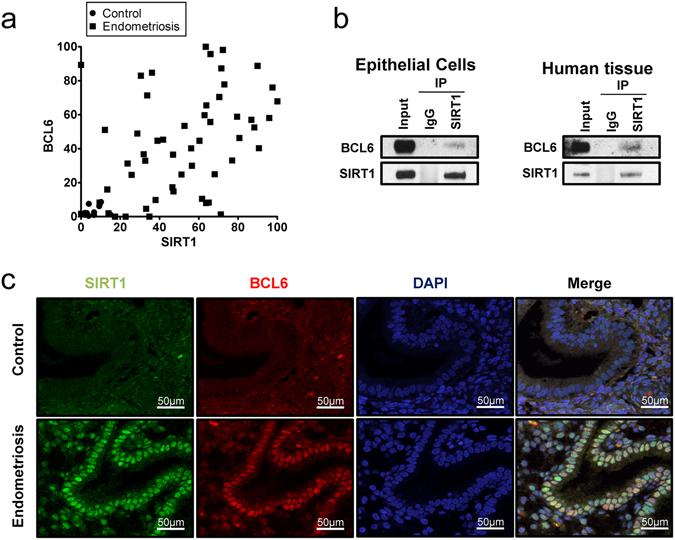
Correlation of between SIRT1 and BCL6. (a) Correlation analysis between SIRT1 and BCL6 in human endometrium with endometriosis. (b) Immunoprecipitation (IP) analysis between SIRT1 and BCL6 in Ishikawa cells and Human endometrium with endometriosis. Representative blots have been cropped to reduce unnecessary area. Full-length blots are presented in Supplementary Fig. S4. (c) Co-localization of SIRT1 and BCL6 in the human endometrium without and with endometriosis by immunofluorescence analysis.
To determine whether SIRT1 physically interacts with BCL6, we performed immunoprecipitation with SIRT1 antibody in total protein lysates from Ishikawa human endometrial adenocarcinoma cell line and endometrium from endometriosis patients. The immunoprecipitation result showed that endogenous SIRT1 physically interacts with BCL6 in human endometrium (Fig. 3b). However, no BCL6 was detected within the immune-precipitate of the IgG negative control. To determine whether SIRT1 proteins co-localize with BCL6 proteins, we performed double immunofluorescence for SIRT1 and BCL6. The immunofluorescence results show that SIRT1 and BCL6 proteins were co-localized in endometrial epithelial cells of endometriosis patients (Fig. 3c). These finding leads to the conclusion that these proteins may be acting in concert and contribute to the development or maintenance of endometriosis.
Aberrant activation of SIRT1 and BCL6 expression in a baboon model of endometriosis progression
We have used primate models to study temporal sequence of events involved in endometriosis establishment and progression34. To determine that SIRT1 and BCL6 proteins are overexpressed as part of endometriosis development, we performed immunohistochemical analysis of SIRT1 and BCL6 in eutopic baboon endometrium sequentially after the experimental induction of the disease (n = 4 per time point). As in human endometrium, the expression of SIRT1 and BCL6 proteins were not evident in the endometrium of pre-inoculation (control) baboons. The levels of SIRT1 and BCL6 proteins were significantly increased at 9 and 15 months post-inoculation during endometriosis progression (Fig. 4). These data suggest that the ontogeny of BCL6 and SIRT1 expression occur synchronously, and that they require time after initiation of endometriosis to develop. The timing of the appearance of SIRT1 and BCL6 corresponds to the increase in inflammation seen in this model.
Figure 4.
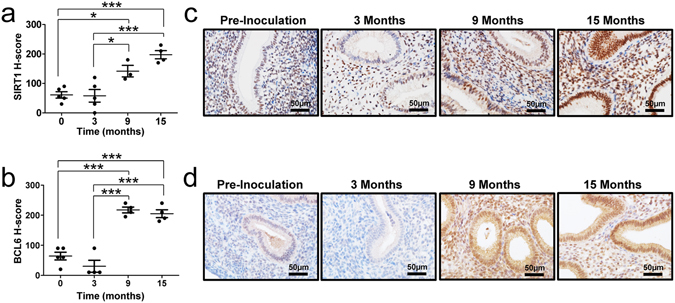
Levels of SIRT1 and BCL6 proteins during progression of endometriosis in a baboon model. (a and b) H-score of SIRT1 (a) and BCL6 (b) expression in endometriosis baboon model induced by intraperitoneal inoculation of menstrual endometrium during progression of endometriosis. The results represent the mean ± SEM. *p < 0.05 and ***p < 0.001. (c and d) Representative photomicrograph of immunohistochemical staining of SIRT1 (c) and BCL6 (d) in the baboon endometrium of pre-inoculation and 3, 9 and 15 months post-inoculation during endometriosis progression.
