Abstract
Relatively high 15N abundances in bone collagen of early anatomically modern humans in Europe have often been interpreted as a specific consumption of freshwater resources, even if mammoth is an alternative high 15N prey. At Buran-Kaya III, access to associated fauna in a secured archaeological context and application of recently developed isotopic analyses of individuals amino acids offer the opportunity to further examine this hypothesis. The site of Buran-Kaya III is located in south Crimea and has provided a rich archaeological sequence including two Upper Palaeolithic layers, from which human fossils were retrieved and directly dated as from 37.8 to 33.1 ka cal BP. Results from bulk collagen of three human remains suggests the consumption of a high 15N prey besides the contribution of saiga, red deer, horse and hare, whose butchered remains were present at the site. In contrast to bulk collagen, phenylalanine and glutamic acid 15N abundances reflect not only animal but also plant protein contributions to omnivorous diet, and allow disentangling aquatic from terrestrial resource consumption. The inferred human trophic position values point to terrestrial-based diet, meaning a significant contribution of mammoth meat, in addition to a clear intake of plant protein.
Introduction
Anatomically modern humans (AMHs) colonized Europe around 45-43 ka cal BP replacing Neanderthals after ca. 40 ka cal BP1–3 with potential cultural and/or biological interactions between these two human groups4. The exploitation by AMHs of a large diversity of ecosystems and food items is one of the highly debated scenarios to explain their successful expansion to the detriment of the possibly less flexible ecology of Neanderthal5, 6. The hypothesis of a broader dietary spectrum for AMH was fueled by observations of higher bone collagen 15N abundances in AMH compared with Neanderthals, which was interpreted as due to the addition of freshwater resources7–9 in contrast to a terrestrial-based diet typical of the Neanderthals10–12. However, the discrepancy in the location of the studied specimens, the overwhelming influence of meat in the protein contribution reflected in bulk collagen and non-systematic validation of the local stable isotope baseline may have contributed in oversimplifying the picture delivered by isotopic data12.
Several explanations can be evoked for high δ15N values in ancient human remains: a) high δ15N values in the terrestrial resources, especially in typical large herbivores whose meat provide the majority of dietary proteins over plants13, b) significant contribution of a given prey with higher δ15N values than the herbivores usually found in the archaeological sites, this resource being fish7, 8 or mammoth13. Freshwater fish is a 15N-enriched source of food compared with most of the terrestrial large herbivores, while their abundances in 13C are comparable14. On the other hand, Late Quaternary mammoth has been recognized as systematically 15N-enriched in comparison to other herbivores in the same environment, probably as the result of diet specialization14. The possible overlapping in 13C and 15N abundances between freshwater food and mammoth hinders accurate estimation solely from bulk collagen. Recent advances in stable nitrogen isotope analysis on individual amino acids should help disentangle the respective intake of resources from terrestrial and freshwater ecosystems15, 16 and may even reveal the contribution of plants to the human diet that is generally not detectable through bulk isotopic data17, 18. Phenylalanine (Phe) and glutamic acid (Glu) were specifically examined since the Phe 15N abundances reflect the local baseline (small trophic 15N-enrichment of 0.4 ± 0.4‰), while Glu 15N abundances depend not only on the baseline but also on the trophic position (large trophic 15N-enrichment of 8.0 ± 1.1‰)19–21. The difference in 15N of Glu-Phe (Δ15NGlu-Phe) is used to estimate the trophic position (TP) of a specimen (TP = 2 for herbivores, TP = 3 for primary carnivores, TP = 4 for secondary carnivores).
Here we consider the remains of AMHs found in Buran-Kaya III, a rock shelter located on the eastern bank of the Burulcha river in the Belogorsk region of south Crimea (Fig. 1; Supplementary Data 1, Supplementary Fig. 1). The site was discovered in 1990 by A. Yanevich (National Academy of Sciences of Ukraine) and excavated until 2001 by a team under the direction of A. Yanevich and A. Marks. The excavation was then resumed between 2009 and 2011 under the supervision of A. Yanevich and S. Péan. The stratigraphy of the site ranges from the Middle Palaeolithic to Neolithic with a complete sequence over the Middle to Upper Palaeolithic transition22–26 (Supplementary Fig. 2). Six Upper Palaeolithic archaeological layers, dated from 34.1 to 29.4 ka BP, i.e. 40.4 to 33.5 ka cal BP26 have provided a rich assemblage of lithic tools and faunal remains. In addition, bone tools, body ornaments (shells, mammoth ivory, red deer and fox teeth), and human fossils were retrieved from the Upper Palaeolithic layers 6-2 and 6-1 ranging from 32.5 to 29.4 ka BP (38.4-33.5 ka cal BP). Convergent palaeoecological results based on pollen, microfauna, large mammals and sediment studies points to a cold and dry climate during the establishment of layers of 6-2 and 6-126. This corresponds to repeated and short occupations mainly devoted to the seasonal hunting of saiga antelopes during their summer migration27 (Supplementary Data 2). The human remains are currently the oldest AMHs from far south Eastern Europe26, 28. Anthropogenic modifications were observed in some of these human remains, which were not explained by dietary cannibalism28, 29 (Supplementary Data 3). This represents the oldest evidence of a complex treatment of the dead by anatomically modern humans in Eastern Europe.
Figure 1.
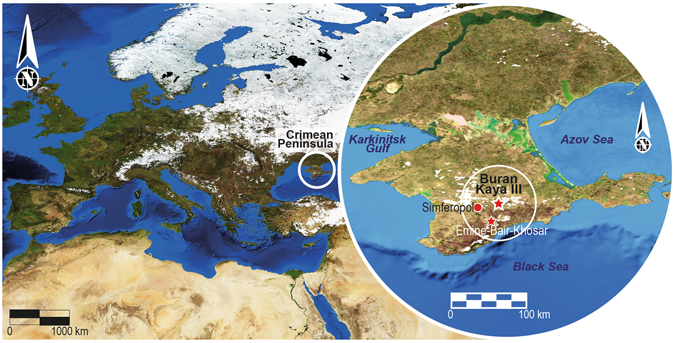
Current map of Europe with the location of Buran Kaya III site and Emine-Bair-Khosar cave in the Crimean Peninsula (map was designed by S. Puaud using open access NASA resources at http://eoimages.gsfc.nasa.gov/images/imagerecords/73000/73580/world.topo.bathy.200401.3×21600×21600.C1.jpg).
This context offers an unique opportunity to investigate the diet of AMHs during the early phases of their expansion in Eastern and Central Europe. Human and animal specimens considered in this study come from layers 6-1 and 6-2, which were formerly attributed to the Gravettian culture30, 31. Direct AMS radiocarbon dating on the human remains demonstrated a human occupation ranging from 37.8 to 33.1 ka cal BP for these two layers26, 28. Preliminary data showed a higher difference than expected in bulk δ15N values between one of the dated human individual and two red deer samples of layer 6-128.
We aim at verifying and identifying the prey causing the unusually high relative enrichment in 15N of the AMHs of Buran-Kaya III compared to their usual game species, namely saiga antelope, red deer and horse. We thus intend to test the relative probability that such a high 15N foodstuff would be of terrestrial – mammoth – rather than of aquatic – fish – origin through the 15N abundance on specific amino acids. The difference between the δ15N values of Glu and Phe will be especially examined to test how compatible is the trophic position (TP) deduced from the Δ15NGlu-Phe with different theoretical combinations of terrestrial and aquatic dietary proteins.
