Abstract
Background
Previous studies on the effect of zolpidem or zopiclone use on the risk of road traffic crashes (RTCs) have shown mixed results.
Objective
Our objective was to determine the association between zolpidem or zopiclone use (as separate drugs or combined) and the occurrence of injurious RTCs among older adult drivers.
Methods
This was a population-based matched case–control and case–crossover study based on secondary data linked together from Swedish national registers. Cases were drivers aged 50–80 years involved in a vehicle crash resulting in injuries between January 2006 and December 2009 for the case–control study (n = 27,096) and from February 2006 to December 2009 for the case–crossover study (n = 26,586). For the first design, four controls were matched to each case by sex, age, and residential area, and exposure was categorized into new, occasional, and frequent use of zolpidem only, zopiclone only, and combined zolpidem and zopiclone. For the case–crossover study, newly dispensed zolpidem or zopiclone users were assessed during the 28 days prior to the crash and compared with an equally long control period using a 12-week washout period. Matched adjusted odds ratios (OR) were computed using conditional logistic regression.
Results
Increased ORs for all users were observed. In the case–control study, the highest odds were seen among newly initiated zolpidem-only users involved in single-vehicle crashes (adjusted OR 2.27; 95% confidence interval [CI] 1.21–4.24), followed by frequent combined zolpidem and zopiclone users [adjusted OR 2.20; CI 1.21–4.00]. In the case–crossover, newly initiated treatment with zolpidem or zopiclone showed an increased risk that was highest in the 2 weeks after the start of the treatment (OR 2.66; 95% CI 1.04–6.81).
Conclusions
These results provide more compelling evidence for the role of zolpidem or zopiclone in the occurrence of RTCs among older adults, not only in frequent users, but also at the beginning of treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s40263-017-0445-9) contains supplementary material, which is available to authorized users.
Key Points
| Previous studies of the effect of zolpidem or zopiclone on the risk of road traffic crashes have shown mixed results. |
| This large population-based matched case–control and case–crossover study found an association with a higher occurrence of injurious road traffic crashes among older adults, in not only frequent users but also new users. |
| Users of both zolpidem and zopiclone have relatively higher risks than those using zolpidem only or zopiclone only. |
Introduction
Hypnotics are one of the most commonly prescribed drug classes in older adults [1]. For decades, benzodiazepines have been the main class used for insomnia treatment, but concerns about adverse effects have led to the use of non-benzodiazepine hypnotics such as zolpidem or zopiclone [2]. Currently, the consumption of these drugs among older adults in Norway, Sweden, and the USA exceeds that of benzodiazepines [3–5]. Furthermore, a recent study from Canada showed that the incidence of use of these medications among older adults continued to increase, whereas the opposite trend was observed for benzodiazepine [6].
While both drugs are considered to have better pharmacological profiles and safer residual effects than benzodiazepines [2], studies reveal side effects such as drowsiness, dizziness, and somnolence [7, 8], which are likely to impair cognitive function [9] and consequently driving performance [10–15]. Age-related changes in the pharmacokinetics and pharmacodynamics of these medications [16, 17] put elderly people at higher risk of such effects. Thus, older adults dependent on their driving ability [10, 18] should be considered a potentially exposed group for the occurrence of road traffic crashes (RTC) when under treatment with such medications.
Previous studies investigating associations between zolpidem or zopiclone intake and driving impairment [10–15] or RTCs [19–28] among younger adults [10], older adults [11, 14, 15, 28], or both groups combined [12, 13, 19–27] have reported mixed results. The discrepancy observed seems to relate mainly to the frequency of use. For instance, while most studies report driving impairments after newly initiated zolpidem or zopiclone use [10–13], the effect is less evident for occasional or frequent users [14, 15]. For elderly drivers, the evidence is also contentious, with some studies showing an increased risk [19–22] or no risk [25–29] among current users (i.e. zolpidem or zopiclone prescribed 1 day prior to the crash). Of those reporting increased risks, one was with drivers taking more than one tablet per day for the past 5 months prior to the crash [23] and another was with individuals using a high dosage [29], adding to the variability of the results. However, so far, researchers have neither focused on elderly drivers nor specifically looked at newly initiated zolpidem or zopiclone use in this group.
Therefore, this study was undertaken to determine the association between zolpidem or zopiclone use and the occurrence of injurious RTCs among older adult drivers, focusing on new, occasional, and frequent users.
Methods
Study Design
A population-based matched case–control design was used linking data from various Swedish registers. To determine the association between newly initiated zolpidem or zopiclone and the occurrence of injurious RTCs, a case–crossover study was also carried out, as this design is suitable to assess short-term effects of intermittent exposures for the risk of acute outcomes while inherently adjusting for time-invariant confounders [30, 31].
The Swedish Traffic Accident Data Acquisition (STRADA) register [32] was used to identify cases. This nationwide register routinely collects police and hospital information about RTCs occurring on Swedish roads [32]. Data extracted included RTC date and place, person’s seating position (driver or passenger), type of crash, and suspected driver alcohol use. Data on medication use, including type of drugs dispensed, anatomical therapeutic chemical (ATC) classification codes, and date of dispensation were obtained from the Swedish Prescribed Drug Register (SPDR) [33]. All prescriptions dispensed in Sweden, excluding drugs used during hospitalization and over-the-counter medications, are recorded in this register [33]. The Total Population Register and National Driving Licence Register [34, 35] were used to select potential controls for the case–control study using information on age, sex, and type and validity of driving licence. The Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA), an annual register that records socioeconomic data of residents aged ≥16 years, was used to obtain information about marital status, occupation, and education [36]. Data on medical conditions, particularly the discharged diagnosis based on International Classification of Diseases and Related Health Problems, tenth version (ICD-10) codes, was extracted from the National Inpatient Register [37], which contains information on inpatient care in all Swedish hospitals. All these registers are updated regularly, and information between registers was linked using the unique ten-digit personal identification number assigned to all Swedish residents [38].
Cases were car, bus, or truck drivers aged 50–80 years involved in an RTC leading to at least one injured person from 1 January 2006 to 31 December 2009 for the case–control and between 1 February 2006 and 31 December 2009 for the case–crossover. We included drivers aged from 50 years to capture older adults with various levels of driving exposure (i.e. older adults who had not retired might drive more than those who had retired). Furthermore, age-related physiological changes might already be occurring around this age, which could affect the pharmacokinetics and pharmacodynamics of the drugs studied [39]. To prevent reverse causality, we used only the first crash in the study period (i.e. to ensure the medications were not prescribed as a result of the crash). The crash date was considered the index date. Individuals suspected of driving under the influence of alcohol according to the police were excluded, as this is a known factor for the occurrence of RTCs, particularly in combination with medications [26, 40].
