Abstract
Neuronal excitabilities behave as the basic and important dynamics related to the transitions between firing and resting states, and are characterized by distinct bifurcation types and spiking frequency responses. Switches between class I and II excitabilities induced by modulations outside the neuron (for example, modulation to M-type potassium current) have been one of the most concerning issues in both electrophysiology and nonlinear dynamics. In the present paper, we identified switches between 2 classes of excitability and firing frequency responses when an autapse, which widely exists in real nervous systems and plays important roles via self-feedback, is introduced into the Morris-Lecar (ML) model neuron. The transition from class I to class II excitability and from class II to class I spiking frequency responses were respectively induced by the inhibitory and excitatory autapse, which are characterized by changes of bifurcations, frequency responses, steady-state current-potential curves, and nullclines. Furthermore, we identified codimension-1 and -2 bifurcations and the characteristics of the current-potential curve that determine the transitions. Our results presented a comprehensive relationship between 2 classes of neuronal excitability/spiking characterized by different types of bifurcations, along with a novel possible function of autapse or self-feedback control on modulating neuronal excitability.
Introduction
Neuronal electronic activities, such as firing and resting states, play basic and important roles in achieving biological functions of the nervous system1, 2 (for example, information encoding, transmission, and processing2–5). Early in 1948, Hodgkin distinguished different firing frequency responses of the resting state to external constant depolarization current stimulations, and then defined 3 classes of neuronal excitability6. As the depolarization current increases, the firing frequency continuously increases from 0 to a certain non-zero value for class I excitability, whereas it switches from 0 to a nearly fixed value for class II excitability. Except for the firing frequency response to external constant current, class I and II neurons exhibit different phase response curves to excitatory impulsive perturbations7, 8, different frequency response properties (integrator or resonator) to periodic stimulus2, 9, different coefficients of variation, or different histograms of interspike intervals to noise or stochastic stimulus10–12. Furthermore, neuronal excitabilities can affect spatiotemporal behaviors of the nervous system. For example, it is easier to obtain synchronization for a neuronal network with class II neurons than that with class I neurons7, 13–15. Neurons with class I and II excitabilities have been observed in biological experiments. For example, pyramid neurons in the hippocampus exhibit class I excitability2, 16, while interneurons in the neocortical and entorhinal cortex manifest class II excitability15, 17, 18.
In nonlinear dynamics, a relationship between neuronal excitability and bifurcation was built1–3. For example, class I excitability corresponds to a resting state (stable equilibrium) changed to firing (limit cycle) via saddle-node on invariant circle (SNIC) bifurcation as the depolarization current increases; meanwhile, class II excitability corresponds to Andronov-Hopf bifurcations1–3, which have been widely investigated in the classical 2-dimensional Morris-Lecar (ML) model with variables (V, w)3, 16, 19–21. The position relationship between 2 nullclines is different for the 2 classes of excitability. The nullcline is tangent to at saddle-node for class I excitability, and intersects at focus for class II excitability. Furthermore, the transitions between 2 classes of neuronal excitability can be well interpreted by the high-codimension bifurcations. For example, a codimension-2 Bogdanov-Takens bifurcation1–3, 19–28 related to both Hopf and saddle-node bifurcations, and a codimension-3 bifurcation29, 30 (degenerate pitchfork bifurcation) have been investigated. In addition, the classes of spiking2, which are characterized by firing frequency responses to a decrease in depolarization current, have attracted research attention. For example, if the firing or spiking is changed to resting state via an SNIC as the depolarization current is decreased, the spiking is named as class I since the spiking exhibits a nearly zero frequency. If the firing or spiking is changed to resting state via a fold bifurcation of limit cycle (LPC) near a subcritical Hopf (SubH) bifurcation point, the spiking is called class II because of a non-zero firing frequency. The classes of spiking corresponding to bifurcations of the limit cycle, combined with the classes of excitability, are helpful for understanding the dynamics of transitions between resting and firing states1–3, 19–28.
In biophysics, neuronal excitability is related to the balance between different ionic currents and the competition between inward and outward currents, especially for mammalian central neurons with rich ion channels, which is characterized by current-voltage (I–V) curves16, 19, 28–30. For SNIC, net current at perithreshold potentials is inward at steady-state (the steady-state I–V curve is non-monotonic), but for SubH, net current at steady state is outward (the steady-state I–V curve is monotonic decreases), and the inward current activates are faster than outward current activities16, 19. The I–V curves have relationships to the excitability classes2, 16, 19. For example, the increase in the outward current contributed by the M-type potassium current can well interpret the transition from class I excitability in vitro to class II excitability under vivo-like condition16. The increase of A-type potassium conductance or the decrease of calcium conductance, which corresponds to the increase in the outward current or decrease in the inward current, respectively, can both induce the transition from class II to class I, and vice versa31.
The modulations that can induce a neuron to switch between class I and II excitabilities are from external influences outside the neuron. However, there are only a few32 investigations on the switch between class I and II excitabilities induced by an important self-modulation to the current across the membrane–autapse33. Autapse of a neuron is a special synapse that connects to the neuron itself33, and has been observed anatomically in nervous systems such as in the cerebral cortex, neocortex, cerebellum, hippocampus, striatum, and visual cortex33–39. Through biological experiments34–36, autapses have been found to play many roles, such as in the persistent firings induced by positive self-feedback mediated by excitatory autapses34, and repetitive firing with precise spike timing induced by inhibitory autapses35, 36. Model simulations also found that autapses have other roles in neuronal systems32, 40–61, for example, autapse-induced stochastic resonance, coherence resonance, and formations in isolated neurons or neuronal networks. A recent study32 shows that inhibitory autapse can induce interneuron switches from class I excitability to class II excitability/spiking characterized by codimension-1 bifurcations and frequency responses. Inhibitory and excitatory autapses of a neuron can generate “negative” autaptic current and “positive” autaptic current, respectively, i.e., an outward current and inward current, to the neuron itself. The current mediated by the autapse may induce transition between the 2 classes of excitability through changes in competition between outward and inward currents, the position relationship between nullclines, bifurcations, as well as firing frequency.
In the present paper, we proposed a comprehensive viewpoint that autapses can induce transition between class I and II excitabilities/spikings in the ML model. That is, inhibitory autapses can induce transition from class I to II excitability due to the outward current mediated by the autapse, wherein the role of autaptic current is identified as the M-type potassium (K +) current. Excitatory autapses can induce transition from class II to class I spiking because of the inward current mediated by the autapse, and the role of autaptic current is the same as that of the L-type potassium (Ca 2+) current. In this paper, we described the transitions between class I and II excitabilities/spikings by firing frequency responses, net current at steady-state, and different bifurcations. Except the well-known SNIC corresponding to class I excitability/spiking and subcritical Hopf bifurcation to class II excitability, more complex codemension-1 and -2 bifurcations related to excitability/spiking classes were identified. The results of the present work not only provided a novel function of the inhibitory autapse in regulating neuronal firing dynamics, which is helpful for understanding the roles of different kinds of autapses in neuroscience and neurodynamics, but also presented a comprehensive relationship between the classes of neuronal excitability/spiking and bifurcations, firing frequency responses, current-potential curves at steady-state, and nullclines. Different from the investigation32, the codimension-2 bifurcations and biophysical basis of the transition between classes of neuronal excitability and the excitatory autapse were investigated in the present study.
Results
The influence of the inhibitory and excitatory autapses on the nullcline
The introduction of the autapse to the ML model changed the nullcline , and therefore changed the position relationship between and , which provided the possibility of bifurcations changes and a transition between excitability/spiking classes. This is because the position relationship between and determined the bifurcations.
The function Γ(V − Θsyn) = 1/ in the autapse model is shown in Fig. 1(a), which implies that autaptic current in the present study (Θsyn = −20 mV, λ = 1, 2) took effect in the range of V > −30 mV. As g inh (g exc) increased, the curve shape of exhibited 2 characteristics when I app was fixed at I app = 39.96 mA/cm2 and I app = 52.765 mA/cm2, respectively, as shown in Fig. 1(b,c). The first is that the part when V > −30 mV shifts down for the inhibitory autapses and up for the excitatory autapses. The other characteristic is that the shape of nullcline around V = −30 mV changes for both inhibitory and excitatory autapses, as shown in Fig. 1(b,c). As I app increased or decreased, the nullcline moved up or down, leading to changes in the position relationship between and , which implied that transitions between bifurcations could be induced by changes in g inh (g exc) and I app.
Figure 1.