SIRT1 overexpression and dysregulation of PGR target genes in mice with uterine specific KRAS activation
In order to effectively investigate the effects of KRAS activation in endometrium, mice with loxP-Stop-loxP-KrasG12D/+ (LSL-K-ras G12D/+)35 were bred to the PGRCre mouse (Pgr cre/+ LSL-K-ras G12D/+ mouse)36. Introduction of the oncogenic K-ras mutation in all PGR-positive cells did not show any pathological phenotype in the uterus37. We investigated whether KRAS activation altered the expression of SIRT1 in the mouse uterus using immunohistochemistry (n = 3 per group). Interestingly, SIRT1 expression was highly increased in endometrium of the mutant mice compared to control mice (Fig. 5a). We performed real-time RT-PCR to assess the expression of PGR and its target genes in the mutant mice. Pgr expression was not changed in the mutant mice. The mRNA expression level of P4 target genes, Fst, Klf15, Lrp2, and Calb1, were highly downregulated in the mutant mice compared to the control mice. Interestingly, the expression of Ihh, Patch1, and Gli1 which are known as P4-target and Indian Hedgehog pathway genes were significantly downregulated in the mutant mice (Fig. 5b). These results suggest that KRAS suppresses transcriptional activity of PGR by regulating SIRT1 expression.
Figure 5.
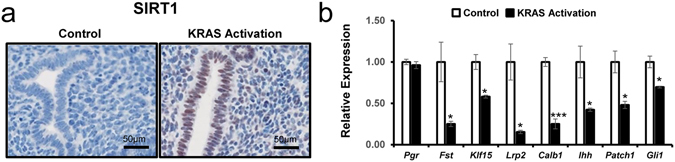
Levels of SIRT1 in the KRAS activation mouse model. (a) Representative photomicrograph of immunohistochemical staining of SIRT1 in the control and KRAS activation mouse. (b) The mRNA expression level of P4 target genes in the uterus from control and KRAS activation mice (n = 9). The results represent the mean ± SEM. *p < 0.05 and ***p < 0.001.
Transcriptional Repression of GLI1 by SIRT1 and BCL6 proteins
E2 stimulates proliferation of uterine epithelial cells while P4 is inhibitory to E2-mediated proliferation of the epithelium38, 39. The major pathologic phenomenon of uterine disease is the loss of ovarian steroid hormone control over uterine epithelial cell proliferation and apoptosis40–43. Resistance to P4 treatment, via loss of progesterone receptors (PGR) or its signaling pathways, is a major hurdle in the treatment of a variety of diseases in the endometrium of women such as endometriosis and endometrial cancer36, 44–46. To gain insight into the underlying molecular mechanisms of SIRT1/BCL6 action in an epithelial cell model of endometrium, Ishikawa cells were treated with E2 + MPA and subsequently used Western blot analysis to examine the expression levels of BCL6 and SIRT1. The level of BCL6 was increased gradually after 6 hours by E2 + MPA (Fig. 6a). SIRT1 levels were consistently strong in Ishikawa cells. Interestingly, the expression of GLI1 was significantly decreased after 12 hours treated with E2 + MPA (Fig. 6b). These results suggest that E2 + MPA, a known inducer of BCL6, results in repression of GLI1 expression.
Figure 6.
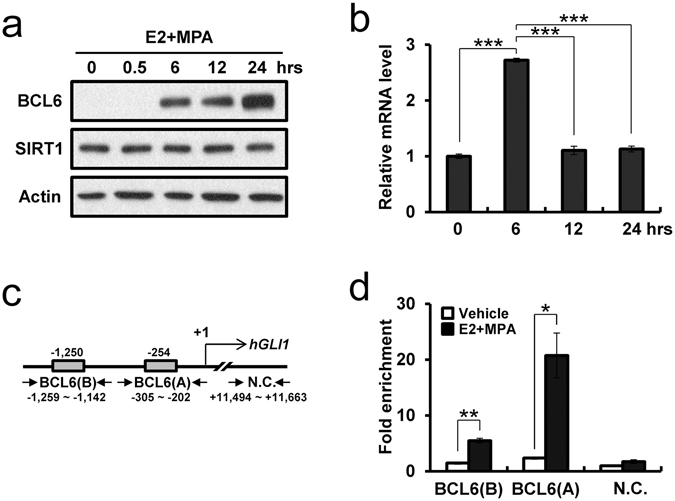
Regulation of GLI1 gene expression by SIRT1 and BCL6 proteins. (a) Western blot analysis of BCL6 and SIRT1 in Ishikawa cells treated with E2 + MPA for 0, 30 min, 6, 12, and 24 hours. β-actin was used as sample-loading control. Representative blots have been cropped to reduce unnecessary area. Full-length blots are presented in Supplementary Fig. S5. (b) Quantitative real time PCR analysis of GLI1 gene expression in Ishikawa cells treated with E2 + MPA for 0, 6, 12, and 24 hours. (c) Map of BCL6 binding site on the GLI1 promoter (Gray boxes). Negative control (N.C.) region on the GLI1 gene was used as negative control of ChIP assay. Primers used in ChIP assay are presented by arrows. (d) ChIP assay using anti-SIRT1 antibody on GLI1 promoter in Ishikawa cells treated with or without E2 + MPA for 24 hours. The results represent the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001.