For the case–control study, four controls were randomly chosen from the population and the National Driver’s Licence Register [34, 35] and individually matched to each case by sex, age, and area of residence. Differences in RTC risks have been reported [41], as well as patterns of medication use, including Z-drugs [3, 42], by sex and age. Area of residence was matched to adjust for geographical differences, which relate to the availability of transportation means and to road and weather conditions that can affect crash risks. Eligible controls were Swedish residents aged 50–80 years with a valid driving licence who were not involved in an RTC during the study period. Once a control was assigned to a case, that individual could no longer be matched to another case. More details about the selection of cases and controls can be obtained from the Electronic Supplementary Material [ESM] 1.
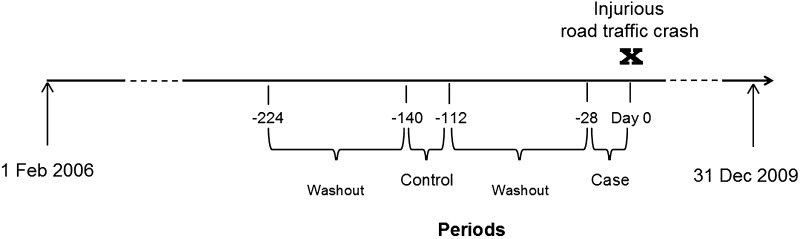
The same cases were used for the case–crossover analyses but also served as their own controls by comparing case and control periods [30]. The case period was defined as the 4 weeks prior to the crash, preceded by a 12-week washout interval. The control period also entailed 4 weeks with the corresponding preceding washout interval. The exposure period was chosen considering that the prescription duration of zolpidem and zopiclone usually ranges from a few days to up to 4 weeks [22, 25]. The washout interval was chosen considering the usual dispensation for a prescription in Sweden and to ensure that the medications were newly initiated [31]. Figure 1 provides an illustration of the periods used for the case–crossover analyses.
Fig. 1.
Graphical presentation of case-crossover design
Exposure Assessment
In Sweden, medications containing zolpidem or zopiclone cannot be purchased over the counter. Data on these medications were obtained using ATC codes N05CF02-zolpidem and N05CF01-zopiclone.
For the case–control analysis, exposure was categorized into one of the following mutually exclusive groups:
Newly initiated use: Dispensation of at least one prescription of zolpidem or zopiclone 1–30 days before the index date but none within 31–180 days prior to the index date.
Occasional use: Dispensation of one or two zolpidem or zopiclone prescriptions within 31–180 days prior to index date, or when one was given within 1–30 days prior to the index date and the other within 31–180 days prior to index date.
Frequent use: Dispensation of three or more prescriptions of zolpidem or zopiclone within 1–180 days prior to index date, with at least one prescription within 31–180 days prior to index date.
Non-zolpidem, non-zopiclone use: Dispensation of medications other than zolpidem and zopiclone within the 1- to 180-day period prior to the index date; the number of other medications was categorized into one to two, three to four, and five or more medications.
No medication use: No dispensation of medications 1–180 days prior to the index date.
For the analyses, exposure was further categorized as only zolpidem use, only zopiclone use, and both zolpidem and zopiclone combined for each of the exposure groups listed above.
For the case–crossover analyses, exposure in the case period was defined as days 1–28 prior to the crash, preceded by a 12-week (84 days) washout period. As a control period, a matching period was used prior to the crash (i.e. days 113–140), which was also preceded by an equal washout period of 12 weeks. To prevent reverse causation, cases with dispensations of zolpidem or zopiclone on the index date were not included in the analysis (n = 43).
Potential Confounders
In addition to age, sex, and area of residence, adjusted by the matched design, the following variables were also considered potential confounders in the case–control analyses.
Marital Status
Defined as the latest recorded marital status prior to the index date according to LISA. Previous studies have suggested an association between marital status and the prevalence of insomnia and use of hypnotics [43, 44], as well as with RTCs [45]. In this study, marital status was categorized as never married, married, divorced, and widowed.
Occupation
Defined as the latest recorded occupation prior to the RTC, and categorized into professional, technician, and skilled worker based on the first digit of the International Standard Classification of Occupations (ISCO-08) [45, 46]. We used this as a proxy for socioeconomic status. Information was obtained from the LISA register.
Comorbidity
Comorbidity was assessed using the weighted Charlson Comorbidity Index and the total number of different medications dispensed. The weighted Charlson Comorbidity Index [47] was computed using the main discharge diagnosis for hospitalizations based on records from the National Patient Register the year prior to the index date. Due to the limited variability within the data, the score was grouped into 0 (no comorbidity) and ≥1 (at least one comorbidity). The total number of different medications dispensed was assessed for the 30-day period prior to the index date and excluded dispensations of zolpidem, zopiclone, benzodiazepine, and opioid analgesics, as these have been associated with increased risks of RTCs in older drivers [46]. All dispensations were recorded based on the fifth-digit level of the ATC classification, and categorized into none, one to two, three to four, and five or more medications.
Benzodiazepines and opioid analgesics are among the most prominent medications linked to the risk of RTCs [18, 48–50]. Thus, dispensations of any of the following benzodiazepines within 30 days prior to the index date were considered: alprazolam, clonazepam, diazepam, flunitrazepam, lorazepam, nitrazepam, midazolam, oxazepam, and triazolam. Opioid analgesics included morphine, hydromorphone, oxycodone, oxycodone combinations, codeine combinations excluding psycholeptics, opium, nicomorphine, dihydrocodeine, papaveretum, morphine combinations, dihydrocodeine combinations, and codeine combinations with psycholeptics. Exposures to benzodiazepine or opioid analgesics were dichotomized.
For the case–crossover analyses, adjustments were made for newly prescribed benzodiazepine and opioid analgesics. This was defined as dispensation of any benzodiazepine or opioid analgesic drug mentioned previously using the same definition as for zolpidem or zopiclone.
Statistical Analysis
The sociodemographic characteristics of cases and controls were presented using proportions. For the case–control analyses, matched odds ratios (ORs) with 95% confidence intervals (CIs) were computed using conditional logistic regression. Matched ORs were adjusted for marital status, occupation, weighted Charlson Comorbidity Index, total number of different medications dispensed, and benzodiazepine and opioid analgesic use. Multicollinearity assumption for comorbidity variables was dismissed as the standard errors of the coefficients were small and variance inflation factors (VIFs) were <2.5.