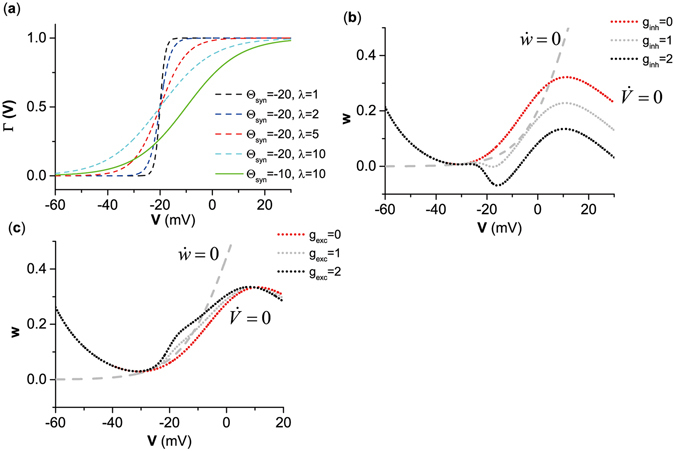
Effects of autaptic currents on nullcline . (a) The curves of Γ(V) with different Θsyn and λ. (b) Changes of the nullcline induced by the inhibitory autapse and I app = 39.96 μA/cm2. (c) Changes of the nullcline induced by the excitatory autapse and I app = 52.765 μA/cm2. In (b,c), the gray dashed curve represents nullcline of , the dotted curve with different colors represent nullcline at different autaptic strengths.
Notations of nonlinear dynamics or bifurcations
For convenience, notations about codim-1 and codim-2 bifurcations encountered in the present paper are summarized in Table 1.
Table 1.
Notations and abbreviations of the bifurcations.
| Co-dimension | Bifurcations | Abbreviations |
|---|---|---|
| Codim-1 | Supercritical Hopf bifurcation | SupH |
| Saddle node bifurcation | or , i = 1, 2 | |
| Saddle node on invariant circle | SNIC | |
| Saddle homoclinic orbit | BHom and SHom | |
| Saddle node bifurcation of limit cycle | LPC | |
| Codim-2 | Cusp bifurcation | CP |
| Bautin bifurcation | GH | |
| Bogdanov-Takens bifurcation | BT | |
| saddle-node separatrix-loop | SNHO |
For codim-1 bifurcations, SubH and SupH respectively represent subcritical and supercritical Hopf bifurcations. and are the saddle-node bifurcation of equilibrium, where the superscript “S” (“U”) means that a stable (unstable) node collides with a saddle, and the subscript “i” is an integer to indicate the sequence of the appearance of this kind of bifurcation. SNIC is saddle-node on invariant circle bifurcation. BHom and SHom represent saddle homoclinic bifurcation, where “B” and “S” are used to distinguish the size of the saddle homoclinic orbit. LPC is saddle-node (fold) bifurcation of the limit cycle.
For codim-2 bifurcations, CP represents cusp bifurcations of equilibrium, GH is Bautin (degenerate Hopf) bifurcations of equilibrium, BT represents Bogdanov-Takens bifurcations of equilibrium, and SNHO is a saddle-node separatrix-loop.
Transition from class I to class II excitability/spiking induced by the inhibitory autapse
In this subsection, the ML model without inhibitory autapse exhibited class I excitability/spiking. We investigated the dynamics of the ML model after the introduction of an inhibitory autapse.
Six examples of one-parameter bifurcations and firing frequency responses
In the ML model with and without inhibitory autapses, 6 different cases of bifurcations related to excitability/spiking and transition from class I to class II excitability/spiking were identified, as shown in Table 2. Six representative examples corresponding to the 6 cases are introduced here in details.
Table 2.
Six cases of bifurcations corresponding to excitability/spiking classes induced by inhibitory autapse.
| Case | g inh | Resting state → Spiking (I app increases) | Spiking → Resting state (I app decreases) | ||||
|---|---|---|---|---|---|---|---|
| Bifurcation | Frequency | Excitability | Bifurcation | Frequency | Spiking | ||
| 1 | (0, 0.3645) | SNIC | Zero | I | SNIC | Zero | I |
| 2 | (0.3645, 0.385) | Nonzero | II | SNIC | Zero | I | |
| 3 | (0.385, 0.55) | Nonzero | II | BHom | Zero | I | |
| 4 | (0.55, 3.032) | SubH | Nonzero | II | BHom | Zero | I |
| 5 | (3.032, 4.05) | SubH | Nonzero | II | LPC | Nonzero | II |
| 6 | (4.05, 5.0) | SupH | Nonzero | II | SupH | Nonzero | II |
The 6 examples of the bifurcation and firing frequency response with g inh = 0, 0.372, 0.5, 1.0, 3.5, and 4.4 μS/cm2 are shown in Fig. 2. For case 1 and case 6, changes from the resting state to spiking (and vice versa) were through the same bifurcation point, and no coexisting behaviors were detected. For cases 2 to 5, the bifurcation via which the resting state changes to spiking was different from the bifurcation from the spiking to resting state showed the coexistence of a resting state and spiking. For case 1 to case 6, the bifurcations related to excitability classes, corresponding to bifurcation from the resting state to spiking, were SNIC (I app ≈ 39.96 μA/cm2), (I app ≈ 40.21 μA/cm2), (I app ≈ 44.85 mA/cm2), SubH (I app ≈ 64.67 mA/cm2), SubH (I app ≈ 174.85 mA/cm2), and SupH (I app ≈ 217.39 mA/cm2), respectively; meanwhile, bifurcations associated with the spiking classes, corresponding to bifurcation from the spiking to resting state, were SNIC (I app ≈ 39.96 μA/cm2), SNIC (I app ≈ 39.96 μA/cm2), BHom (I app ≈ 43.57 μA/cm2), BHom (I app ≈ 62.49 μA/cm2), LPC (I app ≈ 174.71 μA/cm2), and SupH (I app ≈ 217.39 μA/cm2), respectively.
Figure 2.
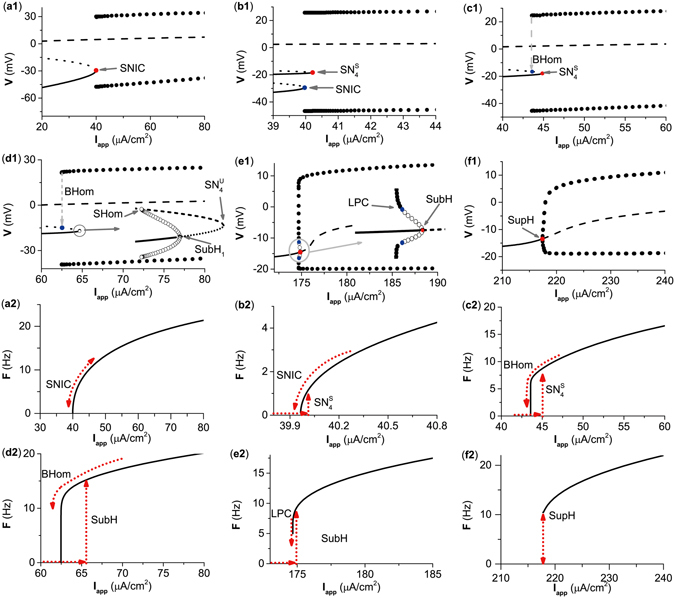
Dynamics of the ML model with respect to I app at different g exc values. (a1–f1) Bifurcation diagrams showing voltage at equilibriums and at max/min of limit cycle as I app is increased when g inh at different values. (a2–f2) Frequency-current (F-I) curves showing firing frequency as I app is increased when g inh at different values. (a1,a2) g inh = 0 μS/cm2. (b1,b2) g inh = 0.372 μS/cm2. (c1,c2) g inh = 0.5 μS/cm2. (d1,d2) g inh = 1.0 μS/cm2. (e1,e2) g inh = 3.5 μS/cm2. (f1,f2) g inh = 4.4 μS/cm2. In panels (a1–f1), the solid, dotted and dashed lines represent stable equilibrium, saddle and unstable equilibrium, respectively. The upper and lower solid (open) circles are the maximum and minimum values of the stable (unstable) limit cycle, respectively. SNIC represents saddle-node bifurcation on an invariant circle. SubH and SupH represent subcritical Hopf bifurcation and supercritical Hopf bifurcation, respectively. BHom and SHom represent saddle homoclinic orbit bifurcation. and represent saddle node bifurcation of equilibrium. LPC represents saddle node bifurcation of limit cycle.
For case 1, as I app approached the SNIC, the firing frequency exhibited a nearly 0 value, corresponding to the infinite period of the invariant cycle, as depicted in Fig. 2(a2), which shows that the excitability/spiking was class I. For cases from 2 to 6, the firing frequency exhibited a non-zero value as I app increased across the bifurcation point from the resting state to spiking, showing that the excitability was class II (Fig. 2(b–f)). Furthermore, the bifurcation SNIC was related to class I excitability, and bifurcations SN, SubH, and SupH were related to class II excitability. Transition from class I excitability to class II excitability occurred when case 1 changed to case 2. It should be emphasized that 2 stable nodes corresponding to the resting state appeared in case 2. One was related to the bifurcation SNIC appearing at I app ≈ 39.96 μA/cm2, while the other corresponded to the located at I app ≈ 40.21 mA/cm2, as shown in Fig. 2(b1). Since the bifurcation was located right next to the SNIC, the , across which the resting state changed to spiking, is suggested to be related to the excitability class in case 2.