To determine that BCL6 and SIRT1 bind to the putative GLI1 promoter, we performed ChIP analysis on chromatin from Ishikawa cells treated with E2 + MPA. Our ChIP results exhibited that both BCL6 and SIRT1 proteins were significantly accumulated on two sites (BCL6 (A) and (B)) of GLI1 promoter in Ishikawa cells treated with E2 + MPA compared to vehicle control. Interestingly, the accumulated SIRT1 protein closely parallels what BCL6 protein accumulated on GLI1 promoter (Fig. 6c and d). These results suggest that BCL6 regulates transcriptional repression of GLI1 expression through direct interaction with SIRT1 in endometrial epithelial cells. Further, elevated levels of SIRT1 and BCL6 in secretory phase endometrium of women with endometriosis likely accounts for the decrease noted in GLI1 protein expression, as a sign of P4 resistance.
Attenuation of GLI1 expression in endometrium from women with endometriosis
SIRT1/BCL6 proteins act as a transcriptional repressor of GLI effectors in the Hedgehog pathway for neurogenesis and tumor suppression of medulloblastoma33. Therefore, we examined GLI1 expression in eutopic endometrium from women with (n = 20) and without (n = 13) endometriosis by immunohistochemistry. Our immunohistochemistry analysis found that GLI1 protein levels are significantly reduced, specifically in the endometrial epithelial cells comparing women with and without endometriosis (Fig. 7).
Figure 7.
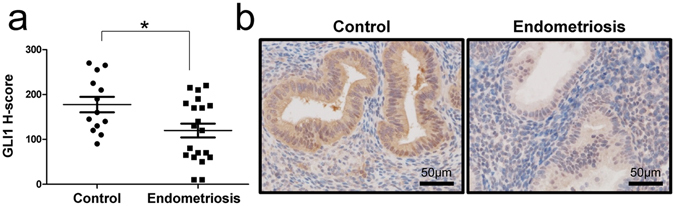
Levels of GLI1 in endometrium from women with and without endometriosis. (a) H-score of GLI1 expression in endometrium from women with and without endometriosis. The results represent the mean ± SEM. *p < 0.05. (b) Representative photomicrograph of immunohistochemical staining of GLI1 in endometrium from women without and with endometriosis.
Discussion
KRAS, a well characterized oncogene, has been implicated in the pathogenesis of endometriosis12. While mutational changes to KRAS appears to be a pivotal change in endometriosis-related ovarian cancers47, we demonstrate for the first time, that KRAS activation is a common finding and a key biomarker in the endometrium of most women with endometriosis. We show that its activation is highly correlated to the over-expression of SIRT1 (member of the sirtuin family) and contributes to the upregulation of this histone deacetylase. Further, we postulate that inflammatory changes associated with endometriosis provide the milieu for activation of KRAS mediated BCL6/SIRT1 complexes that participate in the early stages of P4 resistance, which contributes to infertility and a key to the pathophysiology of endometriosis growth and pathogenesis48.
In the present study, we report that BCL6 and SIRT1 are over-expressed and co-localize in the nuclei of endometrial cells from women with endometriosis. SIRT1 is a nicotinamide adenosine dinucleotide (NAD)-dependent deacetylase that is responsible for a wide variety of vital functions in the cell by removing acetyl groups from histone and non-histone proteins controlling gene expression49. While the regulatory controls for endometrial SIRT1 remain unknown, BCL6 is up-regulated by inflammatory stimuli including IL-6 and STAT3 activation50, that we recently examined22. The number of known SIRT1 targets are many and include genes involved in endometrial function and P4 action, including GLI1, FOX01, PPARγ, CTIP2 (chicken ovalbumin upstream promoter transcription factor interacting protein 2 (COUP-TFII), and p30051.
SIRT1 has been shown to pair with other transcription factors including BCL6. BCL6 is a transcriptional repressor involved in B cell development and oncogenesis52. We showed that BCL6 is over-expressed in eutopic endometrium of women with endometriosis53. We report for the first time that BCL6 and SIRT1 interacting through the IHH pathway both bind to and inactivate the GLI1 promoter. Tiberi et al. had reported similar findings in the Sonic Hedgehog pathway, showing that BCL6/BCOR/SIRT1 complex suppresses growth of human medulloblastoma cells through GLI1 and GLI2 repression33. Collectively, these data suggest thatBCL6/SIRT1 could influence chromatin acetylation patterns at the GLI1regulatory regions and thereby contribute to epigenetic repression of GLI154. Identification of these BCL6/SIRT1-recruiting factors and the mechanism of protein-protein interaction will be of importance in future investigations.