Conditional logistic regression was used in the case–crossover analyses to calculate crude and adjusted ORs (AORs). Adjustment was made for newly prescribed benzodiazepines and opioid analgesics. Analyses were also stratified by each 7-day period within the 28-day period.
Analyses were stratified by sex, age group, and type of crash. For type of crash, the purpose was to estimate the effect in single-vehicle crashes where driver’s responsibility could be assumed [18]. Results were also stratified by sex and age. ORs were used as an approximation of the relative risk, considering that RTCs are relatively rare events [51, 52]. All analyses were conducted using IBM SPSS Statistics version 22.
Results
Table 1 shows the distribution of demographic characteristics, morbidity status, and medication use, along with crude matched ORs for cases and controls. A total of 27,096 non-alcohol-related RTCs were identified, of which 14.6% were single-vehicle crashes. Most drivers involved were men (69.7%), aged between 50–64 years (69.7%), and resided in the south and central regions of Sweden (90.3%). Compared with controls, cases were more often unmarried (44.4 vs. 36.7%), and had a lower socioeconomic position (33.2 vs. 28.5%). Cases also had slightly higher proportions of comorbidities either assessed using the weighted Charlson Comorbidity Index (3.1 vs. 2.2%) or by the number of different medications dispensed (34.3 vs. 31.4%). Cases were also more likely to use benzodiazepines (4.5 vs. 3.2%) or opioid analgesics (4.2 vs. 2.2%). Regarding the frequency of zolpidem and zopiclone use (Table 1), cases had a higher proportion of dispensation of zolpidem (3.7 vs. 2.8%) or zopiclone (3.8 vs. 3.1%) than controls, and only 0.4% of cases and 0.2% of controls used both medications 1–180 days prior to the crash.
Table 1.
Distribution of demographic characteristics, morbidity status, medication use, and crude matched odds ratios for all injurious road traffic crashes among older adult drivers in Sweden (case–control analysis)
| Characteristics | Categories | Casesa
(N = 27,096) |
Controlsa
(N = 108,384) |
Crude matched OR |
|---|---|---|---|---|
| Sex | Male | 69.7 | 69.7 | Matched |
| Female | 30.3 | 30.3 | ||
| Age group | 50–64 years | 69.7 | 69.7 | Matched |
| 65–80 years | 30.3 | 30.3 | ||
| Area of residence | North | 9.5 | 9.5 | Matched |
| Central | 43.1 | 43.1 | ||
| South | 47.2 | 47.2 | ||
| Marital status | Married | 55.3 | 62.9 | 1.00 |
| Never married | 14.9 | 14.5 | 1.17 (1.13–1.22) | |
| Divorced | 23.2 | 17.5 | 1.51 (1.46–1.56) | |
| Widowed | 6.3 | 4.7 | 1.57 (1.48–1.67) | |
| Occupation | Professional | 21.2 | 23.6 | 1.00 |
| Technician | 31.7 | 34.7 | 1.00 (0.97–1.04) | |
| Skilled worker | 33.2 | 28.5 | 1.32 (1.27–1.37) | |
| CCI scoreb | 0 | 96.9 | 97.8 | 1.00 |
| ≥1 | 3.1 | 2.2 | 1.44 (1.33–1.56) | |
| Number of different medications dispensedc | 0 | 65.8 | 68.6 | 1.00 |
| 1–2 | 21.4 | 20.2 | 1.11 (1.08–1.15) | |
| 3–4 | 7.4 | 6.9 | 1.12 (1.06–1.18) | |
| ≥5 | 5.5 | 4.3 | 1.35 (1.27–1.43) | |
| Benzodiazepinesd | No | 95.5 | 93.6 | 1.00 |
| Yes | 4.5 | 3.2 | 1.34 (1.25–1.43) | |
| Opioid analgesicsd | No | 95.8 | 97.8 | 1.00 |
| Yes | 4.2 | 2.2 | 1.96 (1.82–2.10) | |
| Zolpidem or zopiclone usee | No medications | 27.6 | 31.0 | 0.93 (0.89–0.97) |
| Non-zolpidem or non-zopiclone users (1–2 other medications) | 21.7 | 22.8 | 1.00 | |
| Non-zolpidem or non-zopiclone users (3–4 other medications) | 14.3 | 14.4 | 1.05 (1.00–1.10) | |
| Non-zolpidem or non-zopiclone users (≥5 other medications) | 28.6 | 25.7 | 1.19 (1.15–1.24) | |
| Zolpidem only users | 3.7 | 2.8 | 1.39 (1.28–1.50) | |
| Zopiclone only users | 3.8 | 3.1 | 1.33 (1.23–1.43) | |
| Both zolpidem and zopiclone users | 0.4 | 0.2 | 1.91 (1.53–2.40) |
Data are presented as percentages or as OR (95% confidence interval)
CCI Charlson Comorbidity Index, OR odds ratio
aTotal might not add up to 100% due to missing data
bWeighted CCI
cWithin 1–30 days prior to index date excluding zolpidem, zopiclone, benzodiazepines, opioid analgesics
dExposure to at least one medication dispensed within 1–30 days prior to the index date
eWithin 1–180 days prior to index date
The proportions of zolpidem or zopiclone use were also higher in females and among older people aged 65–80 years (see Table 5 in ESM 2). Females used zolpidem more often than men, but the opposite was true for zopiclone.
Table 2 shows the proportions of zolpidem or zopiclone users along with the estimated crude and AORs for the occurrence of injurious RTCs. The majority of users were occasional or frequent users. After adjustment for marital status, occupation, comorbidity, and benzodiazepine and opioid analgesic use, it could be seen that the risks for RTCs were higher among those using both zolpidem and zopiclone combined than among those using the medications separately. Occasional combined zolpidem and zopiclone users had the highest risk of RTC (AOR 2.14; 95% CI 1.26–3.63), followed by frequent combined zolpidem and zopiclone users (AOR 1.53; 95% CI 1.17–2.01). Among users of individual medications (zolpidem or zopiclone only), frequent users had the highest risks (AOR frequent zolpidem 1.33; 95% CI 1.14–1.56; AOR frequent zopiclone 1.31; 95% CI 1.14–1.56), although the risks were still lower than for those using both medications. No differences were seen in risk estimates in the stratified analyses by age. However, women seemed to have a higher risk than men (see Table 6 in ESM 2).
Table 2.