Additionally, the class of spiking in case 2 was still related to SNIC and still class I, as depicted in Fig. 2(b2). For case 3 and case 4, the firing frequency exhibited a nearly 0 value as the spiking changed to the resting state via the BHom, which shows that the spiking was still class I (Fig. 2(c2–d2)). Different from the SNIC (case 1), wherein the firing frequency increased relative slowly with increasing I app, the firing frequency exhibited a fast increase with a logarithmic scale near the BHom2. For case 5 and case 6, the firing frequency exhibited a non-zero value as I app decreased across the bifurcation point from the spiking to resting state, thus showing that the spiking was class II (Fig. 2(e2–f2)). Moreover, the transition from class I spiking to class II spiking appeared when case 4 changed to the case 5. The SNIC and BHom were associated with class I spiking, while LPC and SupH corresponded to class II spiking. With decreasing across the LPC, a stable limit cycle and an unstable limit cycle collided with each other and disappeared, as shown in Fig. 2(f1). The stable limit cycle exhibited a non-zero firing frequency. For example, the firing frequency was 7.5 Hz (non-zero) when I app = 174.72 μA/cm2, as shown in Fig. 2(f2).
Position relationship between nullclines, dynamics in the phase plane, and firing frequency near the bifurcation point
The SNIC is a standard saddle-node bifurcation point located on an invariant circle with an infinite period. The saddle-node point (half hollow circle) and the invariant circle (gray solid line) for the SNIC (Fig. 2(a1)) are shown in Fig. 3(a1). The saddle-node bifurcation point corresponds to the tangent point (−29.39, 0.0085) between nullclines (red dashed curve) and (red dotted curve) in the phase plane. As I app approached the SNIC point (I app ≈ 39.96 μA/cm2), the spiking exhibited a long period, i.e., a low firing frequency because of being near the SNIC. For example, the spiking frequency with (I app = 39.97 μA/cm2) was very low (0.72 Hz, Fig. 3(a2)). This is because that SNIC corresponded to class I excitability/spiking.
Figure 3.
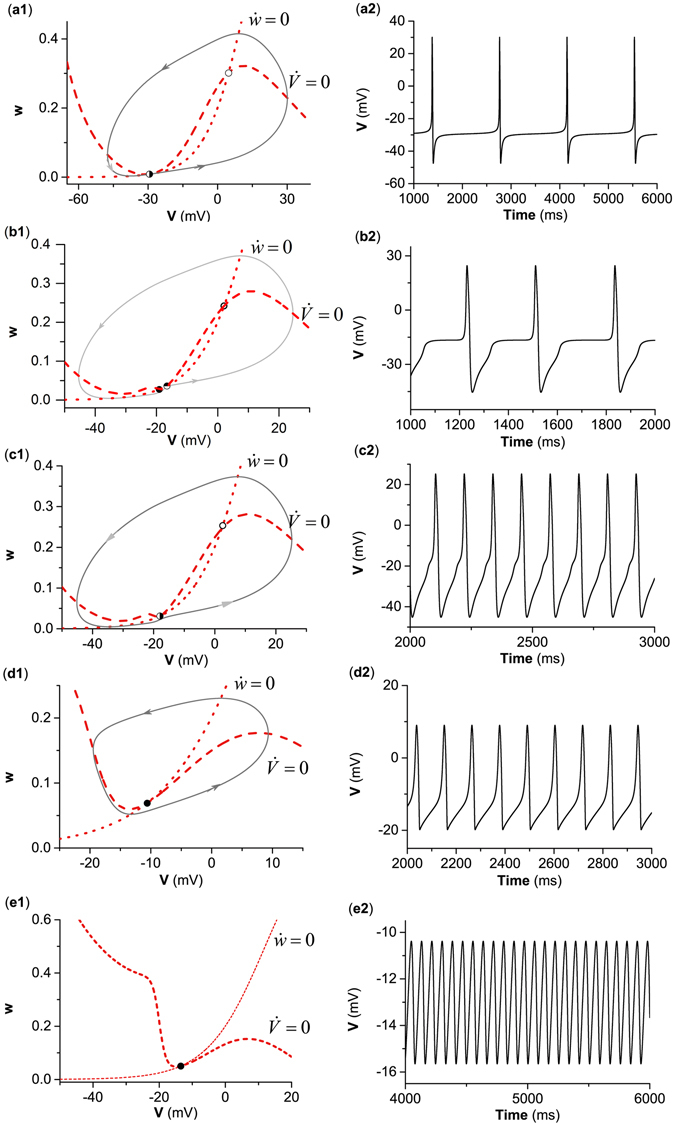
Dynamics corresponding to or near the different bifurcation points. (a) SNIC with g inh = 0 μS/cm2; (b) BHom with g inh = 0.5 μS/cm2; (c) with g inh = 0.5 μS/cm2; (d) SubH with g inh = 3.5 μS/cm2; (e) SupH at = g inh = 4.4 μS/cm2. Left: nullclines, equilibriums, and limit cycles or orbits in the phase portraits; Right: Spike trains. Parameter values: (a2) I app = 39.98 μA/cm2; (b2) I app = 43.572 μA/cm2; (c2) I app = 44.8461 μA/cm2; (d2) I app = 179.2 μA/cm2; (e2) I app = 217.39 μA/cm2. In panels (a1–e1), the red dotted line and dashed line represent nullcline and nullcline , respectively. The solid line represents trajectory and arrow indicates direction.
The BHom corresponds to a saddle contacted with a stable limit cycle, which is a homoclinic orbit with an infinite period. If the limit cycle is unstable, the saddle homoclinic bifurcation is subcrtical. For the BHom shown in Fig. 2(c1), the saddle (half hollow circle) is the intersection points between the nullclines (red dashed curve) and (red dotted curve), as shown in Fig. 3(b1). The homoclinic orbit is shown by the gray curve. In addition, a stable node and an unstable focus are shown by the solid black point and the hollow circle, respectively. If a spiking was close to the BHom (I app ≈ 43.57 μA/cm2), the spiking also exhibited a low firing frequency due to the homoclinic orbit. For example, when I app = 43.57 μA/cm2, the spiking exhibited a low frequency (3.3 Hz), as shown in Fig. 3(b2). This is because that BHom was related to class I spiking.
The (I app ≈ 44.8461 μA/cm2) shown in Fig. 2(c1) corresponds to the tangent point (−17.936, 0.031) (half hollow circle) between the nullclines (red dashed curve) and (red dotted curve), which is a standard saddle-node bifurcation off limit cycle, as illustrated in Fig. 3(c1). When I app = 40.21 μA/cm2, the spiking exhibited non-zero frequency (8.55 Hz) (Fig. 3(c2)). This is because that was associated with class II excitability.
For SubH (I app ≈ 174.85 μA/cm2) shown in Fig. 2(d), the equilibrium corresponds to the intersection point (−10.59, 0.069, solid dot) between nullclines (red dashed curve) and (red dotted curve), as shown in Fig. 3(d1). In addition, a spiking (gray solid curve) appeared at I app = 174.85 μA/cm2. This spiking was a limit cycle bifurcated from the LPC that appeared at I app = 174.71 μA/cm2 and near the SubH. When I app = 174.85 μA/cm2, the spiking exhibited a fixed non-zero firing frequency (8.82 Hz) (Fig. 3(d2)). This is because that SubH was associated with class II excitability.
SupH (I app ≈ 217.3 μA/cm2) shown in Fig. 2(e) corresponds to the intersection point (−13.481, 0.0507) (the solid dot) between nullclines (red dashed curve) and (red dotted curve) in the phase plane, as shown in Fig. 3(e1). The amplitude of the limit cycle increased from 0 as I app increased, i.e., there was a transition from subthreshold oscillation to spiking with nearly fixed, non-zero firing frequency. For example, when I app = 217.6 mA/cm2, the neuron exhibited a fast subthreshold oscillation (12 Hz) (Fig. 3(e2)). This is because that SupH was associated with class II excitability.
