ABSTRACT
Immunotherapy with passive administration of broadly neutralizing HIV-1 envelope-specific antibodies (bnAbs) in the setting of established infection in vivo has yielded mixed results. The contribution of different antibodies toward the direct elimination of infected cells is poorly understood. In this study, we determined the ability of 12 well-characterized anti-HIV-1 neutralizing antibodies to recognize and eliminate primary CD4 T cells infected with HIV-1 belonging to clades A, B, C, and D, via antibody-dependent complement-mediated lysis (ADCML) and antibody-dependent cell-mediated cytotoxicity (ADCC), in vitro. We further tested unique combinations of these antibodies to determine the optimal antibody cocktails to be tested in future clinical trials. We report that antibody binding to infected CD4 T cells is highly variable and correlates with ADCML and ADCC processes. Particularly, antibodies targeting the envelope glycan shield (2G12) and V1/V2 site (PG9, PG16, and PGT145) are best at recognizing HIV-1-infected CD4 T cells. However, only PG9 and PG16 and their combinations with other bnAbs sufficiently induced the elimination of HIV-1-infected CD4 T cells by ADCML, ADCC, or both. Notably, CD4 binding site antibodies VRC01, 3BNC117, and NIH45-46 G54W did not exhibit recognition of infected cells and were unable to induce their killing. Future trials geared toward the development of a cure for HIV/AIDS should incorporate V1/V2 antibodies for maximal clearance of infected cells. With the use of only primary immune cells, we conducted a comprehensive cross-clade physiological analysis to aid the direction of antibodies as therapeutics toward the development of a cure for HIV/AIDS.
IMPORTANCE Several antibodies capable of neutralizing the majority of circulating HIV-1 strains have been identified to date and have been shown to prevent infection in animal models. However, the use of combinations of such broadly neutralizing antibodies (bnAbs) for the treatment and eradication of HIV-1 in infected humans remains uncertain. In this study, we tested the ability of bnAbs to directly recognize and eliminate primary human CD4 T cells infected with diverse HIV-1 strains representative of the global epidemic by antibody-dependent pathways. We also tested several combinations of bnAbs in our assays in order to maximize the clearance of infected cells. We show that the ability of bnAbs to identify and kill infected cells is highly variable and that only a few of them are able to exert this function. Our data will help guide the formulation of bnAbs to test in future human trials aimed at the development of a cure.
KEYWORDS: ADCC, ADCML, AIDS, antibody function, complement-mediated lysis, human immunodeficiency virus, monoclonal antibodies, neutralizing antibodies
INTRODUCTION
The past decade of human immunodeficiency virus type 1 (HIV-1) research has witnessed a surge in the identification and characterization of several HIV envelope-specific antibodies that exhibit high potency and remarkable neutralization breadth across the global clades of HIV-1 (1–4). Aptly named, such broadly neutralizing antibodies (bnAbs) have rekindled optimism regarding the generation of new preventative and therapeutic strategies to end the global HIV-1 epidemic. Several bnAbs characterized to date exhibit specificity toward unique sites on the viral envelope, such as the CD4 binding site, the membrane-proximal external region (MPER), the viral glycan shield, and the V1/V2 and V3 loops. Many of these antibodies have been demonstrated to prevent acquisition of infection if administered prior to viral challenge (5–9) or by vectored prophylaxis in animal models (10). Less is known, however, about the use of these antibodies, particularly their combinations, as therapeutics to cure established HIV-1 infection.
Antibodies employ diverse mechanisms, such as Fc receptor (FcR)-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-mediated complement-mediated lysis (ADCML), to eliminate infected targets. In order to develop a cure, it is imperative that any therapy substantially eliminate or entirely eradicate all cells of the HIV-1 reservoir to achieve a functional or sterilizing cure, respectively. To this end, antibody based therapies may be superior to current highly active antiretroviral therapy (HAART) regimens in that along with viral suppression they may directly induce the clearance of infected cells by the above-mentioned mechanisms (11). Recently it was demonstrated that passive administration of the potent CD4 binding site-specific antibodies VRC01 (12) and 3BNC117 (13) as well as the V3-specific monoclonal antibody 10-1074 (14) was safe and when given to viremic HIV-infected individuals off treatment resulted in a 0.8- to 2.5-log10 reduction in plasma viremia. Notably, administration to HIV-1-infected volunteers on suppressive HAART antibody therapy resulted in a delayed rebound of viremia following treatment interruption (15, 16). Administration of these antibodies, and of the V3-specific monoclonal antibody PGT121 into simian immunodeficiency virus (SIV)-infected macaques, also improved host adaptive immune responses, which contributed to the beneficial effects of antibody therapy (17, 18). These data indicate that the use of broadly neutralizing antibodies as an immunotherapy toward the eradication of the viral reservoir must be explored.
Since the antibodies were delivered as monotherapies in these clinical trials, resistance mutations developed commonly. Hence, similar to the premise of triple HAART for exerting maximal fitness cost to develop resistance, combinations of antibodies should be tested clinically to not only maintain durable suppression of viremia but also explore potential antibody-mediated eradication of the HIV reservoir. In support of this argument, it was recently demonstrated that a combination of 3BNC117 and 10-1074 in a simian-human immunodeficiency virus (SHIV) model allowed for enhanced long-term control of viremia in a CD8 T cell-dependent manner (19). Additionally, a combination of 3BNC117, 10-1074, and PG16 monoclonal antibodies in HAART-treated humanized mice reduced the size of the viral reservoir as measured by cell-associated DNA (20). Additionally, the coexistence of autologous broadly neutralizing antibodies in an HIV-infected individual was associated with long-term viremic control (21). In the moderate and only successful HIV-1 vaccine trial, RV 144, high levels of V1/V2-specific antibodies and ADCC responses conferred protection from HIV-1 acquisition (22). ADCC responses have also been demonstrated to correlate inversely with disease progression (23). Finally, antibodies have longer half-lives in vivo than HAART (24, 25) and could therefore serve as long-lasting therapeutics to not only prevent new infections by neutralization but also directly induce the eradication of HIV-infected cells by antibody-mediated effector functions. Collectively, these lines of evidence demonstrate the in vivo efficacy of broadly neutralizing antibodies toward the elimination of the HIV-1 viral reservoir and suggest that combinations of broadly neutralizing antibodies may be utilized toward the development of a functional cure of HIV/AIDS.
In this study, we aimed to determine ideal antibodies, and their combinations, from a panel of 12 well-characterized antibodies specific to various regions of the HIV-1 envelope to eliminate primary HIV-1 CD4 T cells by two antibody-mediated effector functions, ADCML and ADCC. Importantly, we conducted all experiments on primary human CD4 T cells, natural targets of HIV-1, infected with 10 primary isolates and one lab-adapted strain of HIV-1 representative of four global HIV-1 clades as well as primary natural killer (NK) cells as effector cells for ADCC-mediated elimination of targets. The use of primary CD4 T cells was a critical determinant for our assays, as these cells express HIV-1 envelope on their surface in its native conformation having undergone glycosylation representative of the complex's native form in vivo available for antibody binding. Similarly, primary NK cells recapitulate the true, physiological immune effectors required to mediate ADCC in vivo. Importantly, we tested each antibody at a final concentration of 2 μg/ml, on par with the average neutralization potency of the antibodies tested in our assays. Thus, our findings form a comprehensive physiological analysis of several broadly neutralizing antibodies and their combinations to be tested in future clinical trials.
RESULTS
Rationale and experimental design.
To determine the optimal bnAbs and their combinations to eliminate primary CD4 T cells infected with diverse clades (A, B, C, and D) of HIV-1 (Table 1) from a panel of 12 well-characterized anti-HIV-1 envelope antibodies (Table 2), we first performed an antibody binding assay as illustrated in Fig. 1A. The neutralization breadth and potency of these antibodies against several viruses tested (range: 176 to 787) have been obtained from the CATNAP (Compile, Analyze and Tally NAb Panels [http://hiv.lanl.gov/catnap]) database (26), which has compiled these data from numerous publications (range: 4 to 45) and is hosted online by the Los Alamos National Laboratory. The neutralization breadth ranges from 8 to 91% (median: 70.5%) at a 50% inhibitory concentration (IC50) of <50 μg/ml, with a potency range of 0.02 to 3.10 μg/ml (median: 1.16 μg/ml), as shown in Table 2. As a control, we used the CD4 binding site-specific monoclonal antibody F105, which is not a bnAb.