Distribution of zolpidem and/or zopiclone users with crude and adjusted matched odds ratios for all injurious road traffic crashes among older adult drivers in Sweden (case–control analysis)
| Exposure definition | Cases (N = 27,096) | Controls (N = 108,384) | Crude matched | Adjusted matcheda | Adjusted matcheda + benzodiazepinesb | Adjusted matcheda + opioid analgesicsb | Adjusted matcheda + benzodiazepinesb and opioid analgesicsb |
|---|---|---|---|---|---|---|---|
| No medicationsc | 27.6 | 31.0 | 0.93 (0.90–0.97) | 0.93 (0.89–0.96) | 0.93 (0.89–0.96) | 0.93 (0.89–0.96) | 0.93 (0.89–0.97) |
| Non-zolpidem or non-zopiclone users (1–2 other medications)c | 21.7 | 22.8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-zolpidem or non-zopiclone users (3–4 other medications)c | 14.3 | 14.4 | 1.05 (1.00–1.10) | 1.04 (1.00–1.09) | 1.04 (1.00–1.09) | 1.04 (0.99–1.09) | 1.04 (0.99–1.09) |
| Non-zolpidem or non-zopiclone users (≥5 other medications)c | 28.6 | 25.7 | 1.19 (1.15–1.24) | 1.16 (1.11–1.22) | 1.17 (1.12–1.22) | 1.14 (1.09–1.19) | 1.15 (1.10–1.20) |
| Newly initiated zolpidem-only usersd | 0.3 | 0.2 | 1.37 (1.06–1.78) | 1.33 (1.02–1.72) | 1.21 (0.92–1.60) | 1.28 (0.98–1.66) | 1.23 (0.94–1.63) |
| Newly initiated zopiclone-only usersd | 0.3 | 0.2 | 1.33 (1.03–1.72) | 1.27 (0.99–1.65) | 1.14 (0.87–1.50) | 1.21 (0.93–1.56) | 1.14 (0.87–1.50) |
| Newly initiated zolpidem and zopiclone usersd | 0.0e | 0.0e | 1.09 (0.12–9.78) | 0.93 (0.10–8.44) | 0.90 (0.10–8.19) | 0.73 (0.08–6.81) | 0.74 (0.08–6.95) |
| Occasional zolpidem-only usersf | 2.4 | 1.9 | 1.31 (1.20–1.44) | 1.28 (1.16–1.41) | 1.26 (1.14–1.39) | 1.24 (1.13–1.37) | 1.23 (1.12–1.36) |
| Occasional zopiclone-only usersf | 2.1 | 1.9 | 1.20 (1.09–1.32) | 1.15 (1.04–1.26) | 1.14 (1.03–1.26) | 1.12 (1.01–1.23) | 1.12 (1.01–1.23) |
| Occasional zolpidem and zopiclone usersf | 0.1 | 0.0 g | 2.14 (1.27–3.62) | 2.11 (1.24–3.57) | 2.17 (1.28–3.68) | 2.07 (1.22–3.51) | 2.14 (1.26–3.63) |
| Frequent zolpidem-only usersh | 1.0 | 0.7 | 1.60 (1.39–1.84) | 1.48 (1.29–1.71) | 1.39 (1.19–1.62) | 1.37 (1.18–1.58) | 1.33 (1.14–1.56) |
| Frequent zopiclone-only usersh | 1.3 | 0.9 | 1.60 (1.42–1.82) | 1.46 (1.29–1.66) | 1.36 (1.18–1.57) | 1.35 (1.19–1.54) | 1.31 (1.14–1.52) |
| Frequent zolpidem and zopiclone usersh | 0.3 | 0.2 | 1.89 (1.47–2.43) | 1.76 (1.36–2.27) | 1.63 (1.25–2.13) | 1.59 (1.23–2.05) | 1.53 (1.17–2.01) |
Data are presented as percentages or as odds ratios (95% confidence interval)
aMatched by sex, month and year of birth, and place of residence; adjusted for marital status, occupation, weighted Charlson Comorbidity Index, total number of different medications dispensed (excluding zolpidem, zopiclone, benzodiazepines, and opioid analgesics) within 1–30 days prior to index date
bWithin 1–30 days prior to index date
cWithin 1–180 days prior to index date
dDispensation of one or more zolpidem or zopiclone prescriptions within 1–30 days prior to index date, but none within 31–180 days prior to index date
eNewly initiated zolpidem and zopiclone users (n cases = 1; n controls = 4)
fDispensation of one to two zolpidem or zopiclone prescriptions within 31–180 days, or dispensation of one zolpidem or zopiclone prescription within 1–30 days and another within 31–180 days prior to index date
gOccasional zolpidem and zopiclone users (n controls = 42)
hDispensation of three or more zolpidem or zopiclone prescriptions within 1–180 days with at least one dispensation given within 31–180 days prior to index date
Table 3 presents AORs among single-vehicle crashes. Newly initiated zolpidem users showed the highest risks (AOR 2.27; 95% CI 1.21–4.24), followed by frequent combined zolpidem and zopiclone users (AOR 2.20; 95% CI 1.21–4.00). While increased risks were also seen for occasional combined zolpidem and zopiclone users, the CI was relatively wide (AOR 2.14; 95% CI 0.65–7.08).
Table 3.