Steady-state I–V curves for 5 types of codimension-1 bifurcations
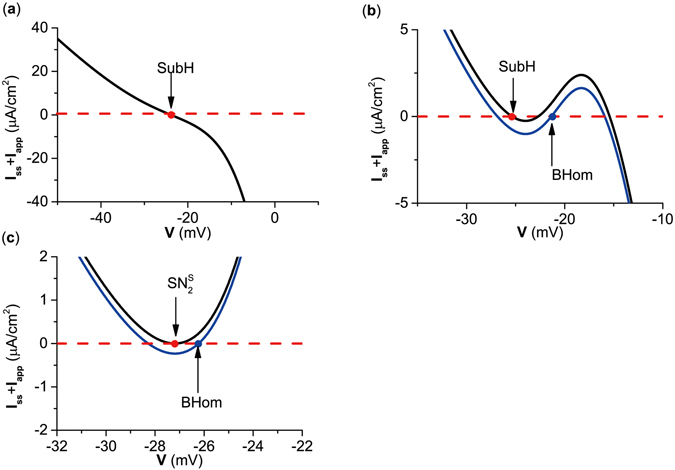
In previous studies for SNIC and SubH, ∂I/∂V = 0 and ∂I/∂V < 0, respectively, were used to characterize class I and II excitability. In fact, the inhibitory autapse could induce changes in I–V curves because .
In the present paper, the I–V curves for 5 types of codimension-1 bifurcations related to equilibrium point corresponding to Fig. 1(a–f) are respectively shown in Fig. 4(a–f). For SNIC and , ∂I/∂V = 0, which corresponds to the net perithreshold current at steady-state being inward. Meanwhile, ∂I/∂V < 0 for SubH and SupH, which shows that current at steady-state monotonically decreased with respect to the increase in V, and the net perithreshold current at steady-state was outward. For BHom, ∂I/∂V > 0, which corresponds to the net perithreshold current at steady-state being inward. Investigations for the SNIC and SubH can can be found in the literature21, while the I–V curves for saddle-node bifurcations, SupH, and BHom are new findings. ∂I/∂V = 0, ∂I/∂V < 0, and ∂I/∂V > 0 were proved as the necessary conditions for SNIC and SN bifurcations, SubH and SupH bifurcations, and for BHom bifurcations, respectively. The detailed proofs are provided in the Methods section.
Figure 4.
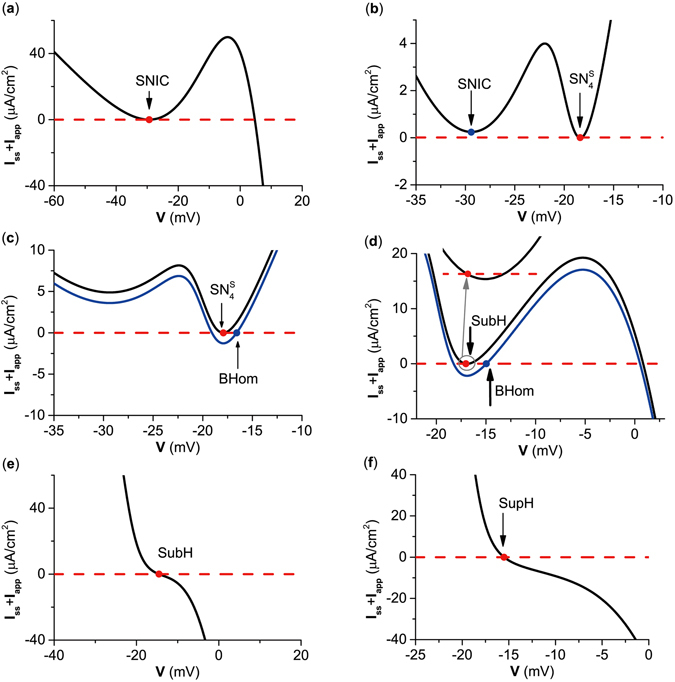
Steady state I–V curves for 5 bifurcation points related to equilibrium. (a) g inh = 0 μS/cm2; (b) g inh = 0.372 μS/cm2; (c) g inh = 0.5 μS/cm2; (d) g inh = 1.0 μS/cm2; (e) g inh = 3.5 μS/cm2; (f) g inh = 4.4 μS/cm2. SNIC represents saddle-node bifurcation on an invariant circle. SubH and SupH represent subcritical Hopf bifurcation and supercritical Hopf bifurcation, respectively. BHom represents saddle homoclinic orbit bifurcation. represents saddle node bifurcation of equilibrium.
Codimension-2 bifurcations related to transitions from class I to II excitability/spiking
We then investigated the bifurcation diagrams in the plane (I app, g inh), and acquired comprehensive views of transitions from class I to class II excitability/spiking (Fig. 5). The detailed dynamics of special or codimension-2 bifurcation points and codimension-1 bifurcation curves related to class I to class II excitability/spiking are introduced firstly, and then the whole framework of the bifurcations in the plane (I app, g inh) are provided. The resting state, coexisting behaviors of the resting and spiking states, and spiking state, are labeled with gray horizontal lines, red or pink sloping lines, and gray vertical lines, respectively.
Figure 5.
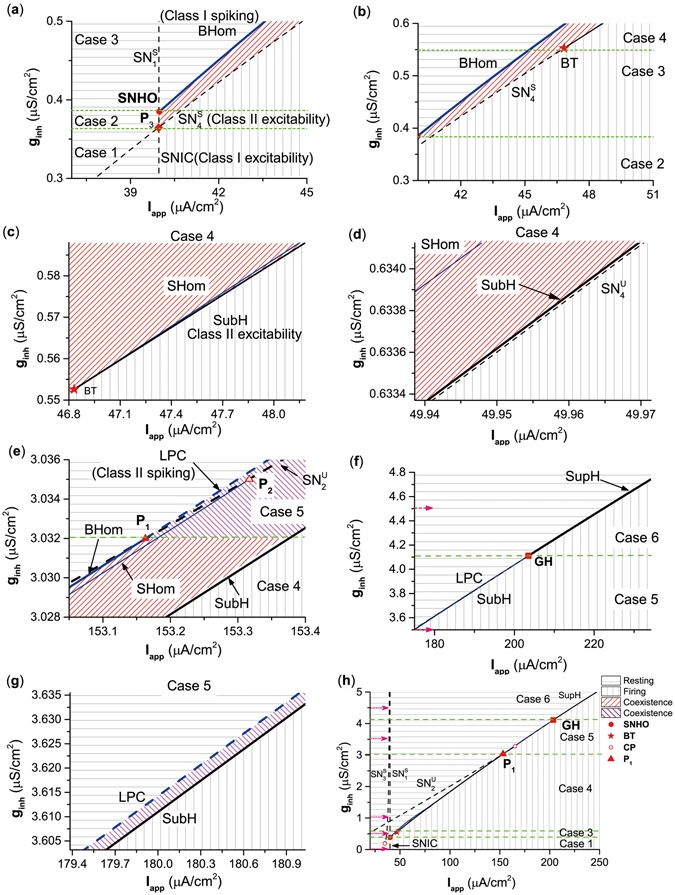
Two-parameter bifurcations in the plane (I app, g inh) of the ML model with an inhibitory autapse. (a) Partial enlargement of the bifurcation diagram around SNHO and P 3. (b) Partial enlargement of the bifurcation diagram around BT. (c) Partial enlargement of the bifurcation diagram is up and to the right of BT in (b). (d) Further amplification is up and to the right of BT in (b). (e) Partial enlargement of the bifurcation diagram around P 1. (f) Partial enlargement of the bifurcation diagram around GH in (h). (g) Partial enlargement of the bifurcation diagram is up and to the right of GH. (h) Global view of the bifurcation diagram. Figure (a–f) is a part of Fig. (h). SNIC represents the curve of saddle-node bifurcation on an invariant circle. SubH and SupH are subcritical Hopf curve and supercritical Hopf curve, respectively. BHom and SHom are saddle homoclinic orbit bifurcation curves. and (i = 1, 2, 3, 4) are curves of saddle node bifurcation of equilibrium. LPC represents the curve of saddle node bifurcation of limit cycle. CP is cusp bifurcation. BT is Bogdanov-Takens bifurcation. SNHO is saddle-node separatrix-loop. GH is Bautin bifurcation.
The borders between 2 neighboring cases from cases 1 to 6 are horizontal green lines across 5 special points, P 3, SNHO, BT, P 1, and GH, with increasing g inh. P 3 (I app ≈ 36.96 μA/cm2, g inh ≈ 0.3645 μS/cm2) is the intersection point between the bifurcation curves and SNIC, as shown in Fig. 5(a). The SNHO (red solid circle) (I app ≈ 39.98 μA/cm2, g inh ≈ 0.385 μS/cm2) is the intersection point between the SNIC curve and curve, and is the origin of the BHom curve (Fig. 5(a)). The BT (red star) point (I app ≈ 46.83 μA/cm2, g inh ≈ 0.55 μS/cm2) corresponds to the tangent point between the curves of SubH and saddle-node bifurcations ( and ), and is the origin of the SHom curve, as shown in Fig. 5(b,c). The position relationship between the 3 bifurcation curves, SubH, SHom, and are shown in Fig. 5(d). The P 1 point (I app ≈ 151.163 μA/cm2, g inh ≈ 3.032 μS/cm2) is the termination of the bifurcation curve BHom and the origin of the LPC curve, and is located on the saddle-node bifurcation curve of , as shown in Fig. 5(e). The GH point (I app ≈ 203.55 μA/cm2, g inh ≈ 4.11 μS/cm2) is the transition point between curves SubH and SupH, and is the termination of the LPC curve, as shown in Fig. 5(f). The position relationship between bifurcation curves SubH and LPC are shown in Fig. 5(g).