The concept of P4 resistance in endometriosis is now well-established55, 56, though the underlying mechanism has remained elusive. Several mechanisms of cellular resistance to P4 have been suggested including alterations in progesterone receptor chaperone proteins FKBP5257–63, progesterone receptor coactivator Kruppel-like factor 9 (KLF9)64, MIG-6 alterations65, progesterone coactivator Hic-566, and direct alterations of PGR subunits67. We believe SIRT1/BCL6 represent a more proximal defect in endometrium of women with endometriosis that interferes with early signaling of P4. As recently reviewed68, P4 initiates a complex series of paracrine signaling steps involving the Indian Hedgehog (IHH) expression by endometrial epithelium. GLI1 has been shown to play an integral role in this pathway69–72.
In this study, we demonstrated for the first time that SIRT1 is over-expressed in women with endometriosis compared to controls by western blot and immunohistochemistry, correlating directly with elevated BCL6 expression. Co-localization using immunofluorescence and co-immunoprecipitation confirmed direct interaction of SIRT1 with BCL6 in the nucleus of affected individuals. Perhaps most striking was the concurrent up-regulation of both proteins in baboon model of endometriosis, both BCL6 and SIRT1 appearing within 9 months of induction of the disease. Animal models are useful for studying the temporal sequence of events involved in disease establishment and progression. Autologous inoculation of autologous menstrual blood establishes endometriotic lesions that are histological and morphological similar to human disease. Together, these data support an inflammatory-driven phenomenon. Interestingly, BCL6 appears to be regulated by different pathways.
We show that IL-6 as well as other inflammatory mediators are higher in women with endometriosis. In P4 resistance, the normally repressive effect of STAT5 on BCL6 appears to be reduced, while the activation of STAT3 seen in endometriosis22 drives BCL6 over-expression50. SIRT1, on the other hand, is regulated by other factors. Estrogen has been shown to increase SIRT173, as well as inflammation-driven miRNAs74. miRNA34 has been shown to inhibit SIRT175 and we previously reported that miR34 levels are markedly reduced in women with endometriosis13, likely regulated by inflammation76. Thus, both SIRT1 and BCL6 over-expression can be regulated through inflammatory cytokines known to be present in women with endometriosis.
Furthermore and importantly, we show that KRAS activation in the mouse uterus is associated with increased SIRT1 proteins and suppressed expression of P4 target genes including Indian hedgehog pathway genes. P4 resistance implies a decreased responsiveness of target tissue to bioavailable P477, and such an impaired P4 response is seen in the endometrium of women with endometriosis78. P4 resistance is associated with early secretory phase deficiency, early pregnancy loss, or infertility due to endometriosis. Understanding the molecular mechanisms of P4 resistance is critical to developing better therapeutic approaches to infertility and endometriosis. Therefore, our results suggest KRAS activation causes P4 resistant through SIRT1 in endometrium.
In summary, this is the first time that non-mutated KRAS activation has been shown to be strongly correlated with endometrium-associated endometriosis and that this activation triggers specific changes in histone deacetylase, SIRT1 which we postulate is a key driver of P4 resistance. SIRT1 is highly expressed in the endometrium of patients with endometriosis and appears to be an excellent biomarker in endometrium of women with this disorder. Transcriptional repression of GLI1 relies on recruitment of SIRT1 and BCL6 onto the promoter. These studies identify a primary mechanism of inflammatory dysfunction that contributes to the pathogenesis of endometriosis and may have a role in infertility and pregnancy loss associated with this disease. A lack of defined pathways has hampered the development of targeted pharmacological approaches that might benefit women with this disease. These novel findings regarding coordinated expression of SIRT1 and KRAS and the colocalization of BCL6 and SIRT1may improve treatment options for this enigmatic disease.
Methods
Human endometrial tissue samples
The study has been approved by Institutional Review Committee of Michigan State University, Greenville Health System and University of North Carolina, and written informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations. The human endometrial samples were collected from Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository (Grand Rapids, MI), the Greenville Hospital System (Greenville, SC), and the University of North Carolina (Chapel Hill, NC). Samples were collected as previously reported22, 79, 80. Briefly, we used eutopic endometrium derived from women with endometriosis and compared it to endometrium from women that did not have endometriosis. Subjects reported regular cycles and were between the ages of 18 and 45. We confirmed the presence of disease at the time of laparoscopy in the endometriosis group. Women who were laparoscopically negative for this disease were placed into the control group. For control eutopic endometrium, 21 samples were collected from the proliferative (n = 5) and secretory phase (n = 16) for Western blot analysis and 23 samples were collected from the proliferative (n = 6) and secretory (n = 17) phase for immunohistochemistry analysis. For endometriosis eutopic endometrium, 54 samples were collected from the proliferative (n = 16) and secretory (n = 38) phase for Western blot analysis and 57 samples were collected for immunohistochemistry analysis. Use of an intrauterine device (IUD) or hormonal therapies in the 3 months preceding surgery was exclusionary for this study. Histologic dating of endometrial samples was performed by a board certified pathologist (DPS).