Adjusted matched odds ratios for the exposure to zolpidem and/or zopiclone and the risk of injurious single-vehicle crashes among older adult drivers in Sweden (case–control analysis)
| Exposure definition | Adjusted matcheda | Adjusted matcheda + benzodiazepinesb | Adjusted matcheda + opioid analgesicsb | Adjusted matcheda + benzodiazepinesb and opioid analgesicsb |
|---|---|---|---|---|
| No medicationsc | 0.90 (0.80–1.00) | 0.91 (0.82–1.02) | 0.90 (0.81–1.01) | 0.92 (0.82–1.02) |
| Non-zolpidem or non-zopiclone users (1–2 other medications)c | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-zolpidem or non-zopiclone users (3–4 other medications)c | 1.09 (0.96–1.23) | 1.10 (0.97–1.25) | 1.08 (0.95–1.22) | 1.09 (0.96–1.24) |
| Non-zolpidem or non-zopiclone users (≥5 other medications)c | 1.33 (1.19–1.50) | 1.34 (1.19–1.51) | 1.29 (1.14–1.45) | 1.30 (1.15–1.46) |
| Newly initiated zolpidem-only usersd | 3.04 (1.70–5.46) | 2.10 (1.13–3.91) | 2.94 (1.63–5.29) | 2.27 (1.21–4.24) |
| Newly initiated zopiclone-only usersd | 2.04 (1.07–3.89) | 1.48 (0.75–2.90) | 1.85 (0.97–3.52) | 1.50 (0.76–2.94) |
| Newly initiated zolpidem and zopiclone usersd | –e | –e | –e | –e |
| Occasional zolpidem-only usersf | 1.21 (0.95–1.56) | 1.15 (0.89–1.49) | 1.13 (0.88–1.46) | 1.10 (0.85–1.42) |
| Occasional zopiclone-only usersf | 1.39 (1.09–1.77) | 1.27 (0.99–1.64) | 1.33 (1.04–1.70) | 1.25 (0.97–1.61) |
| Occasional zolpidem and zopiclone usersf | 2.19 (0.67–7.19) | 2.27 (0.69–7.47) | 2.07 (0.62–6.85) | 2.14 (0.65–7.08) |
| Frequent zolpidem-only usersg | 2.49 (1.79–3.44) | 1.93 (1.34–2.77) | 2.09 (1.50–2.93) | 1.77 (1.22–2.56) |
| Frequent zopiclone-only usersg | 2.54 (1.91–3.38) | 1.97 (1.42–2.73) | 2.19 (1.64–2.93) | 1.85 (1.33–2.58) |
| Frequent zolpidem and zopiclone usersg | 3.42 (1.96–5.95) | 2.45 (1.36–4.41) | 2.81 (1.59–4.96) | 2.20 (1.21–4.00) |
Data are presented as odds ratio (95% confidence interval)
aMatched by sex, month and year of birth, and place of residence; adjusted for marital status, occupation, weighted Charlson Comorbidity Index, total number of different medications dispensed (excluding zolpidem, zopiclone, benzodiazepines, and opioid analgesics) within 1–30 days prior to index date
bWithin 1–30 days prior to index date
cWithin 1–180 days prior to index date
dDispensation of one or more zolpidem or zopiclone prescriptions within 1–30 days, but none within 31–180 days prior to index date
eEstimates cannot be obtained because of very few observations (n cases = 0, n controls = 1)
fDispensation of one to two zolpidem or zopiclone prescriptions within 31–180 days, or dispensation of one zolpidem or zopiclone prescription within 1–30 days and another within 31–180 days prior to index date
gDispensation of three or more zolpidem or zopiclone prescriptions within 1–180 days with at least one dispensation being made within 31–180 days prior to index date
Table 4 shows crude and AORs for RTCs for newly initiated zolpidem or zopiclone treatment based on the case–crossover analyses. Newly initiated users had higher crude ORs of being involved in RTCs for up to 28 days after the start of the treatment, especially in single-vehicle crashes, compared with periods when no treatment had been initiated (OR 1.79; 95% CI 1.13–2.82). The risk remained significant even after adjusting for other newly initiated benzodiazepine and opioid analgesics for those involved in single-vehicle crashes (AOR 1.78; 95% CI 1.12–2.81). Although no clear patterns were observed in crude analyses at different 7-day length periods, further analysis on drivers involved in single-vehicle crashes showed that risks peaked on the second week after initiation (AOR 2.66; 95% CI 1.04–6.81).
Table 4.
Crude and adjusted odds ratios for all and single-vehicle crashes in older adult drivers following newly initiated zolpidem or zopiclone treatment, stratified by time since start of treatment (case-crossover analysis)
| Hazard perioda | All crashes | Single crashes | ||||||
|---|---|---|---|---|---|---|---|---|
| f10 b | f01 b | Crude | Adjustedc | f10 b | f01 b | Crude | Adjustedc | |
| 1–28 | 218 | 207 | 1.05 (0.87–1.27) | 1.03 (0.85–1.25) | 52 | 29 | 1.79 (1.13–2.82) | 1.78 (1.12–2.81) |
| 1–7 | 62 | 46 | 1.34 (0.92–1.97) | 1.29 (0.88–1.91) | 19 | 9 | 2.11 (0.95–4.66) | 2.20 (0.98–4.92) |
| 8–14 | 56 | 62 | 0.90 (0.62–1.29) | 0.91 (0.63–1.30) | 16 | 6 | 2.66 (1.04–6.81) | 2.66 (1.04–6.81) |
| 15–21 | 51 | 53 | 0.96 (0.65–1.41) | 0.97 (0.66–1.44) | 13 | 8 | 1.62 (0.67–3.92) | 1.66 (0.68–4.03) |
| 22–28 | 69 | 57 | 1.21 (0.85–1.71) | 1.17 (0.82–1.67) | 11 | 7 | 1.57 (0.60–4.05) | 1.50 (0.58–3.90) |
Data are presented as odds ratio (95% confidence interval) unless otherwise indicated
aDays prior to crash
bFrequency of discordant matched pairs: (10) exposed in case period and unexposed in control period, (01) unexposed in case period and exposed in control period
cAdjusted for newly initiated treatment of benzodiazepines and opioid analgesics
Discussion
Results show that newly initiated as well as sustained use of zolpidem and zopiclone is associated with a higher occurrence of injurious RTCs in older Swedish drivers. Although frequent and occasional users may have a higher probability, a noticeable increment was also seen at the initiation of treatment, especially during the second week. The patterns observed were more evident when analyses were restricted to single-vehicle crashes where driver responsibility could be largely assumed. In addition to the frequency of use, this study suggests that the type of medication used also plays a role, with frequent and occasional users of zolpidem and zopiclone combined showing higher risks than those who used these medications separately. On the other hand, while newly initiated zolpidem users had higher risks than zopiclone users, the opposite was true for frequent users.
Although comparisons with previous studies are not straightforward because of methodological differences, results could still be indirectly compared with earlier findings. The increased risks found in frequent and occasional users are in line with the study in which continuous zolpidem use increased the chance of RTCs, especially in the 1- to 4-month period of continuous use [23]. Another study reported that drivers using more than one pill of zolpidem per day, as a measure of high exposure, had a higher risk for being responsible for RTCs, though such an association was not seen for zopiclone [24].
With respect to the relatively small differences seen between the findings concerning newly initiated zolpidem or zopiclone use by study design, one should keep in mind that even though similar exposure definitions were used, the control groups differed between the case–control and the case–crossover. In the case–crossover analyses, time in-variant confounders are inherently adjusted for, both measured and unmeasured, which can explain the differences in the effect estimates. There might also be unmeasured confounding that has not been taken into account in the case–control analyses, such as driving exposure. Altogether, the increased risks observed for newly initiated treatment tend to coincide with one case–control [20] and two case–crossover [19, 22] studies, but they contrasted with the other two studies [24, 25].