Bifurcation curves SNIC, , SubH, and SupH, and bifurcation/special points P 3, BT, and GH are related to excitability classes. Right next to these curves, the behavior is spiking. The SNIC curve was related to class I excitability, while the other curves were associated with class II excitability. The transition from class I to class II excitabilities occurred at point P 3.
Bifurcation curves SNIC, BHom, LPC, and SupH, and special bifurcation points P 3, SNHO, P 1, and GH are related to spiking classes. To the left of these curves, the behavior is resting. The SNIC and BHom curves corresponded to class I spiking, while the LPC and SupH curves were related to class II spiking. The transition from class I to class II spiking occurred at point P 1.
The framework of the dynamic behaviors, codimension-2 points, and bifurcation curves are shown in Fig. 5(h). The different codimension-1 bifurcation curves ran from bottom-left to upper-right to form the borders between resting and firing states. The 5 points, P 3, SNHO, BT, P 1, and GH, are shown by the red rhombus, red solid circle, red star, red triangle, and red solid square, respectively. The horizontal green lines run across the 5 points to form the border between the 6 cases, and the 6 pink arrows with dotted lines represent the positions of the 6 examples of bifurcations. However, the parameter region of the coexisting behaviors was too small to be visible in the scale used in Fig. 5(h). In addition, there were other codimension-1 bifurcation curves ( and ) and bifurcation points (P 2 and CP), that had no direct relationship to the transition between neuronal excitability/spiking classes, and thus were not introduced in the present paper.
Transition from class II to class I spiking and the bifurcations induced by the excitatory autapse
In this subsection, we discuss how the ML model without excitatory autapses exhibited class II excitability/spiking. Then, we present the dynamics of the ML.
Three cases of one-parameter bifurcations and firing frequency responses
We identified 3 cases of bifurcations related to classes of excitability/spiking, as shown in Table 3, and the corresponding examples (g exc = 0, 0.5 and 2.0 μS/cm2) are shown in Fig. 6. All 3 cases exhibited coexisting behaviors lying between the resting state and spiking.
Table 3.
Three cases of bifurcations corresponding to neuronal excitability/spiking classes induced by the excitatory autapse.
| Case | g exc | Resting state → Spiking (I app increases) | Spiking → Resting state (I app decreases) | ||||
|---|---|---|---|---|---|---|---|
| Bifurcation | Frequency | Excitability | Bifurcation | Frequency | Spiking | ||
| 1 | (0, 0. 406) | SubH | Nonzero | II | SubH | Nonzero | II |
| 2 | (0.406, 1.59) | SubH | Nonzero | II | BHom | Zero | I |
| 3 | (1.5977, 2.0) | Nonzero | II | BHom | Zero | I | |
Figure 6.
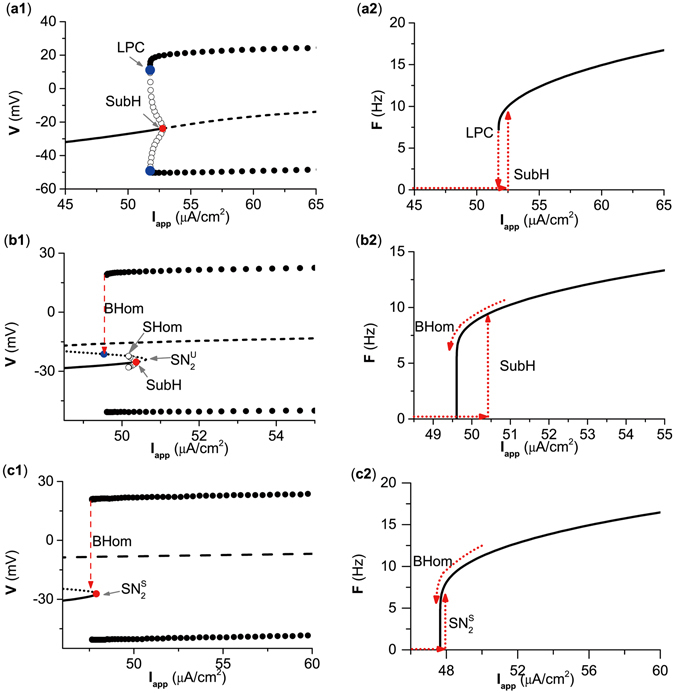
Dynamics of the ML model with respect to I app at different g exc values. (a) g exc = 0.0 μS/cm2; (b) g exc = 0.5 μS/cm2; (c) g exc = 2.0 μS/cm2. Left: bifurcation diagrams of V; Right: firing frequency-current curve. In panels (a1–c1), the solid, dotted and dashed lines represent stable equilibrium, saddle and unstable equilibrium, respectively. The upper and lower solid (open) circles are the maximum and minimum values of the stable (unstable) limit cycle, respectively. BHom and SHom represent saddle homoclinic orbit bifurcation. represents saddle node bifurcation of equilibrium. LPC represents saddle node bifurcation of limit cycle.
For cases 1 to 3, the bifurcations related to the excitability class were SubH (I app ≈ 52.765 μA/cm2), SubH (I app ≈ 50.36 μA/cm2) and (I app ≈ 47.9 μA/cm2). All 3 cases exhibited class II excitability. The bifurcations associated with the spiking class were LPC (I app ≈ 51.75 μA/cm2), BHom (I app ≈ 49.6 μA/cm2), and BHom (I app ≈ 47.66 μA/cm2) for cases 1, 2, and 3, respectively. Case 1 exhibited class I spiking, while case 2 and case 3 had class II spiking. The transition from class I spiking to class II spiking occurred when case 1 changed to case 2.
The steady I–V curves for 5 types of codimension-1 bifurcations
The I–V curve at steady-state exhibited ∂I/∂V < 0 at SubH, ∂I/∂V > 0 at BHom, and ∂I/∂V = 0 at , as show in Fig. 7. These are consistent with the proof shown in the Methods section. Results showed that the transition of bifurcations corresponding to excitability/spiking could be induced by the competition between inward and outward currents, which was achieved by changes in the inward current mediated by the excitatory autapse.
Figure 7.
Steady state I–V curves. (a) g exc = 0.0 μS/cm2; (b) g exc = 0.5 μS/cm2; (c) g exc = 2.0 μS/cm2. SubH represents subcritical Hopf bifurcation. BHom represents saddle homoclinic orbit bifurcation. represents saddle node bifurcation of equilibrium.
Bifurcations and behaviors related to transitions from class II to I spiking in the two-dimensional parameter space
We acquired the dynamic behaviors, codimension-1 bifurcation curves, and special and codimension-2 bifurcation points in the 2-dimensional parameter space (I app, g exc) of the ML model with excitatory autapse (Fig. 8). The left border of the coexisting behavior contains the BHom and LPC curves, and the right border is composed of the SubH and Curves. The LPC curve changed to a BHom curve at the special point P 1 (I app ≈ 49.84 μA/cm2, g exc ≈ 0.41 μS/cm2, red solid triangle), which induced the transition from class II spiking to class I spiking. Meanwhile, the bifurcation curve SubH changed to at the codimension-2 bifurcation point BT (I app ≈ 48.34 μA/cm2, g exc ≈ 1.5977 μS/cm2, red star); however, the excitability remained unchanged as class II. The horizontal green lines across P 1 and BT are the border between case 1 and 2, and between case 2 and 3, respectively. The positions of the 3 examples of bifurcations shown in Fig. 6 correspond to the pink dotted arrows.
Figure 8.
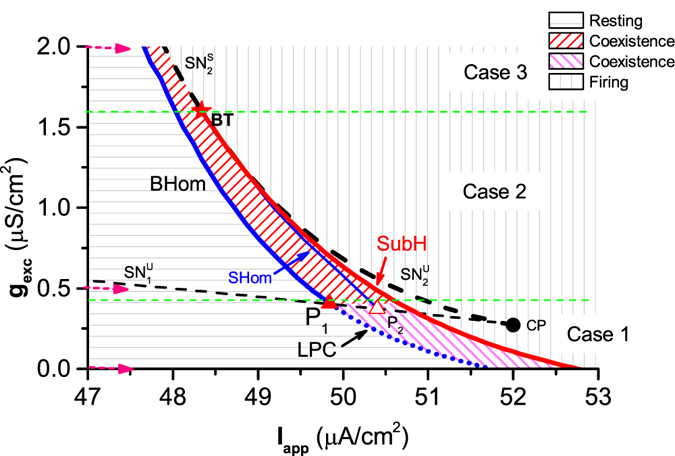
Two parameter bifurcations in the plane (I app, g exc) of the ML model with an excitatory autapse. SubH is subcritical Hopf curve. BHom and SHom are saddle homoclinic orbit bifurcation curves. , , and are curves of saddle node bifurcation of equilibrium. LPC represents the curve of saddle node bifurcation of limit cycle. CP is cusp bifurcation. BT is Bogdanov-Takens bifurcation.