Animals and tissue collection
All the experimental mice were maintained in a designated animal care facility according to Michigan State University’s Institutional Guidelines for the care and use of laboratory animals. All animal procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University. All animal experiments were performed in accordance with the relevant guidelines and regulations. Kras conditional activated mice were generated by crossing Pgr cre/+ with LSL-K-ras G12D/+ mice (Pgr cre/+ LSL-K-ras G12D/+)35, 36. For the study, female control (LSL-K-ras G12D/+ and Pgr cre/+) mice were used.
Cytokine measurements
Plasma samples obtained from endometriosis patients and healthy controls were evaluated using a laser bead technology based commercial multiplex assay for the cytokine analysis by Eve Tech (Eve Technologies, Calgary, AB, Canada). Briefly, color-coded polystyrene beads were coupled with capture antibodies for each respective target cytokine. After washing twice with 100 µL of wash buffer, 50 µL of sample was added to each well. Following 1-hour incubation, wells were washed 3 times with 100 µL of wash buffer prior to adding 25 µL of detection antibody. 50 µL of streptavidin-PE was added to each well and was incubated for 10 minutes. Beads were re-suspended in 125 µL of assay buffer and the plate was read using Bio Plex 200 Suspension Array System. Fluorescent intensity signals in direct proportion to protein bound to specific analyte beads were analyzed. Observed concentration for each target analyte was calculated against standard curve regression.
Baboon endometrium samples
The endometriosis baboon animal model is reviewed and approved by the Institutional Animal Care and Use Committees (IACUCs) of both the University of Illinois at Chicago and Michigan State University. Endometriosis is induced by intraperitoneal inoculation of menstrual endometrium on two consecutive menstrual cycles and harvested using laparotomy via endometriectomy from five female baboons as previously described81. Laparotomies were performed at 3, 9, and 15 months post-inoculation to harvest the eutopic endometrial tissues and these endometrial tissues were used for immunohistochemistry analysis.
Western blot analysis
Western blot analyses were performed as described previously82. Briefly, eutopic endometrial tissues were lysed with lysis buffer (150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 2.5 mM EDTA, 0.125% Nonidet P-40 (vol/vol), a protease inhibitor cocktail (Roche, Indianapolis, IN) and a phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO). Equal amounts of total protein (20 μg) were separated on SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membrane was blocked with 0.5% Casein in phosphate buffered saline (PBS) and incubated with antibodies against SIRT1 (9475; Cell Signaling, Danvers, MA), BCL6 (561520; BD Pharmingen, San Jose, CA), and β-actin (sc1616; Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactivity was visualized by autoradiography and band intensity was determined by relative densitometry using ImageJ (National Institute of Health), and normalized against the bands obtained for β-actin.
Immunohistochemistry and immunofluorescence analyses
Immunohistochemistry and immunofluorescence analysis were performed as previously described22. The paraffin-embedded endometrial tissues were blocked with 10% normal serum in PBS (pH 7.5) and then incubated with antibodies against SIRT1 (9475 for IHC and 8469 for IF, Cell Signaling), BCL6 (14895, Cell Signaling), KRAS (ab55391, Abcam) and GLI1 (sc20687; Santa Cruz Biotechnology). For immunohistochemistry, sections were incubated with secondary antibody conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA). Immunoreactivity was detected using the Vectastain Elite DAB kit (DAB-Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin. A semi-quantitative grading system (H-score) was used to compare the immunohistochemical staining intensities as previously described83. For immunofluorescence, the sections were exposed to primary antibodies overnight at 4 °C and secondary antibodies (Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen, Grand Island, NY) and Alexa Fluor 594-conjugated anti-mouse IgG (Invitrogen) for 2 hour at room temperature. 4′,6‐diamidino‐2‐phenylindole (DAPI; Vector Laboratories) was used to enable nuclear visualization. The IgG antibody was intended for use as a negative control with SIRT1 and BCL6 proteins in the women endometrium (Supplementary Fig. S3).
Immunoprecipitation analysis
Immunoprecipitation analysis were performed as previously described80. Protein lysates were immunoprecipitated with anti-SIRT1 (Cell Signaling) antibodies with protein A-agarose (Pierce Biotechnology, Rockford, IL) overnight at 4 °C. Immunocomplexes were subjected to Western blot analysis using anti-BCL6 (561520, BD Pharmingen) and anti-SIRT1 antibodies (Cell Signaling) antibodies.