TABLE 1.
Panel of diverse HIV-1 strains tested in the study
| HIV-1 strain | Clade | Coreceptor used |
|---|---|---|
| KNH1088 | A | CCR5 |
| KER2008 | A | CCR5/CXCR4 |
| NL4-3 | B | CXCR4 |
| 90THBK132 | B | CXCR4 |
| YU-2 | B | CCR5 |
| BaL | B | CCR5 |
| GS007 | B | CCR5 |
| SM206354 | C | CCR5 |
| MSC5016 | C | CCR5 |
| J32228M4 | D | CCR5 |
| 98UG57128 | D | CCR5 |
TABLE 2.
Characteristics of all anti-HIV-1 envelope antibodies used in the study
| Antibody | Target site on viral envelope | Neutralization breadtha at IC50 of <50 μg/ml (%) | Neutralization potencya (μg/ml) |
|---|---|---|---|
| VRC01 | CD4 binding site | 84 | 0.41 |
| 17b | CD4-induced epitope | 8 | 2.23 |
| 4E10 | gp41 MPER | 91 | 2.82 |
| PG9 | V1/V2 | 74 | 0.18 |
| 2F5 | gp41 MPER | 47 | 2.35 |
| PGT145 | V1/V2 | 69 | 0.18 |
| F105 | CD4 binding site | 9 | 1.54 |
| PG16 | V1/V2 | 72 | 0.11 |
| PGT121 | V3 | 64 | 0.07 |
| 2G12 | Glycan shield | 20 | 3.10 |
| 3BNC117 | CD4 binding site | 78 | 0.16 |
| NIH45-46 G54W | CD4 binding site | 84 | 0.02 |
Data obtained from CATNAP, an online database hosted by the Los Alamos National Laboratory, USA (http://hiv.lanl.gov/catnap).
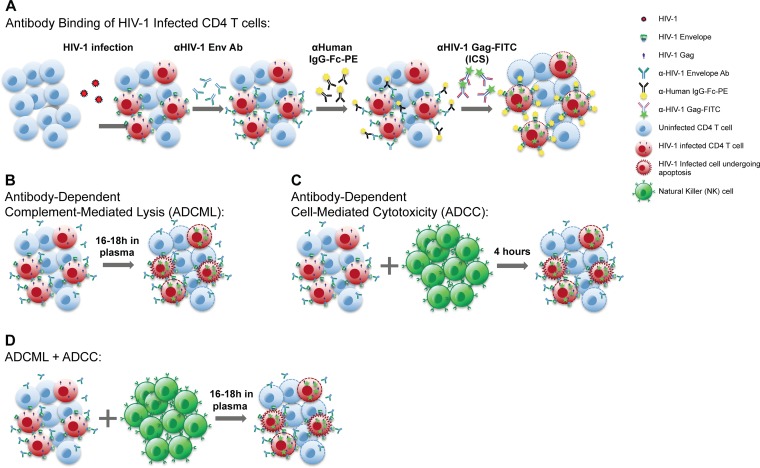
FIG 1.
Schematic illustrations of assays. HIV-1 envelope-specific antibodies were employed at 2 μg/ml in each assay. (A) Binding assays of HIV-1-infected CD4 T cells. HIV-1-infected cells were stained with HIV-1 envelope-specific antibodies for 30 min, followed by PE-labeled anti-human IgG Fc-specific antibody. Cells were permeabilized and stained for intracellular HIV-1 Gag. Data were analyzed via flow cytometry. (B) Antibody-dependent complement-mediated lysis (ADCML) assays. HIV-1-infected CD4 T cells were cultured overnight in undiluted pooled plasma from four healthy HIV-1-negative donors with the antibodies being tested. Cells were stained for intracellular HIV-1 Gag and analyzed by flow cytometry. (C) Antibody-dependent cell-mediated cytotoxicity (ADCC) assays. Isolated natural killer (NK) cells from an uninfected donor were cocultured with HIV-1-infected CD4 T cells in a 1:1 coculture in R-10 medium, and cells were incubated for 4 h. Cells were then stained for intracellular HIV-1 Gag and analyzed via flow cytometry. (D) In some experiments, the ADCC and ADCML assays were combined. HIV-1-infected CD4 T cells were cocultured in undiluted pooled human plasma with NK cells at a 1:1 ratio and cultured overnight. The frequency of HIV-1 Gag-expressing cells was determined by flow cytometry.
We next tested the ability of these antibodies to eliminate HIV-1-infected CD4 T cells via two effector functions, antibody-dependent complement-mediated lysis (ADCML) and antibody-dependent cell-mediated cytotoxicity (ADCC), or both, as illustrated in Fig. 1B, C, and D, respectively.
Given the recent evidence that monotherapy with the broadly neutralizing antibodies VRC01 (12, 16) and 3BNC117 (13) resulted in a failure to impart long-term control of viremia due to the emergence of resistance mutations, we further postulated that combinatorial treatment with antibodies could result in enhanced clearance of HIV-1-infected CD4 T cells via ADCML, ADCC, or both. Therefore, based on the results obtained from the antibody recognition of HIV-1-infected CD4 T cells and given the safety profile of CD4 binding site-specific antibodies, we also tested multiple combinations of antibodies to investigate their ability to eliminate infected CD4 T cells in vitro.
Cross-clade antibody recognition of primary HIV-1-infected CD4 T cells is highly variable.
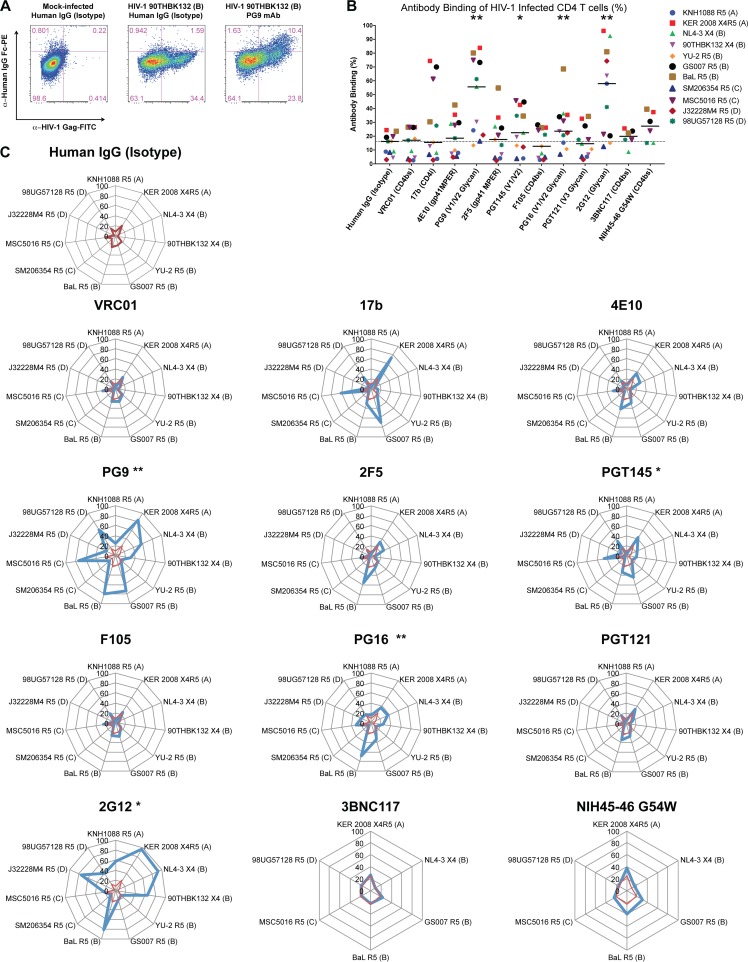
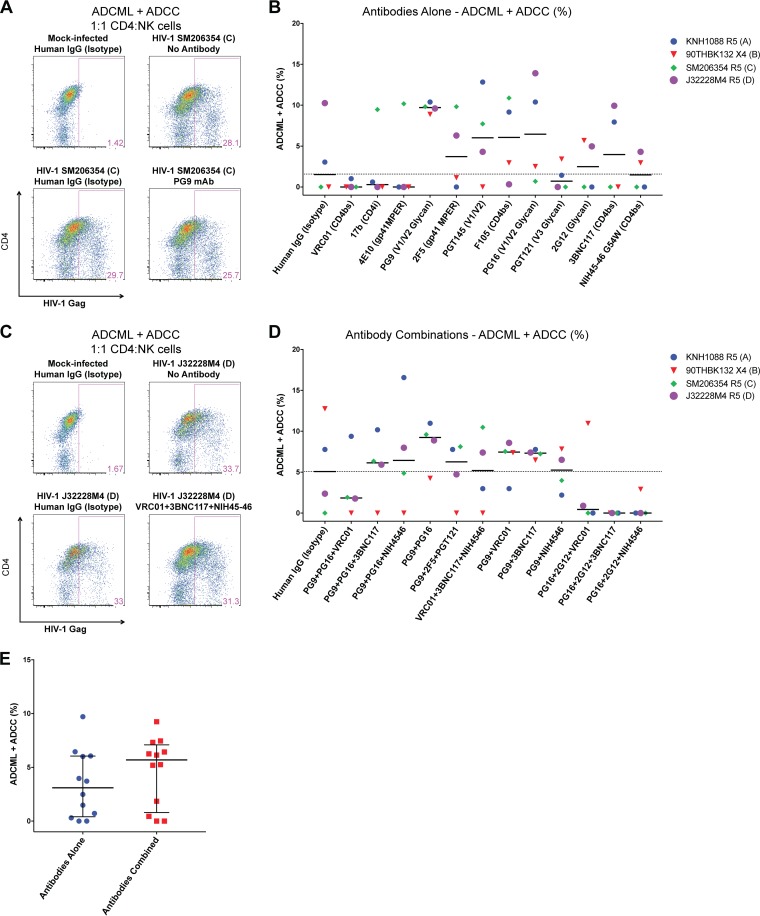
Primary CD4 T cells were isolated from peripheral blood mononuclear cells (PBMCs) of healthy HIV-1-seronegative donors and infected with HIV-1 strains. The frequency of infected cells was monitored by staining for intracellular HIV-1 Gag protein and was typically ∼15 to 40% 4 days following infection. Mock- and HIV-1-infected CD4 T cells were washed extensively and coincubated with HIV-1 envelope-specific monoclonal antibodies at a final concentration of 2 μg/ml for 30 min, followed by 30 min of staining with a phycoerythrin (PE)-labeled mouse-anti-human IgG Fc secondary antibody. CD4 T cells were subsequently stained for intracellular HIV-1 Gag protein expression. Representative flow cytometry plots obtained for one such experiment with clade B HIV-1 90THBK132 virus along with controls are shown in Fig. 2A. In this particular experiment, the human IgG isotype control staining revealed binding of only 4.42% of total infected CD4 T cells [1.59/(1.59 + 34.4) × 100], whereas the HIV-1 V1/V2/glycan-specific antibody PG9 (27) bound to 30.4% of total HIV-1-infected CD4 T cells.