The bifurcation curves related to excitability/spiking classes induced by the excitatory autapse changed from upper-left to bottom-right, which was different from that induced by the inhibitory autapse (Fig. 5). In addition, the bifurcation points P 2 (the red open triangle) and CP (the red open circle), and the codimension-1 bifurcation curves , (the black dashed line), and SHom, had no direct relationship to the transition between classes of excitability and spiking, and thus was not introduced in the present paper.
Discussion
Two classes of neuronal excitability/spiking exhibit different electrophysiological properties and nonlinear dynamics that may have different effects in both single neurons and networks2, 7–9. This has been one of the most important subjects in both neuroscience and neurodynamics1–3. For example, the transition between classes of neuronal excitability/spiking have been observed in biological experiments, and can be used to interpret physiological phenomenon such as the resonance of pyramidal neurons at theta frequency, and symptoms of demyelination16, 62. The neuronal excitability/spiking excitability is classified into different classes by distinct firing frequency responses to external stimulation, which is determined by bifurcations1–3, 16, 20–24, 28, 62 in nonlinear dynamics and the balance between the inward and outward currents across the membrane16, 19. Class I excitability is related to a nearly 0 firing frequency, while class II excitability is related to a non-zero firing frequency. Transition between these 2 classes, which is induced by the modulation of ionic currents2, 16, 19–28, have been investigated with bifurcations and steady-state I–V curves2, 19.
It is well known that class I excitability is related to SNIC bifurcation, wherein the nullcline is tangent to the nullcline , and ∂I/∂V = 0. Conversely, class II excitability is associated with SubH bifurcation, wherein the nullcline intersects with the nullcline , and ∂I/∂V < 0. The transition between classes of neuronal excitability is related to codimension-2 bifurcation BT2, 16, 19–28. In the present paper, we found a comprehensive relationship between classes of neuronal excitability/spiking and the codimension-1 bifurcations, codimension-2 bifurcations, nullclines, and steady-state I–V curves. Firstly, class I excitability had a relationship to codimension-1 bifurcations such as SNIC, while class II excitability was associated with codimension-1 bifurcations such as SN, SubH, and SupH. Secondly, the nullcline was tangent to the nullcline for SNIC and SN, and intersected with the nullcline for SubH, SupH, and BHom. Thirdly, class I spiking was related to codimension-1 bifurcations such as SNIC and BHom, while class II spiking was related to codimension-1 bifurcations such LPC and SupH. Fourthly, we identified the relationships between steady-state I–V curves and 5 types of codimension-1 bifurcations. For SNIC and SN, ∂I/∂V = 03, 19. For SubH and SupH, ∂I/∂V < 03, 19. For supercritical saddle Homoclinic bifucation BHom, ∂I/∂V > 0. Lastly, we found that multiple codimension-2 bifurcation points, including the BT, CP, and GH points, or special points P 1 and P 3, were related to classes of excitability or spiking.
In the present paper, we found that the inhibitory and excitatory autapses regulate the classes of neuronal excitability/spiking. Different from previous studies wherein the modulations to a neuron are external, the inhibitory autapse and excitatory autapse of a neuron provided “negative” (outward) current and “positive” (inward) current to the neuron via self-feedback, which plays different roles on the generation of an action potential2, 16, 19, 31. Autaptic currents have the same roles as some ion currents with same direction across the membrane. For example, the inhibitory autapse induced the transition from class I excitability/spiking to class II excitability/spiking, which is like other outward currents such as M-type potassium currents; meanwhile, the excitatory autapse induced the transition from class II spiking to class I spiking, resembles other inward currents, such as L-type calcium currents2, 16, 19, 31. The autaptic current has the same effect on variation of net current as those of blocking ion currents in experiments. For example, inhibitory autapse induces the change from class I to class II, which is consistent with blocking low threshold calcium currents and persistent sodium currents in spinal lamina I neurons19; excitatory autapse induces the change from class II to class I, which is consistent with blocking potassium currents in mesencephalic neurons, spinal lamina I neurons and cortical interneuron19, 63. In addition, experiments show that pyramidal and dopamine neurons in vivo exhibit activities different from those in vitro, which may be due to synaptic input which changes the net current16, 27.
The classes of neuronal excitability are influenced by the direction and magnitude of the net current active at perithreshold potentials4, 16, 19. The net current at perithreshold potentials for class I, II and III are weak inward, weak outward and strong outward currents4, 16, 19, respectively. The switches of neuronal excitability caused by regulating ion currents have been studied extensively. Experiment shows that the excitability of mesencephalic neurons switches from class III to class II and to class I through blocking potassium currents (outward current)63. Therefore, it can be speculated that autapse may also induce transition between classes of neuronal excitability related to class III excitability. For example, when an inhibitory autapse is introduced to ML model with class I excitability, the net current at perithreshold potentials may change from weak inward current (class I) to weak outward current (class II) and to strong outward current (class III) as autaptic strength increases. When an excitatory autapse is introduced to ML model with class III excitability, the net current at perithreshold potentials may change from strong outward current (class III) to weak outward current (class II) and to weak inward current (class I) as autaptic strength increases.
Based on the progress of the present paper, there are several questions to be answered in the future studies. Firstly, noise is ubiquitous in nervous systems and can induce phenomenon different from the deterministic system64–69. For example, stochastic integer multiple firing patterns and on-off firing patterns have been identified induced by noise near super-critical and sub-critical Hopf bifurcations, respectively, and have been related to coherence resonance66–69. Correspondingly, the frequency curve also changes. It can be speculated that complex stochastic dynamics appear near the bifurcation points when noise is introduced, which awaits further studied in future. Secondly, time delay is another important factor for the autapse, which can influence the activities of neurons such as firing patterns and firing region32, 45–59. When time delay is introduced, more complex bifurcations will be induced and should be calculated in future investigated. Lastly, the paper present dynamic mechanisms and biophysical basis of the autapses that can regulate the classes of the neuronal excitability/spiking, which awaits the experimental demonstration in future investigation.
Materials and Methods
The Morris-Lecar (ML) model and the autapse model
The ML model is widely used to investigate class I excitability and class II excitability, and is described as follows3, 10, 20:
| 1 |
| 2 |
where C is the membrane capacitance, and V(t) and w(t) are the membrane voltage and activation of delayed rectified K + current, respectively. The first 3 terms in the right-hand side of Eq. 1 represent the Ca 2+ current, the voltage-gated delayed-rectifier K + current, and the leak current. Parameters g Ca, g K and g L are the maximal conductances of the calcium current, potassium current, and leak current, respectively. V Ca and V K and V L are the reversal potentials of the calcium current, potassium current, and leak current, respectively. Lastly, I app is the applied (depolarization) current. , , .
The parameters are set as follows: C = 20 μF/cm2, V K = −84 mV, V Ca = 120 mV, V L = −60 mV, g K = 8 μS/cm2, g Ca = 4 μS/cm2, g L = 2 μS/cm2, V 1 = −1.2 mV, V 2 = 18 mV, V 4 = 17.4 mV, ϕ = 0.067. Furthermore, V 3 = 12 mV for class I excitability, and V 3 = 2 mV for class II excitability.
The autapse model, which was based the sigmoid function of the presynaptic voltage with a threshold Θsyn, is described by the following equation70:
| 3 |
where is the sigmoidal function, V pre and V pos are presynaptic and postsynaptic membrane potentials, respectively, Θsyn is the synapse threshold, and V syn is the reversal potential. Θsyn was set −20 mV to ensure that a spike can cross it. V syn was set 10 mV for excitatory autapses, and −60 mV for inhibitory autapses. g s is the synaptic strength, where the subscript “s” is “exc” for the excitatory autapse and “inh” for the inhibitory autapse. V pre = V pos = V to ensure that the synapse is autapse. Finally, λ determines the release rate of neurotransmitters, and was set as 1 for the inhibitory autapse, and 2 for the excitatory autapse.
Methods
The ML model was integrated with the Runge-Kutta method with a time step of 0.001 ms. Bifurcations were acquired using the bifurcation toolbox Matcont71 and XPPAUT72.
For the ML model, the steady state I–V curve is described as
and then
| 4 |
The characteristics of the steady-state I–V curve corresponding to different bifurcations were acquired by stability analysis of steady-state and linearization to the ML model.