Results
We analysed directly dated human and animal specimens from layers 6-2 and 6-1 and an additional human from layer 6-1 for isotopic signature (Table 1). Moreover, we selected remains of saiga antelope (Saiga tatarica, n = 6), red deer (Cervus elaphus, n = 2), horse (Equus sp, n = 2), and hare (Lepus sp, n = 1) from layer 6-1 and 6-2. Small mammoth ivory fragments from layer 6-1 could be used since the reconstruction of a body ornament left a few pieces apart (Mammuthus primigenius, n = 1). Carnivores were represented by wolf (Canis lupus, n = 1) and fox (Vulpes vulpes or Alopex lagopus, n = 5). Fish remains are missing in the site despite systematic sieving of the sediment during the excavations. All the selected specimens provided well-preserved collagen, following established criteria32, 33, and were submitted to stable isotope analysis of bulk collagen (Table 2). All the collagen samples, except for one saiga and one horse specimen that could not be included due to technical constraints, were subsequently prepared for amino acid isotopic analysis.
Table 1.
List of the sampled bone specimens from Buran-Kaya III and related radiocarbon dates.
| Ref lab | Species | Sample | Layer | Excavation reference | 14C BP | cal BP (95.4%) | 14C source |
|---|---|---|---|---|---|---|---|
| BK3-07-01 | Homo sapiens | cranial vault fgmt | 6–1 | 2001 10Б (−155) | 31,900 ± 240/220 GrA-37938 | 36,930-35,503 | 28 |
| BK3-12-01 | Homo sapiens | cranial vault fgmt | 6–1 | 2001 10 A | |||
| BK3-07-03 | Equus sp. | lower cheek tooth R | 6–1 | 2001 10Б | |||
| BK3-07-04 | Cervus elaphus | metacarpal fgmt | 6–1 | 2001 9 A | 31,320 ± 820 GifA-10021/SacA-19018 | 38,357-34,582 | 28 |
| BK3-07-06 | Cervus elaphus | tibia fgmt R | 6–1 | 2001 9Б | |||
| BK3-10-18 | Saiga tatarica | tibia fgmt L | 6–1 | 2009 9Z/272 (−146) | |||
| BK3-08-04 | Saiga tatarica | humerus fgmt L | 6−1 | 2001 10 A | |||
| BK3-07-02 | Saiga tatarica | jawbone fgmt L | 6−1 | 2001 10Б | 31,530 ± 670 GifA-11216/SacA25133 | 37,735-34,749 | 26 |
| BK3-07-05 | Saiga tatarica | proximal phalanx | 6−1 | 2001 9Б | |||
| BK3-11-04 | Saiga tatarica | radius fgmt L | 6−1 | 2010 9Z/1054 (−146) | 29,640 ± 170 GrA-53942/32,200 ± 450 OxA-25669 | 34,781-33,730 (combined) | 26 |
| BK3-08-01 | Lepus sp. | humerus fgmt L | 6−1 | 2001 10 A | |||
| BK3-11-02 | Mammuthus primigenius | processed ivory | 6−1 | 2001 9 A (−152) | |||
| BK3-07-08 | Canis lupus | proximal phalanx | 6−1 | 2001 11 A (−135) | |||
| BK3-08-02 | cf. Vulpes vulpes | tibia fgmt R | 6−1 | 2001 10 A | |||
| BK3-08-03 | Vulpes sp./Alopex lagopus | femur fgmt L | 6−1 | 2001 10 A | |||
| BK3-07-07 | Vulpes sp./Alopex lagopus | metatarsal V L | 6−1 | 2001 11Б | |||
| BK3-11-01 | Homo sapiens | cranial vault fgmt | 6–2 | 2001 10Б | 32450 ± 250/230 GrA-50457 | 37,831–36,450 | 26 |
| BK3-08-05 | Equus sp. | metatarsal fgmt R | 6–2* | 2001 9Б | 34050 ± 260/2s40 GrA-40485/34910 ± 950 GifA-80181/SacA-12260 | 40,078–38,508 (combined) | 28 |
| BK3-10-19 | Saiga tatarica | metacarpal fgmt R | 6–2 | 2009 9Z/636 (−159) | 29440 ± 190/180 GrA-50460 | 34,643–33,486 | 26 |
| BK3-08-07 | cf. Alopex lagopus | humerus fgmt R | 6–2 | 2001 10Б | |||
| BK3-08-09 | cf. Alopex lagopus | ulna fgmt L | 6–2 | 2001 9 A |
Excavation reference corresponds to year square/object, field number and depth in cm; fgmt stands for fragment, L for left and R for right. *Indicates that the former stratigraphy position is now questioned based on the direct radiocarbon date (possible re-attribution to 6–326).
Table 2.
Results of stable isotope analyses of collagen (δ13Ccoll, δ15Ncoll, δ15NPhe, δ15NGlu) from the animal and human samples of Buran-Kaya III.
| Ref lab | Species | Sample | Layer | Ccoll | Ncoll | C:Ncoll | δ13Ccoll | δ15Ncoll | δ15NPhe | δ15NGlu | TP | TP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (‰) | (‰) | C3 | Aqua | |||||||
| BK3-07-01 | Homo sapiens | cranial vault fgmt | 6−1 | 43.2 | 15.3 | 3.3 | −19.4 | 15.4 | 17.4 | 21.2 | 2.6 | 1.1 |
| BK3-12-01 | Homo sapiens | cranial vault fgmt | 6−1 | 41.9 | 15.4 | 3.2 | −18.9 | 16.8 | 17.6 | 20.9 | 2.5 | 1.0 |
| BK3-07-03 | Equus sp. | lower cheek tooth R | 6−1 | 20.6 | 7.3 | 3.3 | −20.4 | 8.1 | 13.1 | 11.8 | 1.9 | |
| BK3-07-04 | Cervus elaphus | metacarpal fgmt | 6−1 | 43.0 | 15.0 | 3.3 | −19.1 | 7.9 | 13.3 | 10.6 | 1.8 | |
| BK3-07-06 | Cervus elaphus | tibia fgmt R | 6−1 | 24.8 | 8.6 | 3.4 | −19.1 | 10.3 | 12.2 | 11.6 | 2.0 | |
| BK3-10-18 | Saiga tatarica | tibia fgmt L | 6−1 | 39.5 | 14.1 | 3.3 | −17.0 | 11.2 | 13.3 | 12.4 | 2.0 | |
| BK3-08-04 | Saiga tatarica | humerus fgmt L | 6−1 | 42.0 | 14.8 | 3.3 | −16.4 | 11.8 | 16.3 | 15.5 | 2.0 | |