FIG 2.
Binding of anti-HIV-1 envelope antibodies to CD4 T cells infected with diverse clades of HIV-1 is highly variable. CD4 T cells from healthy uninfected donors were infected with 11 unique strains of HIV-1, including 2 clade A, 5 clade B, 2 clade C, and 2 clade D strains. Infected cells were stained with 2 μg/ml of each HIV-1 envelope-specific antibody for 30 min at 4°C, followed by 30 min of staining with a secondary PE-labeled anti-HIV-1 IgG-Fc antibody for 30 min. Intracellular HIV-1 Gag (Kc57-FITC) was stained after cells were permeabilized to determine the frequency of infected cells. (A) Representative flow cytometry plots of a binding experiment depicting human IgG (isotype) control staining with mock-infected or HIV-1 90THBK132 (clade B)-infected CD4 T cells as controls relative to PG9 monoclonal antibody (mAb) binding of HIV-1-infected cells. Binding was calculated as the percentage of cells positively stained for anti-human IgG Fc (PE+) within total infected cells (FITC+ PE+). (B) Summary data obtained from experiments testing binding of antibodies against CD4 T cells infected with several HIV-1 clades are reported as the percent binding of each antibody within total infected cells. Each symbol represents an experiment with a unique HIV-1 strain, and the dotted line indicates the median binding of the human IgG (isotype) control data. Medians are shown. Wilcoxon matched-pair signed-rank tests were performed for comparison of the human IgG (isotype) data set for all statistical analyses. (C) Radar graphs depicting breadth of antibody binding in each experiment against individual HIV-1 strains tested for panel B. Antibody binding is illustrated in blue lines, and the isotype control is displayed within each plot with a red line. Virus clades and tropisms are indicated adjacent to the names in the key. X4, CXCR4 tropic; R5, CCR5 tropic. Eleven viruses were tested for all antibodies except 3BNC117 and NIH45-46 G54W (n = 6). *, P < 0.05; **, P < 0.01.
The fraction of HIV-1-infected CD4 T cells (Gag+) that exhibit binding to HIV-1 envelope-specific antibodies was determined for each antibody, and summary data obtained for infections with 11 unique HIV-1 isolates are shown in Fig. 2B. We observed significantly elevated antibody-mediated recognition of surface HIV-1 envelope on CD4 T cells with antibodies PG9 (55.64%; P = 0.0020), PGT145 (22.52%; P = 0.0137), PG16 (23.57%, P = 0.0068), and 2G12 (57.93; P = 0.0029) relative to human IgG (isotype) controls (16.18%), determined by paired analyses (median frequencies reported in parentheses). Surprisingly, the CD4 binding site-specific antibodies VRC01, 3BN117, and NIH45-46 G54W (an engineered version of the parent antibody that exhibits enhanced neutralization breadth and potency [28]; referred to as NIH45-46 here) did not demonstrate significant binding above background in these assays.
We observed highly variable antibody-mediated recognition of primary CD4 T cells infected with various clades of HIV-1, as shown in Fig. 2C. For example, antibody 2G12, specific for an oligomannose cluster on gp120 (29, 30), did not exhibit recognition of CD4 T cells infected with clade C viruses or clade B YU-2, which lack the residue for 2G12 binding (31, 32). The lack of binding observed with any of the highly potent and broad CD4 binding site-specific antibodies VRC01 (1, 2), 3BNC117 (1), and NIH45-46 (1) suggests that the conformation of the HIV-1 envelope on the surface of primary infected CD4 T cells differs from that on cell-free viruses that these antibodies have been demonstrated to neutralize efficiently. The V1/V2-specific monoclonal antibody PG9 displayed the broadest recognition of HIV-1-infected CD4 T cell targets by binding to 10 of 11 viruses tested relative to the human IgG isotype control. PG16 and PGT145, both targeting the V1/V2 domain (27, 33), also displayed enhanced recognition of infected cells. These experiments highlight the V1/V2 loop of the HIV-1 envelope to be interest for future trials, since all three antibodies targeting this domain displayed enhanced recognition of primary HIV-1-infected CD4 T cells.
HIV-1 envelope-specific antibodies induce limited ADCML of primary infected targets.