Cell culture and treatment
Ishikawa cells, epithelial cells of human endometrial adenocarcinoma, were maintained in Dulbecco’s Modified Eagle’s Medium with F12 (Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS; Gibco) and 1% penicillin streptomycin (P/S; Gibco) at 37 °C in 5% CO2. Ishikawa cells were pre-treated with 10 nM estradiol (E2, Sigma-Aldrich, St. Louis, MO) for 1 day and restored. After 2 days, these cells were treated with E2 + 1 μM medroxyprogesterone acetate (MPA; Sigma-Aldrich) and then incubated for the indicated time. All experiments were performed in triplicate.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from mouse uterine tissues or Ishikawa cell pellets using the RNeasy purification kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Then, cDNA were synthesized using quantitative PCR random hexamers and MMLV Reverse Transcriptase (Invitrogen Crop., Carlsbad, CA). The expression levels of GLI1 (TaqMan 00494654) were measured by quantitative real-time PCR using RT-PCR Universal Master Mix reagent (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. mRNA quantities were normalized against the housekeeping gene, 18S RNA using ABI rRNA control reagents.
Chromatinimmunoprecipitation (ChIP)
ChIP analysis was conducted by Active Motif (Carlsbad, CA) using Ishikawa cells treated with vehicle or E2 + MPA for 24 hours. ChIP assays were performed as previously described80. Briefly, 100 μg of chromatin from Ishkawa cells were immunoprecipitated by 4 μg of antibodies against BCL6 (BD Pharmingen). Eluted DNA was amplified with specific primers using SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA). Primers used in PCR were as follows: BCL6 A (forward: 5′-GTCCTGGGGGTGCAATAAG-3′; reverse: 5′-CCCCTCACCTCCCTTCTATT-3′), BCL6 B (forward: 5′-ACTGACCTTCCACACCCAAG-3′; reverse: 5′-GGAGGAAGCATGACAAGGAA-3′), and negative control (N.C.) (forward: 5′-CCTATCCCACCCCTTCACCA-3′; reverse: 5′-TAGCCTGCCCACCTCAGGAT-3′). The resulting signals were normalized to input activity.
Statistical analysis
Statistical analyses were performed using the Student’s t-test for data with only two groups. For data containing more than two groups, we performed an analysis of variance (ANOVA) test and analyzed by Tukey or Bonferroni test for pairwise t-test. All data are presented as means ± SEM. p < 0.05 was considered statistically significant. All statistical analyses were performed using the Instat package from GraphPad (San Diego, CA).
Electronic supplementary material
Acknowledgements
We would like to thank Mark Olson and Angela Houwing for sample preparation and Amanda Sterling for manuscript preparation. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD067721 (to S.L.Y. and B.A.L.), R01HD083273 (to A.T.F.), and R01HD084478 (to J.W.J.), and Canadian Institutes of Health Research (CT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
J.Y.Y. and T.H.K. performed experiments, analyzed the data and wrote the manuscript. WAP provided clinical material from normal fertile controls who had laparoscopically normal anatomy. S.H.A. and C.T. performed cytokine analysis on clinical samples from women with and without endometriosis. A.T.F., D.P.S., S.L.Y., and B.A.L. contributed to sample collection and critical revision of the manuscript. J.W.J. and B.A.L. contributed to the design of the study, interpretation of the data and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04577-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jae-Wook Jeong, Email: jeongj@msu.edu.
Bruce A. Lessey, Email: blessey@ghs.org
References
- 1.Simoens S, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 2.Fourquet J, Baez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril. 2011;96:107–112. doi: 10.1016/j.fertnstert.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulun SE. Endometriosis. The New England journal of medicine. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 4.de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 5.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Mavrogianis PA, Fazleabas AT. Endometriosis is associated with progesterone resistance in the baboon (Papio anubis) oviduct: evidence based on the localization of oviductal glycoprotein 1 (OVGP1) Biology of reproduction. 2009;80:272–278. doi: 10.1095/biolreprod.108.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reproductive biology and endocrinology: RB&E. 2006;4(Suppl 1):S9. doi: 10.1186/1477-7827-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinulescu DM, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nature medicine. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CW, et al. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. The Journal of pathology. 2011;224:261–269. doi: 10.1002/path.2852. [DOI] [PubMed] [Google Scholar]
- 10.Luong HT, et al. No evidence for genetic association with the let-7 microRNA-binding site or other common KRAS variants in risk of endometriosis. Hum Reprod. 2012;27:3616–3621. doi: 10.1093/humrep/des329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santamaria X, Taylor H. MicroRNA and gynecological reproductive diseases. Fertility and sterility. 2014;101:1545–1551. doi: 10.1016/j.fertnstert.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Grechukhina O, et al. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO molecular medicine. 2012;4:206–217. doi: 10.1002/emmm.201100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burney, R. O. et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Molecular human reproduction15, 625–631, doi:gap068 [pii] 10.1093/molehr/gap068 (2009). [DOI] [PMC free article] [PubMed]