| BK3-07-02 | Saiga tatarica | jawbone fgmt L | 6−1 | 34.9 | 12.0 | 3.4 | −15.6 | 9.5 | 14.5 | 12.9 | 1.9 | |
| BK3-07-05 | Saiga tatarica | proximal phalanx | 6−1 | 45.7 | 15.6 | 3.4 | −16.4 | 10.2 | 13.9 | 13.1 | 2.0 | |
| BK3-11-04 | Saiga tatarica | radius fgmt L | 6−1 | 45.7 | 16.0 | 3.3 | −15.5 | 10.0 | ||||
| BK3-08-01 | Lepus sp. | humerus fgmt L | 6−1 | 41.0 | 14.3 | 3.4 | −20.7 | 6.0 | 12.5 | 9.9 | 1.8 | |
| BK3-11-02 | Mammuthus primigenius | processed ivory | 6−1 | 39.6 | 14.1 | 3.3 | −20.7 | 12.6 | 17.5 | 15.5 | 1.8 | |
| BK3-07-08 | Canis lupus | proximal phalanx | 6−1 | 37.1 | 13.1 | 3.3 | −18.7 | 13.0 | 17.4 | 24.7 | 3.1 | |
| BK3-08-02 | cf. Vulpes vulpes | tibia fgmt R | 6−1 | 38.6 | 14.0 | 3.2 | −17.2 | 11.5 | 12.3 | 15.8 | 2.6 | |
| BK3-08-03 | Vulpes sp./Alopex lagopus | femur fgmt L | 6−1 | 42.2 | 15.0 | 3.3 | −17.2 | 13.5 | 13.5 | 17.7 | 2.6 | |
| BK3-07-07 | Vulpes sp./Alopex lagopus | metatarsal V L | 6−1 | 44.4 | 15.2 | 3.4 | −17.9 | 9.4 | 14.2 | 18.9 | 2.7 | |
| BK3-11-01 | Homo sapiens | cranial vault fgmt | 6−2 | 34.6 | 13.2 | 3.1 | −18.8 | 15.8 | 16.6 | 19.7 | 2.5 | 1.0 |
| BK3-08-05 | Equus sp. | metatarsal fgmt R | 6−2* | 32.2 | 11.5 | 3.3 | −19.2 | 9.0 | ||||
| BK3-10-19 | Saiga tatarica | metacarpal fgmt R | 6−2 | 41.8 | 14.7 | 3.3 | −17.8 | 10.7 | 16.1 | 14.0 | 1.8 | |
| BK3-08-07 | cf. Alopex lagopus | humerus fgmt R | 6−2 | 42.6 | 14.8 | 3.4 | −19.2 | 14.2 | 15.7 | 20.3 | 2.7 | |
| BK3-08-09 | cf. Alopex lagopus | ulna fgmt L | 6−2 | 36.2 | 12.8 | 3.3 | −19.6 | 7.8 | 11.7 | 15.7 | 2.6 |
The carbon and nitrogen composition of the collagen is given through elemental composition (Ccoll, Ncoll) and atomic ratio (C:Ncoll). fgmt stands for fragment, L for left and R for right, *indicates that the former stratigraphy position is now questioned based on the direct radiocarbon date (possible re-attribution to 6–326).
Environment and diet reconstruction based on bulk collagen
The animal samples can be divided into two groups based on a cluster analysis of their δ13Ccoll values (Supplementary Fig. 3). The first group exhibited δ13Ccoll values ranging from −20.7 to −18.7‰ and included red deer, horse, hare and mammoth for the herbivorous species, as well as the wolf of layer 6-1 and the two fox specimens of layer 6-2. The second group corresponded to δ13Ccoll values ranging from −17.9 to −15.5‰ and encompassed all the saiga and fox samples of layer 6-1, suggesting a predation relationship between the two species.
The δ15Ncoll values of the fauna exhibited a high variability. Red deer and horse δ15Ncoll values varied from 7.9 to 10.3‰, while hare showed a lower value of 6.0‰ and saiga provided among the highest δ15Ncoll values (9.5 to 11.8‰). Finally, the mammoth ivory displayed the highest δ15Ncoll values with 12.6‰, reaching a comparable range as the wolf (13.0‰). The range of δ15Ncoll values of the fox specimens from layer 6-1 with relatively low δ13Ccoll values was even wider (7.8 to 14.2‰) than for the specimens in the second group with higher δ13Ccoll values from layer 6-2 (9.4 to 17.2‰). Each group corresponded not only to different layers but also possibly to different species (polar fox or red fox). If the foxes of layer 6-1 could clearly have consumed saiga, the foxes of layer 6-2 reflected a drastically different diet. Low abundances in both 13C and 15N can be explained by the specialized hunting of hare, while low 13C with high 15N abundances points to a large proportion of larger herbivore (red deer and/or horse) meat.
The human individuals had δ13Ccoll and δ15Ncoll values ranging from −19.4 to −18.8‰ and from 15.4 to 16.8‰, respectively (Fig. 2). Their δ15Ncoll values were thus at least 1‰ higher than the highest values measured in fox or wolf. This suggests the consumption of prey with higher 15N abundances than those accessible to the animal predators. The δ13Ccoll on the other hand implies the intake of animals mainly dependent on C3 plants, and thus does not fit with a dominant saiga-based protein diet. Beyond the single value available for Buran-Kaya III, the mammoth is a species known to display such low δ13Ccoll for relatively high δ15Ncoll values10–14, and should be considered as a potential meat contributor to human diet. On another hand, the freshwater resource contribution cannot be ruled out, even if no fish remains have been retrieved from the site, despite water sieving of the excavated sediments.
Figure 2.
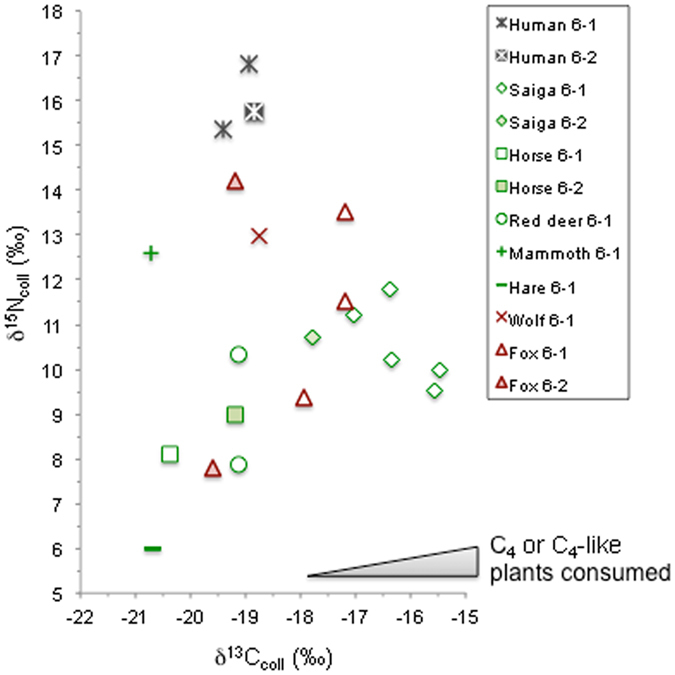
Measured δ13Ccoll and δ15Ncoll values of saiga, horse, red deer, mammoth, hare as herbivores (in green) and wolf, fox and anatomically modern humans from layers 6-1 and 6-2 of Buran-Kaya III (in red).