The ML model with autapse can be described as follows:
| 5 |
| 6 |
Suppose that (V *, w *) is the equilibrium of the ML model with autapse, then, the Jacobian matrix J of the model at resting state (V *, w *) is as follows:
where and . Then the character equation is described as:
where and . Then, we obtained the characteristics of the steady-state I–V curve based on the necessary condition for the different bifurcations of equilibrium.
If the equilibrium (V *, w *) is saddle-node or SNIC bifurcation, then b and c satisfy b > 0 and c = 0. According to Eq. 4, and thus, , and then ∂I/∂V = 0. Therefore, ∂I/∂V = 0 is the necessary condition for SNIC bifurcation or saddle-node bifurcation.
If the equilibrium (V *, w *) is Hopf bifurcation, then b and c satisfy b = 0 and c > 0. From c > 0, we can acquire ∂I/∂V < 0. This indicates that ∂I/∂V < 0 at the equilibrium (V *, w *) is the necessary condition for Hopf bifurcation.
If the equilibrium (V *, w *) is saddle homoclinic bifurcation, then b and c satisfy b < 0 and b 2 − 4c > 0 at the saddle, and the saddle quantity equals c. If the saddle homoclinic bifurcation is supercritical2, 73, the saddle quantity is negative, i.e. c < 0 for the BHom, which implies that ∂I/∂V > 0 at equilibrium (V *, w *) is the necessary condition for supercritical saddle homoclinic bifurcation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos 11372224 and 11572225.
Author Contributions
Z.Z. and H.G. conceived the experiments, Z.Z. conducted the experiments, Z.Z. and H.G. analyzed the results. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Izhikevich, E. M. Neural excitability, spiking and bursting. Int. J. Bifurcat. Chaos10(6), 1171–1266 (2000).
- 2.Izhikevich, E. M. Dynamical systems in neuroscience: The geometry of excitability and bursting (MIT Press, 2007).
- 3.Rinzel, J. Analysis of neuronal excitability and oscillations. In: Koch, C. & Segev, I. editors Methods in Neuronal Modeling: from Synapses to Networks (MIT Press, 1998).
- 4.Ratté S, Hong SG, De Schutter E, Prescott SA. Impact of neuronal properties on network coding: roles of spike initiation dynamics and robust synchrony transfer. Neuron. 2013;78(5):758–772. doi: 10.1016/j.neuron.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bean BP. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8(6):451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 6.Hodgkin AL. The local electric changes associated with repetitive action in a non-medullated axon. J. Physiol. 1948;107(2):165–181. doi: 10.1113/jphysiol.1948.sp004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermentrout GB. Type I membranes, phase resetting curves, and synchrony. Neural Comput. 1996;8(5):979–1001. doi: 10.1162/neco.1996.8.5.979. [DOI] [PubMed] [Google Scholar]
- 8.Smeal RM, Ermentrout GB, White JA. Phase-response curves and synchronized neural networks. Philos. Trans. R. Soc. B Biol. Sci. 2010;365(1551):2407–2422. doi: 10.1098/rstb.2009.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutcheon B, Yarom Y. Resonance. oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23(5):216–222. doi: 10.1016/S0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- 10.Tateno T, Pakdaman K. Random dynamics of the Morris-Lecar neural model. Chaos. 2004;14(3):511–530. doi: 10.1063/1.1756118. [DOI] [PubMed] [Google Scholar]
- 11.Jia B, Gu HG. Identifying type I excitability using dynamics of stochastic neural firing patterns. Cogn. Neurodyn. 2012;6(6):485–497. doi: 10.1007/s11571-012-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SG, Neiman A, Kim S. Coherence resonance in a Hodgkin-Huxley neuron. Phys. Rev. E. 1998;57(3):3292–3297. doi: 10.1103/PhysRevE.57.3292. [DOI] [Google Scholar]
- 13.Hansel D, Mato G, Meunier C. Synchrony in excitatory neural networks. Neural Comput. 1995;7(2):307–337. doi: 10.1162/neco.1995.7.2.307. [DOI] [PubMed] [Google Scholar]
- 14.Bogaard A, Parent J, Zochowski M, Booth V. Interaction of cellular and network mechanisms in spatiotemporal pattern formation in neuronal networks. J. Neurosci. 2009;29(6):1677–1687. doi: 10.1523/JNEUROSCI.5218-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tikidji-hamburyan RA, Martinez JJ, White JA, Canavier CC. Resonant interneurons can increase robustness of Gamma oscillations. J. Neurosci. 2015;35(47):15682–15695. doi: 10.1523/JNEUROSCI.2601-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott SA, Ratté S, De Schutter E, Sejnowski TJ. Pyramidal neurons switch from integrators in vitro to resonators under in vivo-like conditions. J. Neurophysiol. 2008;100(6):3030–3042. doi: 10.1152/jn.90634.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J. Neurosci. 2007;27(8):2058–2073. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J. Neurophysiol. 1999;82(5):2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- 19.Prescott SA, Koninck YD, Sejnowski TJ. Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput. Biol. 2008;4(10):e1000198. doi: 10.1371/journal.pcbi.1000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsumoto K, Kitajima H, Yoshinaga T, Aihara K, Kawakami H. Bifurcations in Morris-Lecar neuron model. Neurocomputing. 2006;69(4):293–316. doi: 10.1016/j.neucom.2005.03.006. [DOI] [Google Scholar]
- 21.Liu CM, Liu XL, Liu SQ. Bifurcation analysis of a Morris-Lecar neuron model. Biol. Cybern. 2014;108(1):75–84. doi: 10.1007/s00422-013-0580-4. [DOI] [PubMed] [Google Scholar]
- 22.Shigeki T, Tetsushi U, Hiroshi K, Hiroshi F, Kazuyuki A. Bifurcations in two-dimensional Hindmarsh-Rose type model. Int. J. Bifurcat. Chaos. 2011;17(3):985–998. [Google Scholar]
- 23.Chen SS, Cheng CY, Lin YR. Application of a two-dimensional Hindmarsh-Rose type model for bifurcation analysis. Int. J. Bifurc. Chaos. 2013;23(3):50055. doi: 10.1142/S0218127413500557. [DOI] [Google Scholar]
- 24.Liu XL, Liu SQ. Codimension-two bifurcation analysis in two-dimensional Hindmarsh-Rose model. Nonlinear Dyn. 2012;67(1):847–857. doi: 10.1007/s11071-011-0030-6. [DOI] [Google Scholar]
- 25.Maesschalck PD, Wechselberger M. Neural excitability and singular bifurcations. J. Math. Neurosci. 2015;5:16. doi: 10.1186/s13408-015-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan LX, Zhai DH, Lu QS. Bifurcation and bursting in Morris-Lecar model for Class I and Class II excitability. Discrete Cont. Dyn. Syst. B. 2011;3(3):391–399. [Google Scholar]
- 27.Morozova EO, Zakharov D, Gutkin BS, Lapish CC, Kuznetsov A. Dopamine neurons change the type of excitability in response to stimuli. PLoS Comput. Biol. 2016;12(12):e1005233. doi: 10.1371/journal.pcbi.1005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeberg H, Blomberg C, Arhem P. Ion channel density regulates switches between regular and fast spiking in soma but not in axons. PLoS Comput. Biol. 2010;6(4):e1000753. doi: 10.1371/journal.pcbi.1000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franci A, Drion G, Sepulchre R. An organizing center in a planar model of neuronal excitability. SIAM J. App. Dyn. Syst. 2012;11(4):1698–1722. doi: 10.1137/120875016. [DOI] [Google Scholar]
- 30.Franci A, Drion G, Seutin V, Sepulchre R. A balance equation determines a switch in neuronal excitability. PLoS Comput. Biol. 2013;9(5):e1003040. doi: 10.1371/journal.pcbi.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drion G, O’Leary T, Marder E. Ion channel degeneracy enables robust and tunable neuronal firing rates. Proc. Natl. Acad. Sci. USA. 2015;112(38):5361–5370. doi: 10.1073/pnas.1516400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo DQ, et al. Firing regulation of fast-spiking interneurons by autaptic inhibition. Europhys. Lett. 2016;114(3):30001. doi: 10.1209/0295-5075/114/30001. [DOI] [Google Scholar]
- 33.Loos HVD, Glaser EM. Autapses in neocortex cerebri: synapses between a pyramidal cells axon and its own dendrites. Brain Res. 1972;48:355–360. doi: 10.1016/0006-8993(72)90189-8. [DOI] [PubMed] [Google Scholar]