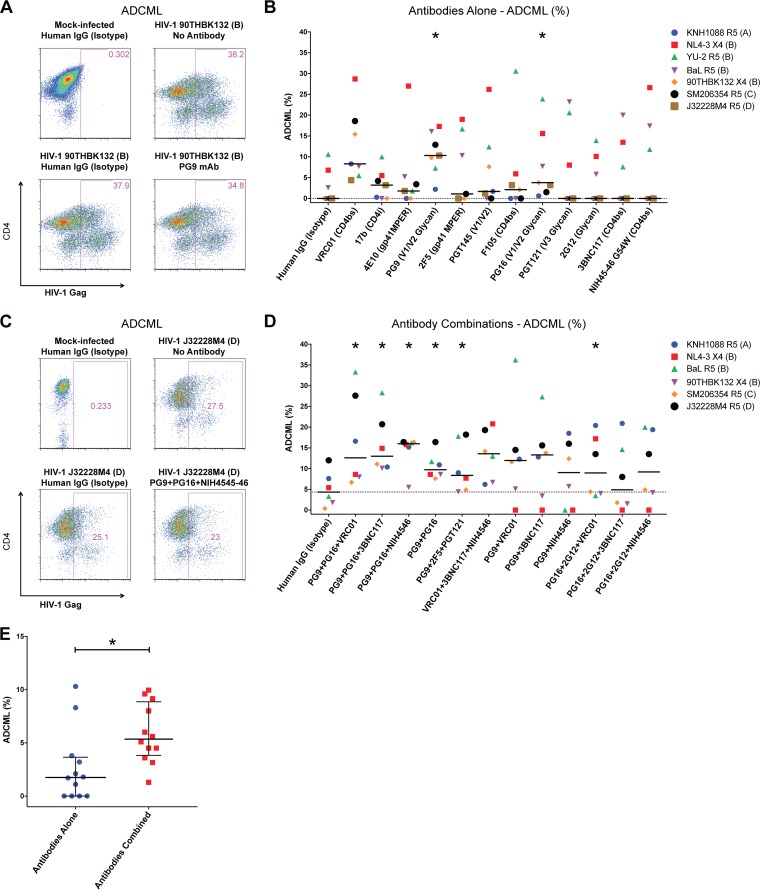
Antibody binding to an HIV-infected cell can trigger complement-mediated lysis (reviewed in reference 34). We next examined the ability of the panel of antibodies to directly eliminate CD4 T cells infected with seven HIV-1 isolates representing clades A, B, C, and D in cultures via complement-mediated lysis. CD4 T cells infected with the viruses were cultured in fresh, undiluted pooled plasma from four healthy human volunteers in the presence of each antibody at 2 μg/ml in an overnight assay. The percent elimination relative to the frequency of infected cells in cultures without any antibody was determined. Results from a representative experiment with controls are depicted in Fig. 3A; the percent elimination of 90THBK132-infected cells with monoclonal antibody PG9 was determined to be ∼9% [(38.2 − 34.8)/38.2 × 100]. Summary data for these experiments with each of the antibodies alone are illustrated in Fig. 3B. Relative to the median ADCML with isotype antibody (0%), we observed significantly enhanced elimination of HIV-1-infected CD4 T cells by antibodies PG9 (10.3%; P = 0.0469) and PG16 (3.8%; P = 0.0156). Surprisingly, despite exhibiting maximal potency in the HIV-1 envelope binding experiments, 2G12 treatment did not induce killing of infected cells (median: 0%; P = 0.2500), suggesting that with this particular antibody, binding did not equate to killing. In contrast to the case with 2G12, we observed a trend toward improved ADCML in the presence of the CD4 binding site antibody VRC01 (8.3%; P = 0.0625) despite the absence of recognition of infected cells in the previous assays.
FIG 3.
Combinations of antibodies elicit enhanced ADCML of HIV-1-infected CD4 T cells. HIV-1-infected CD4 T cells were cultured overnight in freshly collected undiluted pooled plasma with HIV-1 envelope-specific antibodies alone or in combinations in a complement-mediated-lysis assay. The frequency of HIV-1 Gag-expressing cells was determined in each culture; disappearance of Gag+ cells was indicative of ADCML relative to controls. (A) Representative flow cytometry plots depicting the frequency of HIV-1 Gag+ CD4 T cells within mock-infected and HIV-1-infected CD4 T cells with isotype control as well as a no-antibody control. The percentage of infected cells in a culture with the PG9 monoclonal antibody overnight is also shown. (B) ADCML is reported as the percentage difference in HIV-1 Gag+ cells between the no-antibody and respective conditions with seven viruses tested as indicated. (C) Representative flow cytometry plots of controls and HIV-1 J32228M4-infected CD4 T cells treated with a cocktail of three monoclonal antibodies (PG9, PG16, and NIH45-46) are shown. (D) ADCML assays were conducted with the indicated combinations of antibodies tested against CD4 T cells infected with six diverse strains of HIV-1. For panels B and D, each symbol represents an experiment with the indicated HIV-1 strain. Dotted lines indicate the median value of cultures with the human IgG (isotype) wells. Medians are shown. Wilcoxon matched-pair signed-rank tests were performed to compare each condition to the paired isotype controls. (E) Combined ADCML data for experiments using antibodies alone (n = 12) or in various combinations (n = 12) are plotted. Each symbol represents the median of an antibody alone (from panel B) or a particular antibody combination (from panel D) examined. The grand medians of groups are displayed. Nonparametric Mann-Whitney U tests were performed to determine statistical significance. The combinatorial approach increased ADCML of HIV-1-infected CD4 T cells. A total of 2 μg/ml of each antibody was tested per culture. For antibody combination experiments, the isotype control antibody concentration was 6 μg/ml. *, P < 0.05.
We then combined the 3 best bnAbs, PG9, PG16, and VRC01, and tested ADCML mediated by this combination. We also tested combinations of PG9 and PG16 with the two other CD4 binding site antibodies, 3BNC117 and NIH45-46, that were shown to be more potent and broader than VRC01 for neutralization. The results of a representative experiment are depicted in Fig. 3C; in this experiment, HIV-1 J32228M4-infected cells were coincubated with an antibody cocktail comprising 2 μg/ml each of monoclonal antibodies PG9, PG16, and NIH45-46. The percent ADCML was determined as previously described, and in this particular experiment the degree of elimination relative to no-antibody paired wells was ∼16%. Summary data for percent ADCML are shown in Fig. 3D. In these experiments, the PG9-PG16 combination yielded statistically enhanced elimination of primary HIV-1-infected CD4 T cells (median: 9.75%; P = 0.0312) compared to the human IgG isotype control (median: 4.35%). All other combinations of PG9-PG16 with a CD4 binding site-specific antibody resulted in even greater elimination of targets, including the antibodies VRC01 (12.6%; P = 0.0312), 3BNC117 (13%; P = 0.0312), and NIH45-46 (16%; P = 0.0312). A triple combination of VRC01, 3BNC117, and NIH45-46, however, did not significantly enhance elimination of targets (13.6%; P = 0.0625), indicating that antibodies PG9 and PG16 were responsible for the effects observed. Another triple combination targeting three unique epitopes on HIV-1 envelope comprising PG9, the gp41 membrane-proximal external region (MPER)-specific monoclonal antibody 2F5 (35), and the V3 loop-specific monoclonal antibody PGT121 (4) also significantly enhanced clearance of HIV-1-infected CD4 T cells (8.35%; P = 0.0312), suggesting that immunotherapy targeting varied sites on the HIV-1 envelope could be a productive strategy. We also tested PG9 combinations with each of the CD4 binding site antibodies. Although these combinations resulted in elevated median elimination of HIV-1-infected targets, the results were inconsistent. Furthermore, given the enhanced 2G12-mediated recognition of surface envelope on infected CD4 T cells, we tested triple combinations of PG16, 2G12, and either VRC01, 3BNC117, or NIH45-46. Of these, only the combination of PG16, 2G12, and VRC01 induced significantly enhanced clearance of HIV-1-infected targets via ADCML (8.95%; P = 0.0312).
Lastly, we determined that combinatorial administration of antibodies enhanced ADCML of HIV-1-infected CD4 T cells compared to single-antibody treatments alone (Fig. 3E). The median ADCML with each of the 12 antibodies was 1.75%, compared to 5.35% with antibody combinations (P = 0.0114 between groups). Overall the maximal elimination of infected cells by ADCML was 30.6% with singular antibodies versus 36.2% with combinations tested. Hence, passive administration of these HIV-1 envelope-specific antibodies should be tested with combination treatments as an immunotherapy toward the eradication of viral reservoirs.
Antibody cocktails induce effective ADCC of primary HIV-1-infected CD4 T cells.