Diet and trophic position reconstruction based on collagen amino acids
The analysis of compound-specific δ15N values of phenylalanine and glutamic acid serves as a way of detection of possible aquatic contribution in human diet15, 34. The δ15NPhe values of herbivores and carnivores reflect mainly those of the primary producers, at the base of the food chain, due to limited trophic 15N-enrichment19–21. The δ15NPhe values of the herbivores of Buran-Kaya III reflected the observed pattern of the δ15Ncoll with even less overlap between mammoth with the highest value of 17.5‰ and the other herbivores, such as red deer, horse and hare with the lowest values of 12.2 to 13.3‰, and saiga with intermediate values (13.3 to 16.3‰) (Fig. 2). This confirms that the high δ15Ncoll value of the mammoth is linked to the specificity of its diet rather than to physiological traits18, 35, 36. The δ15NPhe values of the foxes (11.7 to 15.7‰) were relatively consistent with those of the saiga, as expected from their comparable δ13Ccoll values. An exception was the fox specimen of 6-2 with low δ15Ncoll value and one individual of 6-1 since both exhibited δ15NPhe values low enough to be comparable to the group of red deer, horse and hare. These foxes had possibly less access to saiga meat than their counterparts. The wolf and the human individuals of layer 6-1 exhibited δ15NPhe values from 17.4 to 17.6‰ that were similar to mammoth (17.4‰), suggesting this large game animal being a significant source of dietary protein. The human sample of layer 6-2 provided a slightly lower value of 16.6‰, which could reflect a lower influence of mammoth meat in the diet and a slightly higher contribution of saiga as source of animal protein.
The calculated trophic positions (TPs) of the herbivores using TP(C3) equation were ranging from 1.8 to 2.0 and averaged at 1.9, slightly lower than the theoretical value for herbivores, which was found at the early Upper Palaeolithic site of Scladina in Belgium (mean TP = 2, excluding cave bears)18. The TP position of the wolf at 3.1 instead of 3 also reflects a possible uncertainty of ± 0.1 in the calculation of the TP value and/or variability in its diet. Interestingly, the foxes’ TP values ranged from 2.6 to 2.7, which is consistent with the omnivorous and opportunistic habits reported for this small canid37, 38.
The calculation of TPs of the human individuals analysed at Buran-Kaya III gave values of 2.5 and 2.6 based on TP(C3) equation, while 1.0 and 1.1 values were obtained using TP(Aqua) equation (Table 2; Fig. 3). If the first result can be interpreted as a terrestrial-based diet with a clear intake of plant protein, the second results would place the human as the same trophic level as aquatic plants, which is unrealistic. In order to test different scenarios including aquatic resources in addition to terrestrial foodstuffs, we have tested a linear model comparing the δ15NGlu measured for the analysed humans with the δ15NGlu values calculated from the measured δ15NPhe at different end-points15 (Supplementary Data 4, Supplementary Fig. 4) for consumption of : aquatic primary consumers (TP(Aqua) = 3), aquatic secondary consumers (TP(Aqua) = 4), terrestrial plants (TP(C3) = 2), terrestrial primary consumers (i.e. herbivore; TP(C3) =3), and a 50:50 mix of terrestrial plant and herbivores (TP(C3) = 2.5). The estimations, with an error of ±10%, should be considered as qualitatively indicative, but underline that the input of plants is necessary (Table 3). The aquatic contribution could go above 10% only in the case that no herbivore meat would be consumed, which is not consistent with the local archaeological evidence of animal hunting by hunter-gatherer at that time. In case of half of the dietary protein being provided by plants, which involves more than half of the diet due to the lower nitrogen content of plant tissues against animal meat, the possible contribution of aquatic resources remains theoretically very low. The intake of human meat due to cannibalism practices has been questioned at Buran-Kaya III due to the occurrence of cut marks testifying to scalping and disarticulation processes, even if the comparison with the human modification on animal remains favours the hypothesis of mortuary practice or ritual cannibalism rather than dietary canibalism28, 29 (Supplementary Data 3). A regular consumption of human meat would have increased the TP over the value of carnivores (TP = 3). The significant consumption of human meat can thus be ruled out for the analysed individuals of Buran-Kaya III.
Figure 3.
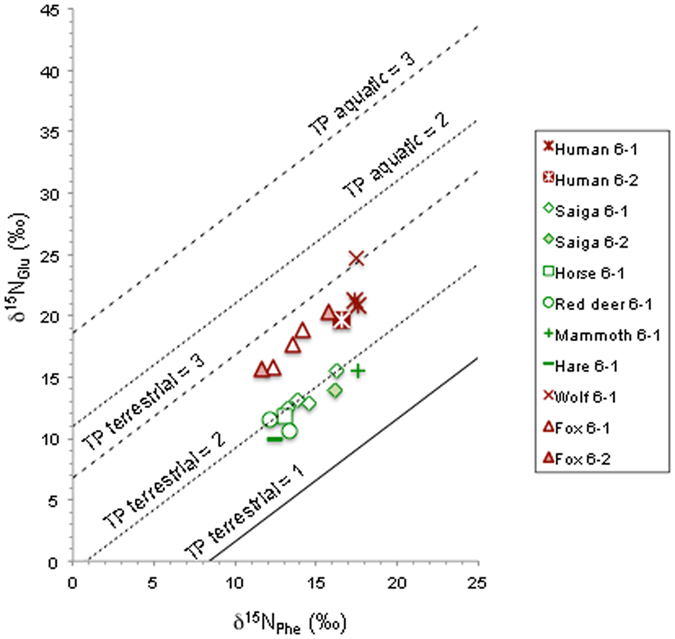
Measured δ15NPhe and δ15NGlu values on saiga, horse, red deer, mammoth, hare as herbivores and wolf, fox and human remains from layers 6-1 and 6-2 of Buran-Kaya III. Solid, dotted, and dashed lines indicate theoretical lines for δ15NPhe and δ15NGlu values of organisms with TP (Trophic Position) in terrestrial ecosystem = 1, 2, and 3, and aquatic ecosystem = 2 and 3, respectively.
Table 3.
Quantitative evaluation of freshwater resource consumption for the human individuals of Buran-Kaya III.
| BK3-07-01 | TP(Aqua) = 3 | TP(Aqua) = 4 |
|---|---|---|
| TP(C3) = 2 | 24.0 | 17.3 |
| TP(C3) = 2.5 | 5.5 | 3.7 |
| TP(C3) = 3 | n/a | n/a |
| BK3-12-01 | TP(Aqua) = 3 | TP(Aqua) = 4 |
| TP(C3) = 2 | 20.8 | 15.0 |
| TP(C3) = 2.5 | 1.6 | 1.1 |
| TP(C3) = 3 | n/a | n/a |
| BK3-11-01 | TP(Aqua) = 3 | TP(Aqua) = 4 |
| TP(C3) = 2 | 20.1 | 14.4 |
| TP(C3) = 2.5 | 0.6 | 0.4 |
| TP(C3) = 3 | n/a | n/a |
Indicated percentages correspond to the contribution of either primary consumers (TP(Aqua) = 3) or secondary consumers (TP(Aqua) = 4) from aquatic ecosystem to the human diet in association to terrestrial plants (TP(C3) = 2), or terrestrial primary consumers (TP(C3) = 3), or a 50:50 mix of terrestrial plant and herbivores (TP(C3) = 2.5). These calculations are indicative since the values of the end-members are based on the theoretical TP lines. n/a (non applicable) stands for unsolvable results.