- 14.Kasinski AL, Slack FJ. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer research. 2012;72:5576–5587. doi: 10.1158/0008-5472.CAN-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahlhut C, Slack FJ. Combinatorial Action of MicroRNAs let-7 and miR-34 Effectively Synergizes with Erlotinib to Suppress Non-small Cell Lung Cancer Cell Proliferation. Cell Cycle. 2015;14:2171–2180. doi: 10.1080/15384101.2014.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada N, et al. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes & development. 2014;28:438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 18.Okabe S, et al. BAZF, a novel Bcl6 homolog, functions as a transcriptional repressor. Mol Cell Biol. 1998;18:4235–4244. doi: 10.1128/MCB.18.7.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan, R. T. & Dalla-Favera, R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature432, 635–639, doi:nature03147 [pii] 10.1038/nature03147 (2004). [DOI] [PubMed]
- 20.Harris MB, et al. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: specific regulation of iepsilon transcription and immunoglobulin E switching. Mol Cell Biol. 1999;19:7264–7275. doi: 10.1128/MCB.19.10.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arguni E, et al. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. International immunology. 2006;18:1079–1089. doi: 10.1093/intimm/dxl041. [DOI] [PubMed] [Google Scholar]
- 22.Kim BG, et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Human reproduction. 2015;30:1069–1078. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans-Hoeker E, et al. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women With Endometriosis. Reprod Sci. 2016;23:1234–1241. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 25.Luo, J. et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell107, 137–148, doi:S0092-8674(01)00524-4 [pii] (2001). [DOI] [PubMed]
- 26.Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852:2442–2455. doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song NY, Surh YJ. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci. 2012;1271:10–19. doi: 10.1111/j.1749-6632.2012.06762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L, et al. SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. Oncol Rep. 2014;32:2831–2835. doi: 10.3892/or.2014.3521. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A, et al. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: a possible role of the sirtuin 1 pathway. J Obstet Gynaecol Res. 2014;40:770–778. doi: 10.1111/jog.12252. [DOI] [PubMed] [Google Scholar]
- 30.Mvunta, D. H. et al. Overexpression of SIRT1 is Associated With Poor Outcomes in Patients With Ovarian Carcinoma. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry, doi:10.1097/PAI.0000000000000316 (2016). [DOI] [PubMed]
- 31.Kriegl L, Vieth M, Kirchner T, Menssen A. Up-regulation of c-MYC and SIRT1 expression correlates with malignant transformation in the serrated route to colorectal cancer. Oncotarget. 2012;3:1182–1193. doi: 10.18632/oncotarget.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnoud, T., Wilkey, D. W., Merchant, M. L., Clark, J. A. & Donninger, H. Proteomics Analysis Reveals Novel RASSF2 Interaction Partners. Cancers8, doi:10.3390/cancers8030037 (2016). [DOI] [PMC free article] [PubMed]
- 33.Tiberi L, et al. A BCL6/BCOR/SIRT1 complex triggers neurogenesis and suppresses medulloblastoma by repressing Sonic Hedgehog signaling. Cancer Cell. 2014;26:797–812. doi: 10.1016/j.ccell.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Molecular human reproduction. 2009;15:577–586. doi: 10.1093/molehr/gap057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson L, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 36.Soyal SM, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 37.Kim TH, et al. The Synergistic Effect of Conditional Pten Loss and Oncogenic K-ras Mutation on Endometrial Cancer Development Occurs via Decreased Progesterone Receptor Action. Journal of oncology. 2010;2010:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 39.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 40.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 41.Sherman ME, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997;10:963–968. [PubMed] [Google Scholar]
- 42.Deligdisch L, Holinka CF. Endometrial carcinoma: two diseases? Cancer Detect Prev. 1987;10:237–246. [PubMed] [Google Scholar]
- 43.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::AID-CNCR2820560233>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Molecular and cellular endocrinology. 2012;358:208–215. doi: 10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Burney RO, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 46.Attia GR, et al. Progesterone receptor isoform A but not B is expressed in endometriosis. The Journal of clinical endocrinology and metabolism. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 47.Zannoni GF, et al. Mutational status of KRAS, NRAS, and BRAF in primary clear cell ovarian carcinoma. Virchows Archiv: an international journal of pathology. 2014;465:193–198. doi: 10.1007/s00428-014-1599-1. [DOI] [PubMed] [Google Scholar]
- 48.Bruner-Tran KL, Herington JL, Duleba AJ, Taylor HS, Osteen KG. Medical management of endometriosis: emerging evidence linking inflammation to disease pathophysiology. Minerva ginecologica. 2013;65:199–213. [PMC free article] [PubMed] [Google Scholar]
- 49.Pillarisetti S. A review of Sirt1 and Sirt1 modulators in cardiovascular and metabolic diseases. Recent Pat Cardiovasc Drug Discov. 2008;3:156–164. doi: 10.2174/157489008786263989. [DOI] [PubMed] [Google Scholar]
- 50.Walker SR, et al. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol Cell Biol. 2013;33:2879–2890. doi: 10.1128/MCB.01620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahman S, Islam R. Mammalian Sirt1: insights on its biological functions. Cell communication and signaling: CCS. 2011;9:11. doi: 10.1186/1478-811X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Advances in immunology. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- 53.Evans-Hoeker, E. et al. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women with Endometriosis. Reprod Sciences in press (2016). [DOI] [PMC free article] [PubMed]