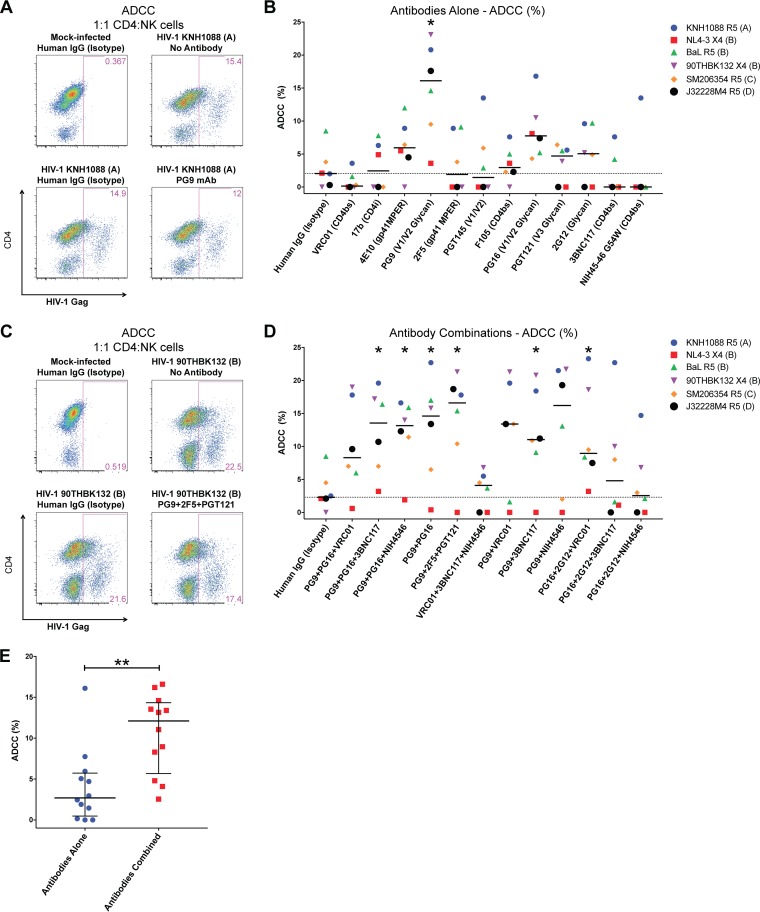
We next determined how effective these HIV-1 envelope-specific antibodies were at eliminating primary CD4 T cells infected with diverse clades of HIV-1 by ADCC. In these assays, HIV-1-infected CD4 T cells were cocultured in a 1:1 ratio with natural killer (NK) cells freshly enriched via negative selection from the PBMCs of a healthy, HIV-1-negative blood donor. A 2-μg/ml concentration of each antibody was added to cultures testing ADCC capabilities of antibodies alone or in combination for 4 h; the difference in frequency of HIV-1-infected CD4 T cells from control cultures without antibodies was determined, and results are reported as percent ADCC in Fig. 4.
FIG 4.
Enhanced ADCC with antibody combinations rather than single-antibody treatment. NK cells from an uninfected donor were isolated and cocultured with HIV-1-infected CD4 T cells in a 1:1 ratio for 4 h in R-10 medium with HIV-1 envelope-specific antibodies. HIV-1 Gag+ cell frequencies were assessed by flow cytometry. (A) Flow cytometry plots of a representative experiment with PG9 monoclonal antibody-treated cocultures of CD4 T cells infected with the clade A HIV-1 strain KNH1088. Cells shown are CD3+ T cells. (B) ADCC was calculated as the percent difference in HIV-1 Gag+ frequencies between the wells receiving antibodies and the control no-antibody wells. Summary data obtained for each antibody tested against HIV-1-infected CD4 T cells generated with six different strains are shown. (C) Representative flow cytometry plots depicting the frequency of HIV-1 Gag+ CD4 T cells in mock-infected and HIV-1 90THBK132-infected CD4 T cells as well as cultures with a triple combination of antibodies (PG9, 2F5, and PGT121). CD3− NK cells were gated out, and only CD3+ CD4 T cells are displayed. (D) Summary data of percent ADCC observed with the indicated antibody combinations tested against six unique HIV-1 strains are shown. For panels B and D, symbols depict individual paired experiments with the indicated virus. Medians are shown; dotted lines depict the median value of the human IgG (isotype) wells, to which all other wells were compared using Wilcoxon matched-pair signed-rank tests to determine statistical significance. (E) Combined ADCC data for experiments using antibodies alone (n = 12) or in various combinations (n = 12) are plotted. Each symbol represents the median percent ADCC obtained with experiments using antibodies alone or in combinations (B and D, respectively). The horizontal line signifies the grand median of groups. Nonparametric Mann-Whitney U tests were performed to determine statistical significance between the two groups. Combination of antibodies resulted in improved killing of HIV-1-infected cells by ADCC compared to antibodies tested alone. Each antibody was tested at 2 μg/ml either alone or in combination. The isotype control antibody concentrations were 2 μg/ml for experiments using antibodies alone and 6 μg/ml for combinatorial experiments. *, P < 0.05; **, P < 0.01.
The results of a representative experiment with KNH1088 HIV-1-infected CD4 T cells cultured with the PG9 monoclonal antibody and NK cells along with controls are shown in Fig. 4A. In this particular example, the PG9 antibody eliminated ∼22% of HIV-1-infected cells [(15.4 − 12)/15.4 × 100]. Summary data for all 12 antibodies tested individually against six HIV-1 isolates are reported in Fig. 4B. PG9 exhibited broad and significant elimination of HIV-1-infected targets (median: 16.1%; P = 0.0312) relative to cultures receiving isotype control antibody (median: 2.05%). Cultures with antibodies 4E10 (5.95%; P = 0.0625) and PG16 (7.75%; P = 0.0938) displayed a trend toward enhanced ADCC in these experiments as well. Consistent with antibody-mediated recognition of infected CD4 T cells and ADCML assays, the CD4 binding site-specific antibodies VRC01 (P = 0.1875), 3BNC117 (P = 0.625), and NIH45-46 (P = 0.625) were unable to effectively induce ADCC of infected targets. Overall, we observed a modest ability of the majority of antibodies tested in our assays to induce elimination of HIV-1-infected CD4 T cells.
Next, we examined whether antibody cocktails could synergistically enhance the clearance of HIV-1-infected targets via ADCC. Figure 4C depicts a representative example of these experiments testing the PG9-2F5-PGT121 combination against HIV-1 90THBK132-infected CD4 T cells. Relative to the no-antibody control wells, this combination resulted in ∼23% [(22.5 − 17.4)/22.5 × 100] elimination of infected cells by ADCC. Similar to the case with the complement-mediated-lysis assays, combination of the antibodies PG9 and PG16 significantly increased the elimination of CD4 T cells infected with HIV-1 strains representative of the four clades tested (median: 14.6%, P = 0.0312) compared to the paired isotype controls (median ADCC: 2.3%). The addition of a few CD4 binding site antibodies—VRC01 (8.3%, P = 0.0938), 3BNC117 (13.55%; P = 0.0156), and NIH45-46 (13.15%; P = 0.0312)—along with PG9 and PG16 also resulted in the greater elimination of targets than with paired isotype controls. However, given that the triple combination comprising VRC01, 3BNC117, and NIH45-46 antibodies did not increase killing of infected cells (4.1%; P = 0.5000), these findings indicate that the CD4 binding site antibodies did not contribute to additional ADCC-dependent lysis of infected targets observed with the PG9-PG16 combination. Combination of PG9 alone with VRC01 (13.4%; P = 0.0781), 3BNC117 (11.05%; P = 0.469), and NIH45-46 (16.2%; P = 0.0781) resulted in moderately enhanced ADCC-mediated clearance of HIV-1-infected CD4 T cells, whereas PG16 combinations without PG9 did not exhibit this effect. Based on the findings that largely the monoclonal antibody PG9 alone (Fig. 4B) induced efficient ADCC of HIV-1-infected cells, it may be inferred that curative approaches involving broadly neutralizing antibodies should incorporate this bnAb in their regimen. As with the ADCML assays, the PG9-2F5-PGT121 combination targeting three unique sites on the HIV-1 envelope also significantly increased the elimination of HIV-1-infected CD4 T cells (16.66%; P = 0.0312). Overall, optimal combinations of antibodies resulted in significantly greater ADCC-mediated elimination targets than did testing antibodies alone (Fig. 4E; P = 0.0029).
ADCML and ADCC target similar populations and are not additive processes for the elimination of HIV-1-infected CD4 T cells.
Since antibodies in vivo could target HIV-1-infected cells for clearance via ADCML and/or ADCC, we next sought to combine the ADCML and ADCC assays to test whether these two distinct mechanisms could additively enhance the elimination of HIV-1-infected CD4 T cells. Assays were performed overnight with antibodies alone, or in combination, with NK cells in a 1:1 ratio in undiluted pooled human plasma from healthy donors' blood. Shown in Fig. 5A is a representative example of results from an experiment with clade C HIV-1 SM206354-infected CD4 T cells treated with monoclonal antibody PG9. In this example, relative to the no-antibody condition, PG9 resulted in killing of 8.5% of infected cells [(28.1 − 25.7)/28.1 × 100]. Summary data for all antibodies tested individually are reported in Fig. 5B. Although we observed increased median elimination of HIV-1-infected CD4 T cells by PG9 (9.71%), PG16 (6.45%), F105 (6.06%), PGT145 (6.01%), 3BNC117 (3.97%), 2F5 (3.72%), and 2G12 (2.49%) antibodies relative to median isotype control (1.43%), none of these findings were significant, likely as a consequence of testing only four HIV-1 strains in these assays: one from each of clades A, B, C, and D. Notably, in agreement with the previous assays, PG9 and PG16 antibodies demonstrated the most elimination of infected cells in these experiments as well.