Discussion
In general, the δ13Ccoll and δ15Ncoll values of the large herbivores from Buran-Kaya III are higher than those observed in other early Upper Palaeolithic mammoth steppe ecosystems11, 39. This difference, in addition to the C4-like plant consumption by the saiga antelope, testifies to the higher aridity of the Crimean context compared with northwest Europe40, 41. Compared with other herbivores of the site, the saiga diet included plants with a higher δ13C values than those expected in C3 plants42. Buran-Kaya III is currently located in a premontane forest-steppe area without favourable conditions for C4 plants development43. From an isotopic point of view, saigas of Buran-Kaya III are comparable with some of the most 13C-enriched modern specimens of Kazahkstan, which are known to consume significant amounts of Chenopodiaceae44 (Supplementary Data 5, Supplementary Fig. 5). The C4-like or C3-C4 intermediate photosynthesis mechanisms of the Chenopodiaceae linked to adaptations to arid environments can explain the high δ13C values of the ancient Crimean saiga. Interestingly, the equation based on C3 terrestrial vascular plants provided sensitive TP values for all the saiga specimens independently of their δ13Ccoll values (Supplementary Data 6 and Table 1). The likely access to desert and salty environments for this migrating species contrasts with the local C3 environment reflected by the horse and red deer of the site. At the extreme points of the variability in herbivore δ15Ncoll values, the relative low 15N abundance of hares fits with previous findings of a 15N-depleted signature for lagomorphs in contrast to coeval larger herbivores45, 46. On the other hand, the high δ15Ncoll value of the mammoth sample derived from an ornament artefact is consistent with the 15N-enriched signature (ca. 4‰ difference with horse/red deer) typical of this species14, while no other mammoth remains have been found in layers 6-1 and 6-2, which could be explained by the type of activities conducted at the site and carcasses transport decisions11. Indeed, the occupation of Buran-Kaya III was highly seasonal and the human activity was devoted to butchery of small and middle-size mammals during repeated short episodes. Interestingly, only saiga carcasses were brought complete among the middle-size herbivores, while the skeletons of the other species, such as red deer, are partially represented which could reflect far distance hunting. Mammoth meat procurement by humans found at the site would have happened out of the context of the occupation of the site of Buran-Kaya III. Interestingly, only 24 km southwest, a natural accumulation at Emine-Bair-Khosar cave in the Crimean mountains delivered remains of mammoth in a context dated around 38.7-36.6 ka cal BP (level H, 33,500 ± 400 BP)47. Thus, living mammoth could have been encountered in Crimea until the early phases of the Upper Palaeolithic. However, the exact dating of mammoth survival in the neighbouring Buran-Kaya III is hindered by the lack of sites of references in the area, probably accentuated by the loss of territories north and west of the current peninsula due to the rise of the sea-level since the Last Glacial Maximum24, 48.
The fox samples could be separated into two categories based on the δ13Ccoll values: one with values higher than −18‰ (layer 6-1), and a second with values lower than −19‰ (layer 6-2). The δ15NPhe values of the foxes of layer 6-1 generally fit with those of the saiga, except for one individual whose low value makes the saiga contribution difficult to discriminate from the red deer/horse and hare meat intake. The low δ13Ccoll and δ15Ncoll of one fox of layer 6-2 could be interpreted as a high consumption of hare, which is consistent with its low δ15NPhe value. The other fox specimen of layer 6-2 shows a potential important access to large herbivores carcasses. The consumption of saiga antelope could be mainly the result of scavenging, since the fox only occasionally preys on this species, especially on calves, in modern ecosystems44, 49 and traces of small carnivores gnawing was found on some saiga bone remains at Buran-Kaya III. The potential contributions of red deer/horse, saiga or hare as prey of the wolf are wide and largely overlapping based on the SIAR model using bulk collagen isotopic composition, hindering a more precise reconstruction of the diet composition (Supplementary Fig. 6). The mammoth however appears as a significant source of food of up to 30% of the diet of the wolf (Supplementary Data 7), which is consistent with the similar δ15NPhe values between wolf and mammoth ivory.
The TP values of the humans of Buran-Kaya III argue in favour of terrestrial-based subsistence practices, with no significant consumption of freshwater foodstuffs (Supplementary Fig. 7). Hence, the mammoth represents the most likely protein source contributing to the high δ15N values in human collagen and could account for up to 40–50% of the meat protein. In contrast, saiga, the main prey hunted on a seasonal basis at Buran-Kaya III, shows a potential protein contribution to human diet lower than 20%. Interestingly, a lower δ15NPhe value in association with a slightly higher δ13Ccoll value compared with those of the human samples of 6-1 points to a higher intake of saiga antelope for the individual of 6-2. Possible red deer and horse contributions appear comparable if not slightly higher than for saiga. Finally, hare appears as a protein source used to a limited extent by the analysed humans with a maximum of 15% of protein contribution with the highest probability density according to the SIAR Bayesian model (Figs 4 and 5). Such isotopic reconstructions are not only qualitative but also highly difficult to compare directly with the relative amounts of species remains left at the site since collagen reflect several years if not decades of meat intake at the scale of an individual, while the faunal accumulation results from seasonal activities of a group. Moreover, such comparison would require a conversion of the number of remains in relative meat weight, taking into account the large contrast between megaherbivores such as mammoth, and medium to small herbivores, such as saiga and hare. A contribution of up to ca. 20% of plant to the dietary proteins was estimated for late Neanderthals from Spy (Belgium)18 with TPs values between 2.7 and 2.8. Our estimation for the modern humans of Buran-Kaya III suggests a possible higher consumption of plants, which is consistent with the higher availability of such resources in more southern latitudes.
Figure 4.
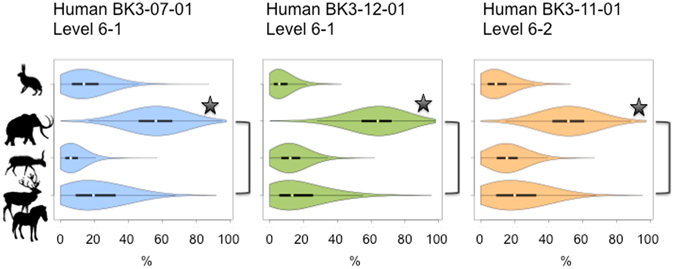
Proportional contribution of Deer&Horse (red deer and horse), Saiga (saiga antelope), Mammoth (woolly mammoth) and Hare (hare) (from bottom to top along the y axis) as estimated by SIAR using the considering both δ13Ccoll and δ15Ncoll values for human remains from Buran-Kaya III layers 6-2 and 6-1. Black boxes and whiskers show the median with 1st and 3rd quartiles and ranges with 1.5 times length of the interquartile range above the 3rd quartile or below the 1st quartile, respectively. The shaded area indicates the Kernel density plot of the probability density of prey proportions. The brackets link the resources with a significant negative correlation in their posterior distribution. Stars are placed close to the food resource whose significant contribution is in accordance with the δ15NPhe values.
Figure 5.