The increased risks found here suggest these medications have both transient and cumulative effects in the body. Drug tolerance or adaptation, which often occurs with repeated use of certain medications, does not seem to be the case with zolpidem and zopiclone. Meta-analyses of randomized controlled trials [10, 12] and pooled analyses of crossover trials [13] looking at the effects of hypnotics on on-the-road driving tests have found that bedtime zopiclone administration impaired driving performance the following morning, indicating the presence of transient or immediate effects of the medications. While zolpidem was deemed safer following bedtime use, middle-of-the-night administration showed this medication can also impair driving performance the morning after [10, 12]. It is important to bear in mind that, whereas the majority of these trials included only healthy young adults, other studies with middle-aged and elderly drivers have also shown similar findings [11, 15].
Unfortunately, there is no experimental evidence assessing the long-term or cumulative effects of zolpidem or zopiclone in terms of driving performance. For now, the evidence relies on observational studies, which are often contradictory. For instance, the results of one study that investigated the driving performance of insomnia patients taking hypnotics occasionally and frequently, which showed no differences compared with healthy controls [14], contrast with another that showed significant driving impairments in individuals who used just a single dose of zopiclone, although of lower magnitude than in frequent users [15].
Apart from the side effects of the medications, the risks observed among new users could also be affected by driving-related behavioural change. While it is generally noted that people with insomnia and drivers taking central nervous system medications [13, 53–55] were usually not aware of their driving impairments, it has also been shown that the level of awareness might differ depending on which drug was being taken. For instance, people taking zolpidem were usually more aware of their reduced alertness than were alprazolam users [55]. Thus, it is possible that newly initiated zolpidem or zopiclone users could have abstained or reduced their driving at the beginning because of warnings of potential side effects and the awareness of their perceived driving ability. However, it is also possible that they drove again as usual some days after, which could explain the risk peaking in the second week after the start of the treatment.
Moreover, there is evidence that chronic use of non-benzodiazepine hypnotics, including zolpidem and zopiclone, is associated with greater anxiety and sleep difficulties in the elderly, the so-called ‘paradoxical effects’ [56]. Thus, it is plausible that such adverse events could influence the risk of RTCs among frequent users, as anxiety can affect driving performance [57], and sleep difficulties have also been associated with impaired driving and with road traffic injuries [53, 58].
This study has various strengths. First, unlike previous studies that investigated the effects of zolpidem and zopiclone rather superficially [19–21, 23–27], we focused this study exclusively on these drugs, using distinct exposure categories to help understand the risks according to periods of use. Second, we conducted analyses using a relatively large nationally representative sample by means of two strong epidemiological designs that allowed us to thoroughly control for potential confounders; we were able to control for many relevant factors in the matched case–control study and nearly all time-invariant confounders in the case–crossover design, including driving behaviour, which is unlikely to have changed within the same individual, as the case and control periods were rather close to each other. Finally, we were able to assess the separate and combined effect of both drugs studied.
Despite its strengths, this study also has some limitations. In spite of the relatively large sample analysed, we still faced sample size limitations in specific subgroups, especially among combined zolpidem and zopiclone users, which can explain the lack of statistical significance of some estimates (i.e. occasional combined zolpidem and zopiclone users in single-vehicle crashes). Given this kind of limitation, we were also unable to compute the risk for the newly initiated combined zolpidem and zopiclone users in single-vehicle crashes. Another limitation is the use of dispensation as a proxy of actual intake, which could lead to non-differential exposure misclassification biasing the estimates towards the null. There was also no information on dosage, precluding comparisons with other studies [20]. Yet, we are aware that, in Sweden, zolpidem is available only in 10 or 5 mg [59] and zopiclone in 7.5 or 5 mg [60] doses. We also had no information on insomnia, the main indication for prescription of zolpidem or zopiclone. As a result, it was not possible to distinguish between the effects of the medications and those of the underlying medical conditions (i.e. confounding by indication).
Further research should focus on determining the extent to which dosage plays a role in the associations reported here. Also, future studies should assess potential epidemiological interactions between benzodiazepine, opioids, and zolpidem and zopiclone using appropriate designs.
Conclusions
Zolpidem and zopiclone are the preferred non-benzodiazepine medications to treat insomnia, as they are believed to have a less dangerous risk profile. However, the results of this study suggest that both new initiation and frequent exposure to these drugs, either when used separately or in combination, can expose older adult drivers to an increased risk of RTCs. It is possible that the associations found here were influenced by potentially transient and cumulative effects of the medications on driving performance and consequently on the risk of RTCs.
Awareness among prescribing physicians regarding the potential risks of these medications should be raised so patients can be informed. However, it is important to keep in mind that decisions made when prescribing medications need to be based on a consideration of the potential consequences of leaving patients untreated as well as their mobility requirements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank statistician Berty Elling for her assistance with data management.
Compliance with Ethical Standards
Ethics approval
This study was approved by the Regional Ethical Review Committee in Stockholm, Sweden (DNR 201/865-31/2). This was a register-based study not directly involving patients. It was not possible to trace individual information in the study.
Funding
No sources of funding were used to conduct this study or prepare this manuscript. Open access was funded by the Karolinska Institutet, as part of the Springer Compact agreement.
Conflicts of interest
Alicia Nevriana, Jette Möller, Lucie Laflamme, and Joel Monárrez-Espino have no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s40263-017-0445-9) contains supplementary material, which is available to authorized users.
Contributor Information
Alicia Nevriana, Email: alicia.nevriana@ki.se.
Jette Möller, Email: jette.moller@ki.se.
Lucie Laflamme, Email: lucie.laflamme@ki.se.
Joel Monárrez-Espino, Phone: +46 8 524 833 84, Email: joel.monarrez-espino@ki.se.