FIG 5.
ADCC and ADCML responses are not synergistic. CD4 T cells infected with four unique clades of HIV-1 were cocultured with NK cells from a healthy donor overnight in undiluted human plasma with HIV-1 envelope-specific antibodies. Frequencies of HIV-1 Gag+ cells within the CD3+ CD4 T cell fraction were determined by flow cytometry. (A) Representative data obtained from cocultures with mock-infected or clade C HIV-1 SM206354-infected CD4 T cells controls and the monoclonal antibody PG9 are shown. Data shown are gated on CD3+ T cells. (B) The difference in the frequency of HIV-1 Gag+ CD4 T cells and that number in the corresponding no-antibody data is reported as the percent combined ADCML and ADCC. Summary data from experiments performed with all four viruses treated with monoclonal antibodies tested individually are shown. (C) Representative flow cytometry plots of data generated with antibodies tested in a triple combination (VRC01, 3BNC117, and NIH45-46) against clade D HIV-1 J32228M4 are shown along with relevant controls. (D) Summary data for percent ADCML plus ADCC are displayed for all antibody combinations tested with each of the viruses. Symbols represent individual paired experiments conducted with a unique virus. Dotted lines represent the median isotype control percent ADCML plus ADCC to which all conditions were compared to determine statistical significance by Wilcoxon matched-pair signed-rank tests. No statistical significance was observed (n = 4). (E) Combined data from experiments using antibodies alone (panel B; n = 12) and in various combinations (panel D; n = 12) tested in the ADCML-ADCC assays are shown. Each symbol represents the median percent ADCML plus ADCC obtained in the respective assays. Mann-Whitney U tests were performed to determine statistical significance between groups. No statistical significance was observed. Each antibody was tested in at a final concentration of 2 μg/ml in experiments testing antibodies alone or in combination. Isotype control antibody was used at 2-μg/ml and 6-μg/ml final concentrations in experiments testing antibodies alone and in combination, respectively.
We then tested identical cocktails of antibodies from Fig. 3D and 4D in the combined ADCML and ADCC assay. The results of an example of one such experiment with the antibody cocktail comprising VRC01, 3BNC117, and NIH45-46 with HIV-1 SM206354-infected CD4 T cells are displayed in Fig. 5C. The triple combination resulted in the killing of ∼7% [(33.7 − 31.3)]/33.7 × 100] HIV-1-infected cells relative to the no-antibody cultures. Summary data for these assays against CD4 T cells infected with four unique clades of HIV-1 are shown in Fig. 5D. Compared to the human IgG isotype control (median: 5.08%), PG9-PG16 resulted in the consistently highest elimination of infected cells recorded in these experiments (9.24%). These effects were largely PG9 mediated, as only combinations including this antibody exhibit clearance of HIV-1-infected CD4 T cells above the median of the control isotype cultures. A combination of VRC01, 3BNC117, and NIH45-46 was unable to induce the killing of HIV-1-infected cells, consistent with our previous findings in the individual ADCML and ADCC assays. Again, these results suggest that the PG9 monoclonal antibody would be an ideal candidate toward the development of new strategies to eliminate the HIV-1 reservoir.
We compared whether combined antibodies induced greater killing of HIV-1-infected CD4 T cells in these assays than did antibodies tested alone, shown in Fig. 5E. We observed that combined antibodies exhibited a marginal increase in the elimination of targets over that with antibodies alone, with medians of 5.7% and 3.1%, respectively. However, this difference was not statistically significant. In these assays, overall, we did not observe greater elimination of infected targets than in the assays with ADCML or ADCC alone. For example, the PG9-PG16 combination demonstrated medians of 9.75%, 14.6%, and 9.24% killing of infected targets via ADCML, ADCC, and ADCML-ADCC, respectively. Thus, it may be inferred that ADCML and ADCC are not synergistic processes and may induce the clearance of a similar population of HIV-1-infected CD4 T cells.
ADCML and ADCC target early-stage-infected cells exclusively.
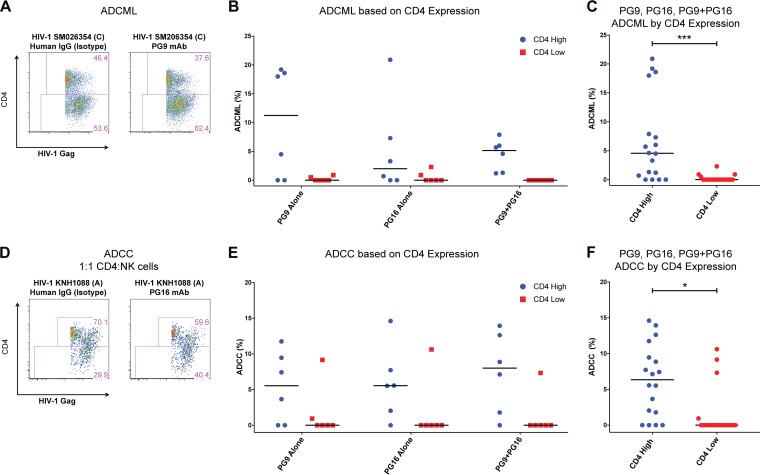
To better understand the characteristics of HIV-1-infected CD4 T cells that may be cleared by antibody-mediated immune pathways, we probed the CD4 expression of infected cells as a “kinetic” marker of infection progression. HIV-1 proteins Env, Nef, and Vpu downregulate the expression of CD4 molecules through distinct mechanisms (36–38). Hence, in our in vitro model of productive infection of primary CD4 T cells, the CD4 expression profile would distinguish early- from late-stage-infected cells. Indeed, we observed two distinct populations of HIV-1 Gag-expressing early-stage (CD4 High) and late-stage (CD4 Low) CD4 T cells (Fig. 6A). We further evaluated the percent elimination of HIV-1-infected CD4 T cells by ADCML based on the CD4 expression profile, i.e., early- or late-stage infection. As seen in Fig. 6A, relative to the isotype control, PG9 treatment resulted in the killing of ∼19% [(46.4 − 37.6)/46.4 × 100] of HIV-1 SM206354-infected targets expressing CD4, whereas no elimination of the cells lacking CD4 expression was observed. Summary data for ADCML experiments with antibodies PG9 and PG16 and the PG9-PG16 combination (Fig. 3B and D) are reported in Fig. 6B. Data from Fig. 6B were combined to determine the overall elimination by ADCML of HIV-1-infected CD4 targets by either of these three conditions stratified by CD4 expression and are shown in Fig. 6C. Only the early-stage (CD4 High) HIV-1-infected CD4-expressing cells were eliminated to some degree under each tested condition, whereas cells infected for a prolonged period, devoid of CD4 expression, did not experience similar killing rates; medians were 4.6% and 0% (P = 0.0010), respectively. Remarkably, we obtained identical findings with these antibodies tested for the ability to eliminate HIV-1-infected CD4 T cells via ADCC as shown in Fig. 6D and summary data in Fig. 6E. In the ADCC assays, the contribution of these three conditions to the clearance of CD4 High HIV-1-infected CD4 T cells was a median of 6.4%, compared to 0% (P = 0.0268) for cells lacking CD4 expression (Fig. 6F). These observations suggest a role for HIV-1 infection in rendering infected cells resistant to antibody-mediated complement-mediated lysis as well as antibody-mediated cell-mediated cytotoxicity pathways in progressively infected cells.
FIG 6.