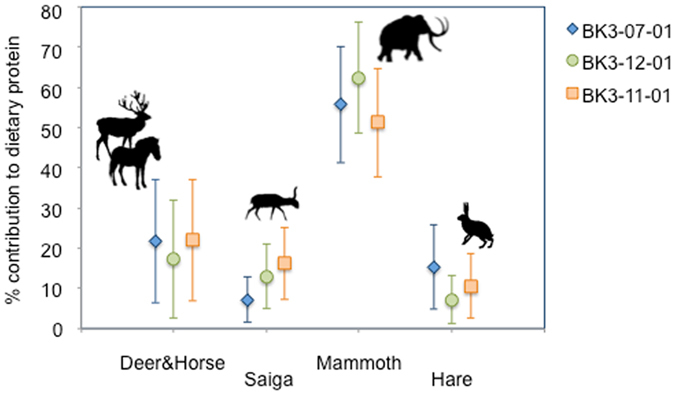
Proportional contribution of Deer&Horse (red deer and horse), Saiga (saiga antelope), Mammoth (woolly mammoth) and Hare (hare) as estimated by SIAR model for human remains from Buran-Kaya III layers 6-2 and 6-1. Each symbol corresponds to the mean protein diet contribution to a given human individual. Bars indicate standard deviations.
The individuals of Buran-Kaya III provide the highest δ15Ncoll values reported so far for east and central Europe during the early Upper Palaeolithic9. A diet incorporating freshwater fish was proposed for other individuals with high 15N from comparable chronological context, such as Oase 1 (13.3‰)50 as well as Muierii 1 and 2 (12.3 and 12.4‰ respectively)51, and Cioclovina 1 from Romania (12.7‰)51. Despite limited sample size, the case of Buran-Kaya III, with its exceptional archaeological context and good collagen preservation, shows that the mammoth could be the source of such high 15N signal and suggests that it should be more systematically considered as an alternative explanation to aquatic resources. Isotopic studies of western European late Neanderthals also point to the significant consumption of mammoth as well11, 39. Thus, the role of mammoth in human subsistence during the early Upper Palaeolithic should be further examined in future research.
Methods
Sample preparation for isotopic analyses
Specimens were chosen from taxonomically and anatomically identified compact bone pieces. Collagen was extracted following previously established protocol52, 53. The extraction process includes a step of soaking in 0.125 M NaOH between the demineralization and solubilization steps to achieve the elimination of lipids and humic acids.
Isotope analyses of bulk collagen
Elemental analysis (Ccoll, Ncoll) and isotopic analysis (δ13Ccoll, δ15Ncoll) were conducted at the Department of Geosciences of Tübingen University using a NC2500 CHN-elemental analyzer coupled to a Thermo Quest Delta+ XL mass spectrometer. The standard, internationally defined, is a marine carbonate (PDB) for δ13C and atmospheric nitrogen (AIR) for δ15N. Analytical error, based on within-run replicate measurement of laboratory standards (albumen, modern collagen, USGS 24, IAEA 305 A), was ±0.1‰ for δ13C values and ±0.2‰ for δ15N values. Reliability of the δ13Ccoll and δ15Ncoll values can be established by measuring its chemical composition, with C/Ncoll atomic ratio ranging from 2.9 to 3.632, percentage of Ccoll and Ncoll above 8% and 3%33, respectively.
Cluster analyses using Ward’s minimum variance method were performed on stable carbon and nitrogen isotopic composition, with the software SAS JMP version 12.2.0.
Isotope analyses of individual amino acids
All amino acid samples were prepared following the established protocols51. The bone and ivory collagen samples were subjected to hydrolysis by 12 N HCl at 110 °C for 12 h, followed by derivatisation with thionyl chloride/2-propanol (1:4, v/v) at 110 °C for 2 h and pivaloyl chloride/dichloromethane (1:4, v/v) at 110 °C for 2 h. The nitrogen isotopic compositions of the individual amino acid derivatives were measured by gas chromatography/combustion/IRMS (GC/C/IRMS) using an Agilent Technology 6890 GC (Agilent) coupled to a Thermo Finnigan DeltaplusXP IRMS (Thermo Fisher Scientific, Waltham, MA, USA) via combustion and reduction furnaces. Instrumental analysis was performed according to previous methods, with a few modifications of the equipment settings54. Standard mixtures of nine amino acids with known δ15N values were injected into the GC/C/IRMS every five runs to confirm the reproducibility of the isotope measurements. The mean accuracy and precision of the reference mixtures were 0.0‰ and 0.4–0.7‰ (mean of 1σ), respectively.
The characteristic Δ15NGlu-Phe value is −8.4‰ for most wild terrestrial plants and 3.4‰ for aquatic - marine and freshwater – plants. Based on these empirical estimations21, 55, equations were established to calculate the trophic position (TP) in terrestrial C3 and aquatic food webs. For C3-plant-based ecosystems: TP(C3) = [(Δ15NGlu-Phe + 8.4)/7.6] + 1. For aquatic ecosystems: TP(Aqua) = [(Δ15NGlu-Phe − 3.4)/7.6] + 1.
SIAR Bayesian model
The relative contribution of the different prey to the average diet of the human individuals was simulated using a Bayesian mixing model approach performed in the Stable Isotope Analysis in R (SIAR) package56, using the R software version 3.3.057. SIAR offers the possibility to work with multiple sources and to incorporate uncertainty in input data, yielding not only a range of possible dietary proportions, but providing also their relative probability distribution52. The relative contributions of four groups of preys as sources of animal protein were tested using the SIAR model: red deer/horse, saiga, mammoth and hare. Mean and standard deviations were calculated for use in the model (Supplementary Table 2). In the case of a single individual, as for mammoth and hare, we attributed a standard deviation value that was calculated from other datasets measured on archaeological context (Supplementary Tables 3 and 4). We considered a trophic enrichment factor (TEF) of +1.1 ± 0.2‰ and +3.8 ± 1.1‰ for δ13C and δ15N values of bulk collagen, respectively58. The food categories to be tested through SIAR model were defined as follow: deer and horse altogether (Deer&Horse), saiga antelope (Saiga), woolly mammoth (Mammoth) and hare (Hare).