References
- 1.Johnell K, Fastbom J. Comparison of prescription drug use between community-dwelling and institutionalized elderly in Sweden. Drugs Aging. 2012;29:751–758. doi: 10.1007/s40266-012-0002-7. [DOI] [PubMed] [Google Scholar]
- 2.Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155–162. doi: 10.1007/s13181-013-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnell K, Fastbom J. The use of benzodiazpines and related drugs amongst older people in Sweden: associated factors and concomitant use of other psychotropics. Int J Geriatr Psychiatry. 2009;24:731–738. doi: 10.1002/gps.2189. [DOI] [PubMed] [Google Scholar]
- 4.Hausken AM, Furu K, Skurtveit S, Engeland A, Bramness JG. Starting insomnia treatment: the use of benzodiazepines versus z-hypnotics. A prescription database study of predictors. Eur J Clin Pharmacol. 2009;65:295–301. doi: 10.1007/s00228-008-0565-8. [DOI] [PubMed] [Google Scholar]
- 5.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343–349. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alessi-Severini S, Bolton JM, Enns MW, Dahl M, Collins DM, Chateau D, et al. Use of benzodiazepines and related drugs in Manitoba: a population-based study. CMAJ Open. 2014;2(4):E208–E216. doi: 10.9778/cmajo.20130076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolder C, Nelson M, McKinsey J. Use of non-benzodiazepine hypnotics in the elderly: are all agents the same? CNS Drugs. 2007;21:389–405. doi: 10.2165/00023210-200721050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hetland AJ, Carr DB, Wallendorf MJ, Barco PP. Potentially driver-impairing (PDI) medication use in medically impaired adults referred for driving evaluation. Ann Pharmacother. 2014;48:476–482. doi: 10.1177/1060028014520881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranks EK, Crowe SF. The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol. 2014;36:691–700. doi: 10.1080/13803395.2014.928268. [DOI] [PubMed] [Google Scholar]
- 10.Verster JC, Veldhuijzen DS, Patat A, Olivier B, Volkerts ER. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63–71. doi: 10.2174/157488606775252674. [DOI] [PubMed] [Google Scholar]
- 11.Bocca M-L, Marie S, Lelong-Boulouard V, Bertran F, Couque C, Desfemmes T, et al. Zolpidem and zopiclone impair similarly monotonous driving performance after a single nighttime intake in aged subjects. Psychopharmacology (Berl) 2011;214:699–706. doi: 10.1007/s00213-010-2075-5. [DOI] [PubMed] [Google Scholar]
- 12.Roth T, Eklov SD, Drake CL, Verster JC. Meta-analysis of on-the-road experimental studies of hypnotics: effects of time after intake, dose, and half-life. Traffic Inj Prev. 2014;15:439–445. doi: 10.1080/15389588.2013.830211. [DOI] [PubMed] [Google Scholar]
- 13.Leufkens TRM, Vermeeren A. Zopiclone’s residual effects on actual driving performance in a standardized test: a pooled analysis of age and sex effects in 4 placebo-controlled studies. Clin Ther. 2014;36:141–150. doi: 10.1016/j.clinthera.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Leufkens TRM, Ramaekers JG, De Weerd AW, Riedel WJ, Vermeeren A. On-the-road driving performance and driving-related skills in older untreated insomnia patients and chronic users of hypnotics. Psychopharmacology (Berl) 2014;231:2851–2865. doi: 10.1007/s00213-014-3455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leufkens TRM, Ramaekers JG, De Weerd AW, Riedel WJ, Vermeeren A. Residual effects of zopiclone 7.5 mg on highway driving performance in insomnia patients and healthy controls: A placebo controlled crossover study. Psychopharmacology (Berl) 2014;231:2785–2798. doi: 10.1007/s00213-014-3447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Routledge PA, O’Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol. 2004;57:121–126. doi: 10.1046/j.1365-2125.2003.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bénard-Laribière A, Noize P, Pambrun E, Bazin F, Verdoux H, Tournier M, et al. Comorbidities and concurrent medications increasing the risk of adverse drug reactions: prevalence in French benzodiazepine users. Eur J Clin Pharmacol. 2016;72:869–876. doi: 10.1007/s00228-016-2044-y. [DOI] [PubMed] [Google Scholar]
- 18.Monárrez-Espino J, Laflamme L, Rausch C, Elling B, Möller J. New opioid analgesic use and the risk of injurious single-vehicle crashes in drivers aged 50–80 years: a population-based matched case–control study. Age Ageing. 2016;45:628–634. doi: 10.1093/ageing/afw115. [DOI] [PubMed] [Google Scholar]
- 19.Barbone F, McMahon A, Davey P, Morris A, Reid I, McDevitt D, et al. Association of road-traffic accidents with benzodiazepine use. Lancet. 1998;352:1331–1336. doi: 10.1016/S0140-6736(98)04087-2. [DOI] [PubMed] [Google Scholar]
- 20.Chang C-M, Wu EC-H, Chen C-Y, Wu K-Y, Liang H-Y, Chau Y-L, et al. Psychotropic drugs and risk of motor vehicle accidents: a population-based case–control study. Br J Clin Pharmacol. 2013;75:1125–1133. doi: 10.1111/j.1365-2125.2012.04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustavsen II, Bramness JGJG, Skurtveit S, Engeland A, Neutel I, Mørland J, et al. Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam. Sleep Med. 2008;9:818–822. doi: 10.1016/j.sleep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y-H, Lai J-N, Lee C-H, Wang J-D, Chen P-C. Increased risk of hospitalization related to motor vehicle accidents among people taking zolpidem: a case-crossover study. J Epidemiol. 2011;21:37–43. doi: 10.2188/jea.JE20090195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen RN, Boudreau DM, Ebel BE, Grossman DC, Sullivan SD. Sedative hypnotic medication use and the risk of motor vehicle crash. Am J Public Health. 2015;105:64–69. doi: 10.2105/AJPH.2015.302723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orriols L, Philip P, Moore N, Castot A, Gadegbeku B, Delorme B, et al. Benzodiazepine-like hypnotics and the associated risk of road traffic accidents. Clin Pharmacol Ther. 2011;89:595–601. doi: 10.1038/clpt.2011.3. [DOI] [PubMed] [Google Scholar]
- 25.Gibson JE, Hubbard RB, Smith CJP, Tata LJ, Britton JR, Fogarty AW. Use of self-controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol. 2009;169:761–768. doi: 10.1093/aje/kwn364. [DOI] [PubMed] [Google Scholar]
- 26.Gjerde H, Normann PT, Christophersen AS, Samuelsen SO, Mørland J. Alcohol, psychoactive drugs and fatal road traffic accidents in Norway: a case–control study. Accid Anal Prev. 2011;43:1197–1203. doi: 10.1016/j.aap.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Gjerde H, Christophersen AS, Normann PT, Mørland J. Associations between substance use among car and van drivers in Norway and fatal injury in road traffic accidents: a case–control study. Transp Res Part F Traffic Psychol Behav. 2013;17:134–145. doi: 10.1016/j.trf.2012.11.004. [DOI] [Google Scholar]
- 28.Booth JN, Behring M, Cantor RS, Colantonio LD, Davidson S, Donnelly JP, et al. Zolpidem use and motor vehicle collisions in older drivers. Sleep Med. 2016;20:98–102. doi: 10.1016/j.sleep.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orriols L, Salmi L-R, Philip P, Moore N, Delorme B, Castot A, et al. The impact of medicinal drugs on traffic safety: a systematic review of epidemiological studies. Pharmacoepidemiol Drug Saf. 2009;18:647–658. doi: 10.1002/pds.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 31.Soderberg KC, Laflamme L, Moller J. Newly initiated opioid treatment and the risk of fall-related injuries. a nationwide, register-based, case-crossover study in Sweden. CNS Drugs. 2013;27:155–161. doi: 10.1007/s40263-013-0038-1. [DOI] [PubMed] [Google Scholar]
- 32.Swedish Transport Agency. STRADA—Swedish Traffic Accident Data Acquisition. 2011. http://www.transportstyrelsen.se/en/road/statistik-ochstrada/STRADA/. Accessed 18 June 2015.