HIV-1 envelope-specific antibodies induce elimination of early-stage but not late-stage HIV-1-infected CD4 T cells. ADCML or ADCC assays were performed with HIV-1-infected cells using PG9 and PG16 antibodies alone or in combination. Cells were stained for CD4 expression and analyzed via flow cytometry. Early-stage-infected cells retain high CD4 expression (CD4 High), whereas late-stage-infected cells drastically downregulate CD4 expression (CD4 Low). We assessed the degree of elimination induced by each antibody treatment based on their CD4 expression within the total infected-cell fraction relative to the paired human IgG isotype control. (A) Representative flow cytometry plots depicting ADCML data with clade C HIV-1 SM206354 are shown. (B) Summary data (n = 6) of ADCML induced under each listed condition are reported stratified by CD4 expression profile of infected cells. (C) Data from PG9, PG16, and PG9-PG16 conditions were combined (n = 18), and percent ADCML was determined based on the CD4 expression profile of HIV-1-infected targets. (D) Results from a representative ADCC experiment with PG16-treated clade A HIV-1 KNH1088-infected cells and control (isotype) are shown. Gates represent the frequency of CD4 High and CD4 Low cells within the total infected-cell population. (E) Summary data for ADCC induced on the CD4 High and CD4 Low cells within total infected cells are reported (n = 6). (F) Data from panel E were combined (n = 18) to determine ADCC by these three treatments based on CD4 expression. Elimination of HIV-1-infected CD4 T cells by either ADCML or ADCC was limited to early-infected (CD4 High) cells, whereas the late-stage-infected (CD4 Low) cells were resistant to killing by these mechanisms. Medians are shown. Nonparametric Wilcoxon matched-pair signed-rank tests were performed for data analysis. *, P < 0.05; ***, P < 0.001.
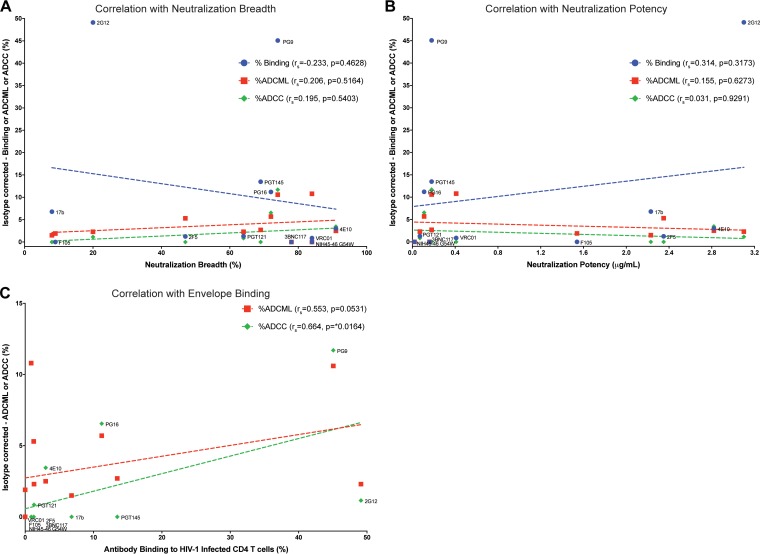
Antibody binding of HIV-1-infected CD4 T cells, but not neutralization breadth or potency, correlates with effector functions.
In order to better understand what factor(s) contributed to antibody-mediated elimination of HIV-1-infected CD4 T cells, correlation analyses were performed for the human IgG isotype-corrected median values obtained for antibody binding of HIV-1-infected cells (Fig. 2B), ADCML (Fig. 3B), ADCC (Fig. 4B), and historical neutralization breadth and potency of each antibody against several viruses (listed in Table 2), as shown in Fig. 7A and B, respectively. We observed that neither of these parameters correlated significantly, indicating that neutralization activities and antibody-mediated clearance of infected cells are distinct mechanisms.
FIG 7.
Antibody binding to HIV-1-infected CD4 T cells correlates with effector functions. Isotype-subtracted, median values obtained in this study for antibody binding and ADCML and ADCC assays are plotted alongside historical neutralization breadth (A) and potency (B) of each neutralizing antibody tested after corresponding human IgG (isotype) subtraction for each antibody. Neutralization breadth and potency data for several HIV-1 strains characterized by numerous groups were obtained from CATNAP (26) and are reported in Table 2. No correlation was observed between neutralization parameters and effector functions or antibody binding of HIV-1-infected primary CD4 T cells. Labels are provided for the data set for antibody binding of infected cells. (C) Linear regression plots of isotype-subtracted median values of antibody binding and ADCML or ADCC are plotted. ADCC assay data are labeled. Nonparametric Spearman correlations (rs) were determined to ascertain any statistical significance. *, P < 0.05.
Next, correlation analysis of isotype-corrected effector functions (ADCML and ADCC) with antibody binding experiments was performed; results are illustrated in Fig. 7C. Recognition of HIV-1-infected CD4 T cells by monoclonal antibodies displayed a strong trend to association with ADCML (Spearman correlation [rs] = 0.553; P = 0.0531) and correlated significantly with ADCC (rs = 0.0664; P = 0.0164). These findings indicate that future trials aimed at the eradication of the viral reservoir with HIV-1 envelope antibodies should test the ability of the antibody cocktails to bind directly to HIV-1-infected CD4 T cells in order to eliminate them via immune-mediated pathways.
DISCUSSION
In this study, we obtained direct experimental data on the ability of anti-HIV-1 envelope antibodies and their combinations to eliminate primary HIV-1-infected CD4 T cells via antibody-mediated effector functions, namely, antibody-dependent complement-mediated lysis (ADCML) and antibody-dependent cell-mediated cytotoxicity (ADCC), in vitro. The activities of 12 well-characterized anti-HIV-1 envelope antibodies, and their combinations, against a broad range of epitopes on the viral envelope were tested for the elimination of primary CD4 T cells infected with HIV-1 isolates from four main circulating HIV-1 clades in different regions of the world. Much is known about the ability of several of these antibodies to prevent viral infection from cell-free HIV-1 virions; however, far less is understood about their potential toward the direct binding and subsequent elimination of HIV-1-infected cells. The ability of an antibody to recognize and bind to an infected cell is critical to initiate clearance via antibody-mediated effector functions through Fc-FcR (Fc receptor) interactions (reviewed in reference 39).
We found that antibodies recognizing carbohydrate epitopes, such as 2G12 and the V1/V2/glycan-specific antibodies PG9, PG16, and PGT145, were the best at binding to HIV-1 envelope protein on the surface of primary HIV-1-infected cells. Except for 2G12, these V1/V2 bnAbs were also best at inducing ADCML and ADCC. Notably, V1/V2-specific antibodies were also determined to be a correlate of protection in the moderately protective HIV-1 vaccine trial RV 144 (22). Remarkably, another predictor of protection in RV 144 was the degree of ADCC-mediated responses between high and low responders to the vaccine (22). Thus, it is plausible that V1/V2-induced antibodies in RV 144 conferred protection by superior ADCC-mediated responses. To further corroborate our findings, a recent report (40) also identified V1/V2-directed antibodies to be superior for binding to envelope on infected cell lines and also exhibit more efficient ADCC than other antibodies. It is pertinent to note, however, that PG9 and PG16 antibodies are specific for glycan epitopes on the HIV-1 trimer, and therefore, their reactivity can be diminished by heterogeneous glycosylation of HIV-1 envelope produced from infected cells (41). Despite this possibility, we observed these two bnAbs to consistently impart the broadest recognition and subsequent antibody-mediated elimination of primary CD4 T cells infected with diverse strains of HIV-1 in this study. Collectively, these observations highlight the need to target the V1/V2 site on the HIV-1 envelope and the incorporation of antibodies such as PG9 and PG16 into future antibody cocktails as therapeutics for HIV-1 control or cure. We report that antibodies in combination were moderately superior to antibodies tested individually for the clearance of primary HIV-1-infected CD4 T cells. Furthermore, we demonstrate that antibody-mediated recognition, and not neutralization breadth or potency, correlated with the elimination of HIV-1-infected CD4 T cells by ADCML or ADCC mechanisms.