- 54.Huynh KD, Fischle W, Verdin E, Bardwell VJ. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 2000;14:1810–1823. [PMC free article] [PubMed] [Google Scholar]
- 55.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Seminars in reproductive medicine. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- 56.Lessey BA, Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Seminars in reproductive medicine. 2014;32:365–375. doi: 10.1055/s-0034-1376355. [DOI] [PubMed] [Google Scholar]
- 57.Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reproductive biology and endocrinology: RB&E. 2006;4(Suppl. 1):S9. doi: 10.1186/1477-7827-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bulun SE, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Molecular and cellular endocrinology. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 59.Pavone ME, et al. Altered retinoid uptake and action contributes to cell survival in endometriosis. The Journal of clinical endocrinology and metabolism. 2010;95:E300–309. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy DB, Janowski BA, Chen CC, Mendelson CR. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol Endocrinol. 2008;22:1812–1824. doi: 10.1210/me.2007-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bulun, S. E. et al. Estrogen production and metabolism in endometriosis. Annals of the New York Academy of Sciences955, 75–85, discussion 86–78, 396–406 (2002). [DOI] [PubMed]
- 62.Li X, et al. COUP-TFII Regulates Human Endometrial Stromal Genes Involved in Inflammation. Mol Endocrinol. 2013;27:2041–2054. doi: 10.1210/me.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson, K. S. et al. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci14, 137–150, doi:14/2/137 [pii] 10.1177/1933719106298409 (2007). [DOI] [PubMed]
- 64.Heard ME, Simmons CD, Simmen FA, Simmen RC. Kruppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology. 2014;155:1532–1546. doi: 10.1210/en.2013-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong JW, et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8677–8682. doi: 10.1073/pnas.0903632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aghajanova, L., Velarde, M. C. & Giudice, L. C. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology150, 3863–3870, doi:en.2009-0008 [pii] 10.1210/en.2009-0008 (2009). [DOI] [PMC free article] [PubMed]
- 67.Igarashi TM, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertility and sterility. 2005;84:67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 68.Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Molecular and cellular endocrinology. 2012;358:155–165. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. The Journal of clinical endocrinology and metabolism. 2010;95:5330–5337. doi: 10.1210/jc.2010-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diehl SA, Schmidlin H, Nagasawa M, Blom B, Spits H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunology and cell biology. 2012;90:802–811. doi: 10.1038/icb.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker SR, et al. Reciprocal effects of STAT5 and STAT3 in breast cancer. Molecular cancer research: MCR. 2009;7:966–976. doi: 10.1158/1541-7786.MCR-08-0238. [DOI] [PubMed] [Google Scholar]
- 72.Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocrine journal. 2008;55:795–810. doi: 10.1507/endocrj.K08E-067. [DOI] [PubMed] [Google Scholar]
- 73.Shen T, et al. SIRT1 Functions as an Important Regulator of Estrogen-Mediated Cardiomyocyte Protection in Angiotensin II-Induced Heart Hypertrophy. Oxidative medicine and cellular longevity. 2014;2014:713894. doi: 10.1155/2014/713894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamakuchi M. MicroRNA Regulation of SIRT1. Frontiers in physiology. 2012;3:68. doi: 10.3389/fphys.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle. 2009;8:712–715. doi: 10.4161/cc.8.5.7753. [DOI] [PubMed] [Google Scholar]
- 76.Olivo-Marston SE, et al. Effects of calorie restriction and diet-induced obesity on murine colon carcinogenesis, growth and inflammatory factors, and microRNA expression. PloS one. 2014;9:e94765. doi: 10.1371/journal.pone.0094765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirota Y, Cha J, Dey SK. Revisiting reproduction: Prematurity and the puzzle of progesterone resistance. Nature medicine. 2010;16:529–531. doi: 10.1038/nm0510-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fox C, Morin S, Jeong JW, Scott RT, Jr., Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertility and sterility. 2016;105:873–884. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoo JY, et al. CRISPLD2 is a target of progesterone receptor and its expression is decreased in women with endometriosis. PLoS One. 2014;9:e100481. doi: 10.1371/journal.pone.0100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim TH, et al. ARID1A Is Essential for Endometrial Function during Early Pregnancy. PLoS Genet. 2015;11:e1005537. doi: 10.1371/journal.pgen.1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Afshar Y, et al. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biology of reproduction. 2013;88:44. doi: 10.1095/biolreprod.112.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim TH, et al. Mig-6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer research. 2014;74:7371–7382. doi: 10.1158/0008-5472.CAN-14-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishibashi H, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88:2309–2317. doi: 10.1210/jc.2002-021353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