Electronic supplementary material
Acknowledgements
ANR “Mammouths” Research Project No. ANR-05-JCJC-0240-01 (dir. St.P.) of the French National Research Agency (Agence Nationale de la Recherche), Project “Transition Paléolithique moyen/Paléolithique supérieur en Crimée” (St.P. & M. Patou-Mathis, dir.) of the Muséum National d’Histoire Naturelle (MNHN, Paris) ATM Program “Relations Sociétés-Nature dans le long terme” and the Fyssen Foundation project “Les premiers hommes anatomiquement modernes du Sud-Est de l’Europe” (coordinated by Sa.P.) supported this work. Y.I.N. was funded by the JSPS Postdoctoral Fellowships for Research Abroad (20120329) and N.O. by a Grant-in-Aid for Scientific Research from MEXT. The European Social Fund and the Ministry of Science, Research and Arts of Baden-Württemberg funded D.G.D. during the preparation of the manuscript. M.L.-G. is funded by a grant of the Czech Science Foundation GAČR 15-06446S “The relationships between humans and larges canids - the dogs and wolves of the Gravettian Předmostí site (Moravia)”. The collagen preparation and analysis benefited from the technical assistance of Sirwan Ali, Andrea Orendi, Catherine Bauer, Bernd Steinhilber and Christoph Wißing (University of Tübingen). We are thankful to James Fellows Yates for proofreading the manuscript and improving the language. The comments of two anonymous reviewers contributed to improve this manuscript. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Author Contributions
D.G.D., Y.I.N., St.P., Sa.P., L.C. and A.Y. designed the research. D.G.D., Y.I.N., Y.C. and N.O. performed the experiments and analysed the data. D.G.D., Y.I.N. and H.B. wrote the paper with input from all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Dorothée G. Drucker and Yuichi I. Naito contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07065-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dorothée G. Drucker, Email: dorothee.drucker@ifu.uni-tuebingen.de
Yuichi I. Naito, Email: ynaito@num.nagoya-u.ac.jp
References
- 1.Benazzi S, et al. Early dispersal of modern humans in Europe and implications for Neanderthal behaviour. Nature. 2011;479(7374):525–528. doi: 10.1038/nature10617. [DOI] [PubMed] [Google Scholar]
- 2.Higham T, et al. The timing and spatiotemporal patterning of Neanderthal disappearance. Nature. 2014;512(7514):306–309. doi: 10.1038/nature13621. [DOI] [PubMed] [Google Scholar]
- 3.Hublin JJ. The modern human colonization of western Eurasia: when and where? Quat. Sci. Rev. 2015;118:194–210. doi: 10.1016/j.quascirev.2014.08.011. [DOI] [Google Scholar]
- 4.Fu Q, et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature. 2015;524(7564):216–219. doi: 10.1038/nature14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockett B, Haws JA. Nutritional ecology and the human demography of Neandertal extinction. Quat. Int. 2005;137(1):21–34. doi: 10.1016/j.quaint.2004.11.017. [DOI] [Google Scholar]
- 6.O’connell, J. F. How did modern humans displace Neanderthals? Insights from hunter-gatherer ethnography and archaeology In: When Neanderthals and Modern Humans Met. (ed. Conard, N. J.) 43–64 (Kerns Verlag, 2006).
- 7.Richards MP, Pettitt PB, Stiner MC, Trinkaus E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc. Natl. Acad. Sci. 2001;98(11):6528–6532. doi: 10.1073/pnas.111155298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards MP, Trinkaus E. Isotopic evidence for the diets of European Neanderthals and early modern humans. Proc. Natl. Acad. Sci. 2009;106(38):16034–16039. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514(7523):445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bocherens H, et al. Isotopic biogeochemistry (13C, 15N) of fossil vertebrate collagen: application to the study of a past food web including Neandertal man. J. Hum. Evol. 1991;20(6):481–492. doi: 10.1016/0047-2484(91)90021-M. [DOI] [Google Scholar]
- 11.Bocherens H, Drucker DG, Billiou D, Patou-Mathis M, Vandermeersch B. Isotopic evidence for diet and subsistence pattern of the Saint-Césaire I Neanderthal: review and use of a multi-source mixing model. J. Hum. Evol. 2005;49(1):71–87. doi: 10.1016/j.jhevol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Bocherens, H. Neanderthal dietary habits: review of the isotopic evidence In The evolution of Hominin diets (eds Hublin, J.-J. & Richards, M. P.) 241–250 (Springer, 2009).
- 13.Bocherens H, Drucker DG, Madelaine S. Evidence for a 15N positive excursion in terrestrial foodwebs at the Middle to Upper Palaeolithic transition in South-western France: implications for early modern human palaeodiet and palaeoenvironment. J. Hum. Evol. 2014;69:31–43. doi: 10.1016/j.jhevol.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Bocherens H. Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quat. Sci. Rev. 2015;117:42–71. doi: 10.1016/j.quascirev.2015.03.018. [DOI] [Google Scholar]
- 15.Naito YI, Chikaraishi Y, Ohkouchi N, Drucker DG, Bocherens H. Nitrogen isotopic composition of collagen amino acids as an indicator of aquatic resource consumption: insights from Mesolithic and Epipalaeolithic archaeological sites in France. World Archaeol. 2013;45(3):338–359. doi: 10.1080/00438243.2013.820650. [DOI] [Google Scholar]
- 16.Naito YI, et al. An overview of methods used for the detection of aquatic resource consumption by humans: Compound-specific delta N-15 analysis of amino acids in archaeological materials. J. Archaeol. Sci.: Reports. 2016;6:720–732. [Google Scholar]
- 17.Naito YI, Chikaraishi Y, Ohkouchi N, Yoneda M. Evaluation of carnivory in inland Jomon hunter–gatherers based on nitrogen isotopic compositions of individual amino acids in bone collagen. J. Archaeol. Sci. 2013;40(7):2913–2923. doi: 10.1016/j.jas.2013.03.012. [DOI] [Google Scholar]
- 18.Naito YI, et al. Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen. J. Hum. Evol. 2016;93:82–90. doi: 10.1016/j.jhevol.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 19.McClelland JW, Montoya JP. Trophic Relationships and the Nitrogen Isotopic Composition of Amino Acids in Plankton. Ecology. 2002;83(8):2173–2180. doi: 10.1890/0012-9658(2002)083[2173:TRATNI]2.0.CO;2. [DOI] [Google Scholar]
- 20.Chikaraishi Y, et al. Determination of aquatic foodweb structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Methods. 2009;7:740–750. doi: 10.4319/lom.2009.7.740. [DOI] [Google Scholar]
- 21.Chikaraishi Y, et al. High-resolution food webs based on nitrogen isotopic composition of amino acids. Ecol. and Evol. 2014;4:2423–2449. doi: 10.1002/ece3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janevic AA. Buran-Kaya 3 – Neue Angaben zur Kulturgliederung des Jungpaläolithikums der Krim. Préhist. Eur. 1998;13:133–148. [Google Scholar]
- 23.Pettitt, P. B. Middle and early upper Palaeolithic Crimea: the radiocarbon chronology in Préhistoire d’Anatolie, Genèse de deux mondes (ed. Otte, M.) 329–38 (ERAUL, 1998).
- 24.Chabai, V. P. The chronological and industrial variability of the Middle to Upper Paleolithic transition in eastern Europe in The chronology of the Aurignacian and of the transitional technocomplexes: Dating, stratigraphies, cultural implications (eds Zilhão, J. & d’Errico, F.) 71–288 (Instituto Português de Arqueologia, 2003).
- 25.Monigal, K. Introduction to the site of Buran-Kaya III In The Middle Paleolithic and Early Upper Paleolithic of Eastern Crimea (eds Chabai, V. P., Monigal, K. & Marks, A. E.), 3–18 (ERAUL, 2004).