- 33.Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 34.Ludvigsson JF, Almqvist C, Bonamy A-KE, Ljung R, Michaëlsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 35.Transportstyrelsen. Körkortsstatistik. 2010. https://www.transportstyrelsen.se/sv/vagtrafik/statistik-ochstrada/Vag/Korkort/. Accessed 18 June 2016.
- 36.Statistics Sweden. Background Facts, Labour and Education Statistics: Integrated database for labour market research. Statistics Sweden, Stockholm, Sweden. 2011. http://www.scb.se/en_/Services/Guidance-for-researchers-and-universities/SCB-Data/Longitudinalintegration-database-for-health-insurance-and-labour-market-studies-LISA-by-Swedish-acronym/. Accessed 18 June 2017.
- 37.Socialstyrelsen. In English—the National Patient Register. 2015 https://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish. Accessed 23 Oct 2015.
- 38.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsberg G, Hattis D, Russ A, Sonawane B. Pharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individuals. Environ Health Perspect. 2005;113:1243–1249. doi: 10.1289/ehp.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor B, Irving HM, Kanteres F, Room R, Borges G, Cherpitel C, et al. The more you drink, the harder you fall: a systematic review and meta-analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend. 2010;110:108–116. doi: 10.1016/j.drugalcdep.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santamariña-Rubio E, Pérez K, Olabarria M, Novoa AM. Gender differences in road traffic injury rate using time travelled as a measure of exposure. Accid Anal Prev. 2014;65:1–7. doi: 10.1016/j.aap.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Johnell K, Fastbom J. Gender and use of hypnotics or sedatives in old age: a nationwide register-based study. Int J Clin Pharm. 2011;33:788–793. doi: 10.1007/s11096-011-9536-8. [DOI] [PubMed] [Google Scholar]
- 43.Doi Y, Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general japanese adult population. J Epidemiol. 2000;10:79–86. doi: 10.2188/jea.10.79. [DOI] [PubMed] [Google Scholar]
- 44.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 45.Johnell K, Laflamme L, Möller J, Monárrez-Espino J. The role of marital status in the association between benzodiazepines, psychotropics and injurious road traffic crashes: a register-based nationwide study of senior drivers in Sweden. PLoS One. 2014;9:e86742. doi: 10.1371/journal.pone.0086742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monárrez-Espino J, Laflamme L, Elling B, Möller J. Number of medications and road traffic crashes in senior Swedish drivers: a population-based matched case–control study. Inj Prev. 2014;20:81–87. doi: 10.1136/injuryprev-2013-040762. [DOI] [PubMed] [Google Scholar]
- 47.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 48.Elvik R. Risk of road accident associated with the use of drugs: a systematic review and meta-analysis of evidence from epidemiological studies. Accid Anal Prev. 2013;60:254–267. doi: 10.1016/j.aap.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Monárrez-Espino J, Möller J, Berg H-Y, Kalani M, Laflamme L. Analgesics and road traffic crashes in senior drivers: an epidemiological review and explorative meta-analysis on opioids. Accid Anal Prev. 2013;57:157–164. doi: 10.1016/j.aap.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Rudisill TM, Zhu M, Kelley GA, Pilkerton C, Rudisill BR. Medication use and the risk of motor vehicle collisions among licensed drivers: a systematic review. Accid Anal Prev. 2016;96:255–270. doi: 10.1016/j.aap.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health. 2008;53:165–167. doi: 10.1007/s00038-008-7068-3. [DOI] [PubMed] [Google Scholar]
- 52.Theofilatos A, Yannis G, Kopelias P, Papadimitriou F. Predicting road accidents: a rare-events modeling approach. Transp Res Proc. 2016;14:3399–3405. doi: 10.1016/j.trpro.2016.05.293. [DOI] [Google Scholar]
- 53.Perrier J, Bertran F, Marie S, Couque C, Bulla J, Denise P, et al. Impaired driving performance associated with effect of time duration in patients with primary insomnia. Sleep. 2014;37:1565–1573. doi: 10.5665/sleep.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verster JC, Roth T. Insomnia and driving ability. Sleep. 2014;37:1411–1412. doi: 10.5665/sleep.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verster JC, Roth T. Drivers can poorly predict their own driving impairment: a comparison between measurements of subjective and objective driving quality. Psychopharmacology (Berl) 2012;219:775–781. doi: 10.1007/s00213-011-2400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nordfjaern T. Prospective associations between benzodiazepine use and later life satisfaction, somatic pain and psychological health among the elderly. Hum Psychopharmacol Clin Exp. 2013;28:248–257. doi: 10.1002/hup.2316. [DOI] [PubMed] [Google Scholar]
- 57.Roidl E, Frehse B, Höger R. Emotional states of drivers and the impact on speed, acceleration and traffic violations—a simulator study. Accid Anal Prev. 2014;70:282–292. doi: 10.1016/j.aap.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Laugsand LE, Strand LB, Vatten LJ, Janszky I, Bjørngaard JH. Insomnia symptoms and risk for unintentional fatal injuries—The HUNT Study. Sleep. 2014;37:1777–1786. doi: 10.5665/sleep.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farmaceutiska Specialiteter i Sverige (Medicines Compendium for healthcare professionals). Zolpidem. http://www.fass.se/LIF/substance?userType=0&substanceId=IDE4POFIUB0C5VERT1. Accessed 23 Mar 2017.
- 60.Farmaceutiska Specialiteter i Sverige (Medicines Compendium for healthcare professionals). Zopiklon. http://www.fass.se/LIF/substance?userType=0&substanceId=IDE4POF0UAMLJVERT1. Accessed 23 Mar 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.