- 34.Saada R, Miller N, Hurwitz I, Susswein AJ. Autaptic excitation elicits persistent activity and a plateau potential in a neuron of known behavioral function. Curr. Biol. 2009;19(6):479–684. doi: 10.1016/j.cub.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 35.Bacci A, Huguenard JR, Prince DA. Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J. Neurosci. 2003;23(3):859–866. doi: 10.1523/JNEUROSCI.23-03-00859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacci A, Huguenard JR. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron. 2006;49(1):119–130. doi: 10.1016/j.neuron.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Cobb SR, et al. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience. 1997;79(3):629–648. doi: 10.1016/S0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 38.Tamás G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J. Neurosci. 1997;17(16):6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pouzat C, Marty A. Autaptic inhibitory currents recorded from interneurones in rat cerebellar slices. J. Physiol. 1998;509(3):777–783. doi: 10.1111/j.1469-7793.1998.777bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yilmaz E, Baysal V, Perc M, Ozer M. Enhancement of pacemaker induced stochastic resonance by an autapse in a scale-free neuronal network. Sci. China Technol. Sci. 2016;59(3):364–370. doi: 10.1007/s11431-015-5984-z. [DOI] [Google Scholar]
- 41.Yilmaz E, Ozer M, Baysal V, Perc M. Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci. Rep. 2016;6:30914. doi: 10.1038/srep30914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song XL, Wang CN, Ma J, Tang J. Transition of electric activity of neurons induced by chemical and electric autapses. Sci. China Technol. Sci. 2015;58(6):1007–1014. doi: 10.1007/s11431-015-5826-z. [DOI] [Google Scholar]
- 43.Qin HX, Ma J, Wang CN, Wu Y. Autapse-induced spiral wave in network of neurons under noise. PLoS One. 2014;9(6):e100849. doi: 10.1371/journal.pone.0100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connelly WM. Autaptic connections and synaptic depression constrain and promote gamma oscillations. PLoS One. 2014;9(2):e89995. doi: 10.1371/journal.pone.0089995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu YN, Gong YB, Wang Q. Autaptic activity-induced synchronization transitions in Newman-Watts network of Hodgkin-Huxley neurons. Chaos. 2015;25(4):043113. doi: 10.1063/1.4918997. [DOI] [PubMed] [Google Scholar]
- 46.Wang HT, Ma J, Chen YL, Chen Y. Effect of an autapse on the firing pattern transition in a bursting neuron. Commun. Nonlinear Sci. Numer. Simul. 2014;19(9):3242–3254. doi: 10.1016/j.cnsns.2014.02.018. [DOI] [Google Scholar]
- 47.Wang HT, Wang LF, Chen YL, Chen Y. Effect of autaptic activity on the response of a Hodgkin-Huxley neuron. Chaos. 2014;24(3):033122. doi: 10.1063/1.4892769. [DOI] [PubMed] [Google Scholar]
- 48.Hashemi M, Valizadeh A, Azizi Y. Effect of duration of synaptic activity on spike rate of a Hodgkin-Huxley neuron with delayed feedback. Phys. Rev. E. 2012;85(2):021917. doi: 10.1103/PhysRevE.85.021917. [DOI] [PubMed] [Google Scholar]
- 49.Yilmaz E, Baysal V, Ozer M, Perc M. Autaptic pacemaker mediated propagation of weak rhythmic activity across small-world neuronal networks. Physica A. 2016;444:538–546. doi: 10.1016/j.physa.2015.10.054. [DOI] [Google Scholar]
- 50.Guo DQ, et al. Regulation of irregular neuronal firing by autaptic transmission. Sci. Rep. 2016;6:26096. doi: 10.1038/srep26096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang HT, Chen Y. Firing dynamics of an autaptic neuron. Chin. Phys. B. 2015;24(12):128709. doi: 10.1088/1674-1056/24/12/128709. [DOI] [Google Scholar]
- 52.Xu Y, Ying HP, Jia Y, Ma J, Hayat T. Autaptic regulation of electrical activities in neuron under electromagnetic induction. Sci. Rep. 2017;7:4352. doi: 10.1038/s41598-017-04022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin HX, Ma J, Jin WY, Wang CN. Dynamics of electric activities in neuron and neurons of network induced by autapses. Sci. China Technol. Sci. 2014;57(5):936–946. doi: 10.1007/s11431-014-5534-0. [DOI] [Google Scholar]
- 54.Yilmaz E, Ozer M. Delayed feedback and detection of weak periodic signals in a stochastic Hodgkin-Huxley neuron. Physica A. 2015;421:455–462. doi: 10.1016/j.physa.2014.10.096. [DOI] [Google Scholar]
- 55.Wang, C. N. et al. Formation of autapse connected to neuron and its biological function. Complexity 5436737 (2017).
- 56.Wang HT, Chen Y. Response of autaptic Hodgkin-Huxley neuron with noise to subthreshold sinusoidal signals. Physica A. 2016;462:321–329. doi: 10.1016/j.physa.2016.06.019. [DOI] [Google Scholar]
- 57.Li YY, Schmid G, Hanggi P, Schimansky-Geier L. Spontaneous spiking in an autaptic Hodgkin-Huxley setup. Phys. Rev. E. 2010;82(6):061907. doi: 10.1103/PhysRevE.82.061907. [DOI] [PubMed] [Google Scholar]
- 58.Gong YB, Wang BY, Xie HJ. Spike-timing-dependent plasticity enhanced synchronization transitions induced by autapses in adaptive Newman-Watts neuronal networks. Biosystems. 2016;150:132–137. doi: 10.1016/j.biosystems.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Gong YB, Wu YN. Autaptic self-feedback-induced synchronization transitions in Newman-Watts neuronal network with time delays. Eur. Phys. J. B. 2015;88(4):103. doi: 10.1140/epjb/e2015-60164-8. [DOI] [Google Scholar]
- 60.Ma, J. & Tang, J. A review for dynamics in neuron and neuronal network. Nonlinear Dyn., doi:10.1007/s11071-017-3565-3 (2017).
- 61.Zhao ZG, Jia B, Gu HG. Bifurcations and enhancement of neuronal firing induced by negative feedback. Nonlinear Dyn. 2016;86(3):1549–1560. doi: 10.1007/s11071-016-2976-x. [DOI] [Google Scholar]
- 62.Coggan JS, Prescott SA, Bartol TM, Sejnowski TJ. Imbalance of ionic conductances contributes to diverse symptoms of demyelination. Proc. Natl. Acad. Sci. USA. 2010;107(48):20602. doi: 10.1073/pnas.1013798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, et al. Membrane current-based mechanisms for excitability transitions in neurons of the rat mesencephalic trigeminal nuclei. Neuroscience. 2009;163(3):799–810. doi: 10.1016/j.neuroscience.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Guo DQ, Li CG. Stochastic and coherence resonance in feed-forward-loop neuronal network motifs. Phys. Rev. E. 2009;79(5):051921. doi: 10.1103/PhysRevE.79.051921. [DOI] [PubMed] [Google Scholar]
- 65.Guo DQ, Wang QY, Perc M. Complex synchronous behavior in interneuronal networks with delayed inhibitory and fast electrical synapses. Phys. Rev. E. 2012;85(6):061905. doi: 10.1103/PhysRevE.85.061905. [DOI] [PubMed] [Google Scholar]
- 66.Paydarfar D, Forger DB, Clay JR. Noisy inputs and the induction of on-off switching behavior in a neuronal pacemaker. J. Neurophysiol. 2006;96(6):3338–3348. doi: 10.1152/jn.00486.2006. [DOI] [PubMed] [Google Scholar]
- 67.Gu HG, Pan BB, Chen GR, Duan LX. Biological experimental demonstration of bifurcations from bursting to spiking predicted by theoretical models. Nonlinear Dyn. 2014;78(1):391–407. doi: 10.1007/s11071-014-1447-5. [DOI] [Google Scholar]
- 68.Gu HG. Different bifurcation scenarios of neural firing pattern in identical pacemakers. Int. J. Bifurcat. Chaos. 2013;23(12):1350195. doi: 10.1142/S0218127413501952. [DOI] [Google Scholar]
- 69.Gu HG. Experimental observation of transitions from chaotic bursting to chaotic spiking in a neural pacemaker. Chaos. 2013;23(2):023126. doi: 10.1063/1.4810932. [DOI] [PubMed] [Google Scholar]
- 70.Somers D, Kopell N. Rapid synchronization through fast threshold modulation. Biol. Cybern. 1993;68(5):393–407. doi: 10.1007/BF00198772. [DOI] [PubMed] [Google Scholar]
- 71.Dhooge A, Govaerts W, Kuznetsov YA. MATCONT: A MATLAB package for numerical bifurcation analysis of ODEs. ACM Trans. Math. Softw. 2003;29(2):141–164. doi: 10.1145/779359.779362. [DOI] [Google Scholar]
- 72.Ermentrout, B. Simulating, analyzing, and animating dynamical systems: A guide to XPPAUT for researchers and students (SIAM Philadelphia, 2002).
- 73.Kuznetsov, Y. A. Elements of applied bifurcation theory (Springer-Verlag, 1995).