- 26.Péan S, et al. The middle to upper Paleolithic sequence of Buran-Kaya III (Crimea, Ukraine): new stratigraphic, paleoenvironmental and chronological results. Radiocarbon. 2013;55(2–3):1454–1469. doi: 10.1017/S0033822200048384. [DOI] [Google Scholar]
- 27.Crépin L, Péan S, Lázničková-Galetová M. Comportements de subsistance au Paléolithique supérieur en Crimée: analyse archéozoologique des couches 6-2, 6-1 et 5-2 de Buran-Kaya III. L’Anthropologie. 2014;118(5):584–598. doi: 10.1016/j.anthro.2014.10.008. [DOI] [Google Scholar]
- 28.Prat S, et al. The Oldest Anatomically Modern Humans from Far Southeast Europe: Direct Dating, Culture and Behavior. PLoS ONE. 2011;6(6):e20834. doi: 10.1371/journal.pone.0020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crépin L, Prat S, Péan S, Yanevich A. Traitement du cadavre des plus anciens hommes anatomiquement modernes de l’extrême sud-est de l’Europe (Buran-Kaya III, Ukraine) Bull. Mém. Soc. Anthropol. Paris. 2012;22(1):11. [Google Scholar]
- 30.Yanevich A. Buran-Kaya culture of the Crimea’s gravett (in Ukrainian) Archeologia. 2000;2:11–20. [Google Scholar]
- 31.Yanevich A. Les occupations gravettiennes de Buran-Kaya III (Crimée): contexte archéologique. L’Anthropologie. 2014;118:554–566. doi: 10.1016/j.anthro.2014.10.006. [DOI] [Google Scholar]
- 32.DeNiro MJ. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature. 1985;317:806–809. doi: 10.1038/317806a0. [DOI] [Google Scholar]
- 33.Ambrose SH. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archeol. Sci. 1990;17(4):431–451. doi: 10.1016/0305-4403(90)90007-R. [DOI] [Google Scholar]
- 34.Styring AK, Sealy JC, Evershed RP. Resolving the bulk δ15N values of ancient human and animal bone collagen via compound-specific nitrogen isotope analysis of constituent amino acids. Geochim. Cosmochim. Acta. 2010;74:241–251. doi: 10.1016/j.gca.2009.09.022. [DOI] [Google Scholar]
- 35.Bocherens H. Isotopic biogeochemistry and the paleoecology of the mammoth steppe fauna. Deinsea. 2003;9(57):57–76. [Google Scholar]
- 36.Schwartz-Narbonne R, Longstaffe FJ, Metcalfe JZ, Zazula G. Solving the woolly mammoth conundrum: amino acid 15N-enrichment suggests a distinct forage or habitat. Sci. Rep. 2015;5:09791. doi: 10.1038/srep09791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jędrzejewski W, Jędrzejewska B. Foraging and diet of the red fox Vulpes vulpes in relation to variable food resources in Biatowieza National Park, Poland. Ecography. 1992;15(2):212–220. doi: 10.1111/j.1600-0587.1992.tb00027.x. [DOI] [Google Scholar]
- 38.Elmhagen B, Tannerfeldt M, Verucci P, Angerbjörn A. The arctic fox (Alopex lagopus): an opportunistic specialist. J. Zool. 2000;251(2):139–149. doi: 10.1111/j.1469-7998.2000.tb00599.x. [DOI] [Google Scholar]
- 39.Wißing, C. et al. Isotopic evidence for dietary ecology of Late Neandertals in North-Western Europe. Quaternary International411, 327–345 (2016).
- 40.Amundson R, et al. Global patterns of the isotopic composition of soil and plant nitrogen. Global biogeochemical cycles. 2003;17(1):1031. doi: 10.1029/2002GB001903. [DOI] [Google Scholar]
- 41.Murphy BP, Bowman DM. Kangaroo metabolism does not cause the relationship between bone collagen δ15N and water availability. Functional Ecology. 2006;20(6):1062–1069. doi: 10.1111/j.1365-2435.2006.01186.x. [DOI] [Google Scholar]
- 42.Kohn MJ. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate. Proc. Natl. Acad. Sci. USA. 2010;107(46):19691–19695. doi: 10.1073/pnas.1004933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordova CE, Rybak AR, Lehman PH. Vegetation patterns and conservation issues in southern Crimea. Post Sov. Geogr. Econ. 2001;42(5):362–385. [Google Scholar]
- 44.Bekenov AB, Grachev IA, Milner-Gulland EJ. The ecology and management of the saiga antelope in Kazakhstan. Mammal Rev. 1998;28(1):1–52. doi: 10.1046/j.1365-2907.1998.281024.x. [DOI] [Google Scholar]
- 45.Bocherens H, et al. Isotopic evidence for dietary ecology of cave lion (Panthera spelaea) in North-Western Europe: prey choice, competition and implications for extinction. Quat. Int. 2011;245(2):249–261. doi: 10.1016/j.quaint.2011.02.023. [DOI] [Google Scholar]
- 46.Drucker, D. G. et al. Aquatic resources in human diet in the Late Mesolithic in Northern France and Luxembourg: insights from carbon, nitrogen and sulphur isotope ratios. Archaeol. Anthropol. Sci. (2016).
- 47.Ridush B, et al. Emine-Bair-Khosar Cave in the Crimea, a huge bone accumulation of Late Pleistocene fauna. Quat. Int. 2013;284:151–160. doi: 10.1016/j.quaint.2012.03.050. [DOI] [Google Scholar]
- 48.Demidenko, Y. E. Crimean Upper Paleolithic in Encyclopedia of GlobalArchaeology (ed Smith, C.) 1782–1791 (Springer, 2014).
- 49.Buuveibaatar B, et al. Factors affecting survival and cause-specific mortality of saiga calves in Mongolia. J. Mamm. 2013;94(1):127–136. doi: 10.1644/11-MAMM-A-077.1. [DOI] [Google Scholar]
- 50.Trinkaus E, et al. An early modern human from the Peştera cu Oase, Romania. Proc. Natl. Acad. Sci. 2003;100(20):11231–11236. doi: 10.1073/pnas.2035108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trinkaus ER, et al. Stable isotope evidence for early modern human diet in southeastern Europe: Peştera cu Oase, Peştera Muierii and Peştera Cioclovina Uscată. Materiale şi Cercetări Arheologice. 2009;5:4–14. [Google Scholar]
- 52.Longin R. New method of collagen extraction for radiocarbon dating. Nature. 1971;230:241–242. doi: 10.1038/230241a0. [DOI] [PubMed] [Google Scholar]
- 53.Bocherens H, et al. Paleobiological implications of the isotopic signature (13C, 15N) of fossil mammal collagen in Scladina cave (Sclayn, Belgium) Quatern. Res. 1997;48:370–380. doi: 10.1006/qres.1997.1927. [DOI] [Google Scholar]
- 54.Chikaraishi Y, Kashiyama Y, Ogawa NO, Kitazato H, Ohkouchi N. Metabolic control of nitrogen isotope composition of amino acids in macroalgae and gastropods: implications for aquatic food web studies. Mar. Ecol. Prog. Ser. 2007;342:85–90. doi: 10.3354/meps342085. [DOI] [Google Scholar]
- 55.Chikaraishi Y, Ogawa NO, Doi H, Ohkouchi N. 15N/14N Ratios of Amino Acids as a Tool for Studying Terrestrial Food Webs: A Case Study of Terrestrial Insects (Bees, Wasps, and Hornets) Ecol. Res. 2011;26(4):835–844. doi: 10.1007/s11284-011-0844-1. [DOI] [Google Scholar]
- 56.Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: Coping with too much variation. PLoS One. 2010;5:e9672. doi: 10.1371/journal.pone.0009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.R Core Team. A language and environment for statistical computing. European Environment Agencyhttp://www.R-project.org (2013).
- 58.Bocherens H, et al. Reconstruction of the Gravettian food-web at Předmostí I using multi-isotopic tracking (13C, 15N, 34S) of bone collagen. Quat. Int. 2015;359:211–228. doi: 10.1016/j.quaint.2014.09.044. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