Since we tested the ability of several bnAbs to bind surface envelope on CD4 T cells infected with diverse HIV-1 clades, it is probable that the limited recognition breadth of some antibodies would prove to be a limiting factor. For example, the majority of clade C viruses and the clade B virus YU-2 lack the glycosylated residue for 2G12 binding (31, 32). This would explain why we did not observe recognition of these viruses by this antibody in our assays. However, the CD4 binding site-targeting antibodies VRC01, 3BNC117, and NIH45-46 are among the broadest and most potent neutralizing antibodies identified to date; surprisingly, however, these did not exhibit recognition of primary HIV-1-infected CD4 T cells or contribute to their direct elimination in our assays. These results suggest that the conformation of the envelope protein on the surface of physiological primary HIV-1-infected CD4 T cells, unlike cell lines (42), is not conducive to binding by these CD4 binding site-specific antibodies. Certainly, the HIV-1 envelope protein undergoes proteolytic cleavage (32) and several other modifications, including receptor ligation (43) and extensive glycosylation (44, 45), that may be of an altered phenotype in comparison to that present on a cell-free virus that these antibodies efficiently bind for neutralization (1). Thus, it is plausible that Env on the surface of infected primary CD4 T cells occludes the epitopes for certain antibodies with specificities for the CD4 binding site. Indeed, the removal of a glycan residue on trimeric envelope proximal to the CD4 binding site enhanced VRC01 binding relative to the wild-type trimer (46). Another potential mechanism at play might be that the viral envelope on the surface of infected CD4 T cells is constitutively engaged with the CD4 receptor, excluding the CD4 binding site antibodies from their epitope. Furthermore, while mature virions budding off from the cellular membrane of an infected cell incorporate several host proteins, the CD4 receptor is not among these (47, 48), indicating that the epitope for CD4 binding site antibodies, although accessible on cell-free virions, is unavailable on the viral envelope on the surface of an infected CD4 T cells as a consequence of gp120 interaction with its ligand. Thus, CD4 binding site antibodies, although potent at virus neutralization, did not exhibit recognition or elimination of primary HIV-1-infected CD4 T cells in our assays. It is also important to note that we tested the binding of 3BNC117 to primary HIV-1-infected CD4 T cells at 2 μg/ml for 30 min in a standard two-step staining protocol which differed from that of Lu et al. (49), who demonstrated copious binding of biotinylated 3BNC117 to infected CD4 T cells using 20 μg/ml of the antibody to stain cells for 90 min in a unique three-step staining protocol. These modifications between the two procedures could explain the discrepancy between our findings. Notably, Pham et al. (40) also demonstrated far weaker staining of envelope protein by 3BNC117 on primary HIV-1-infected CD4 T cells than by PG9. Lastly, while nonfunctional forms of the HIV-1 envelope, such as gp120/gp41 monomers, are readily detected on virus particles, it has been demonstrated that neutralization correlates with binding of antibody to trimeric, functional HIV-1 envelopes (50). Since we tested only neutralizing antibodies in this study, it is plausible that the antibody-mediated effector functions we observed were due to the recognition of functional HIV-1 trimers on the surface of HIV-1-infected CD4 T cells.
Antibody-mediated effector functions are critical for maximal protection of macaques from virus acquisition and are independent of neutralization capacity (51, 52). Reports from human trials of passive administration of broadly neutralizing antibodies 2F5, 4E10, and 2G12 in combination (53–55) and monotherapy with CD4 binding site-specific antibodies VRC01 (12, 16) and 3BNC117 (13, 15) as well as the V3 glycan-specific 10-1074 (14) have been published. Each trial reported the emergence of viral resistance mutations to the active antibody. Immunotherapy was associated with delayed kinetics of viral rebound following analytical treatment interruption compared to historical controls (15, 16, 53). In the more recent VRC01, 3BNC117, and 10-1074 trials, the benefits of therapy were observed primarily when antibodies were administered to viremic individuals off HAART. Notably, no changes in the size of the HIV-1 reservoir were observed by monotherapy with VRC01 as measured by infectious units per million (IUPM) CD4 T cells or cell-associated DNA and RNA measurements (12). When tested, 3BNC117 therapy did not decrease the size of the HIV-1 proviral reservoir measured as total HIV-1 DNA (15), although this parameter is not as robust an assessor of reservoir size as IUPM determination. These observations indicate that successful therapy was dependent on the presence of plasma viremia with viruses that these antibodies have been shown to bind and neutralize efficiently in vitro. It was inferred that the presence of these bnAbs accelerated viral clearance by opsonization of viral particles and the uptake of antibody-virus complexes by immune cells that would have led to the enhancement of anti-HIV-1 adaptive immune responses as a consequence of efficient antigen presentation (39). Indeed, immunological assays following 3BNC117 administration in humans (17) and in a dual combination study of 3BNC117 with V3-specific 10-1074 monoclonal antibody in SHIV-infected macaques (19) have demonstrated that passive immunotherapy with monoclonal antibodies enhanced humoral and cellular immune responses such as autologous antibody neutralizing titers and anti-HIV-1 CD8 T cell responses, respectively, leading to longer control of viremia irrespective of neutralization breadth (17, 19). In relation to this, the elimination of HIV-1-infected cells by 3BNC117 was shown to be dependent on Fc-FcR interactions (49); further supporting these observations, Fc-FcR interactions are crucial for the prevention of SHIV acquisition in macaque models (52). Due to a lack of reduction in the size of the viral reservoir following administration of the bnAbs 2F5, 4E10, 2G12, VRC01, and 3BNC117, it may be inferred that these antibodies did not directly eliminate HIV-1-infected cells. Importantly, our assays recapitulate this inability of these specific bnAbs, namely, 2F5, 4E10, 2G12, VRC01, and 3BNC117, to enhance clearance of virus-infected cells by ADCML or ADCC pathways. Thus, future trials of antibody cocktails should also include antibodies such as PG9 and PG16 along with other potent bnAbs like VRC01, 3BNC117, and 10-1074 in order to maximize the elimination of HIV-1-infected cells in vivo by synergistically triggering direct lysis (ADCML), ADCC, and the generation of improved anti-HIV-1 adaptive immune responses simultaneously.
HIV-1 has evolved several strategies to evade host immune responses. Among the orchestrators of these mechanisms are the viral proteins Nef and Vpu, which target several host proteins, such as major histocompatibility complex class I (MHC-I), for degradation to promote viral persistence (56, 57). Together, Nef and Vpu also directly inhibit antibody-mediated effector functions to interfere with the clearance of infected cells by unique mechanisms such as downregulating CD4 receptor from the surface of HIV-1-infected cells, which retains the envelope in its “closed” unligated trimeric state (58, 59). Several anti-HIV-1 envelope antibodies, such as 17b (60), interact with epitopes exposed upon CD4 ligation with the HIV-1 trimer; thus, by downmodulation of CD4 receptors, the viral epitopes for antibody interaction are masked (43, 61). Nef proteins from elite controllers of HIV-1 are unable to efficiently downmodulate CD4 (62). Thus, CD4-induced epitopes on the envelope trimer are readily available for antibody binding that leads to subsequent ADCC (62). This protective role of ADCC in elite controllers highlights the importance of ADCC in vivo and identifies it as a correlate of control in HIV-1 infection. Vpu and Nef also downregulate BST2 (tetherin), a restriction factor that anchors virions onto the surface of infected CD4 T cells, thus increasing surface density of viral antigens and leading to enhancement of antibody-antigen interactions (40, 58, 63). Additionally, both HIV-1 virions and infected cells utilize surface regulators of complement activation, such as CD55 and CD59, to resist complement-mediated lysis (34, 64–66). These viral mechanistic processes could explain why we observed modest effects by antibody-dependent complement-mediated lysis or cell-mediated cytotoxicity in our assays with primary CD4 T cells. Previous reports have also described only limited efficacy, typically <30%, of ADCML (67) and ADCC of HIV-1-infected cell lines by NK effector cells (40, 42). Blockade of CD59 yields increased complement-mediated lysis (67, 68), whereas attenuated viruses, deficient for Nef and Vpu expression, displayed enhanced susceptibility to ADCC of infected cells in a tetherin-dependent manner (40, 63). Consistent with this, we found that ADCML- and ADCC-sensitive HIV-1-infected CD4 T cells were phenotypically in the early stage of infection, i.e., displayed high CD4 expression. Intriguingly, these lines of evidence suggest that in order to maximize antibody-mediated clearance of HIV-1-infected targets, new therapeutics to antagonize Vpu and Nef as well as CD55 and CD59 function should be explored in combination with antibody treatment. However, it must be noted that in vitro, both ADCML and ADCC are short-term culture assays, whereas in vivo bnAbs could elicit a greater degree of clearance of infected cells by recurrent elimination of targets.
The Fc fragment of HIV envelope-specific antibodies too may be engineered to increase their in vivo half-lives, enhance localization to lymphatic tissues, and augment their ability to interact with FcRs (69). An Fc-modified version of VRC01 with two amino acid substitutions, dubbed VRC01-LS, had an extended in vivo half-life and conferred protection to macaques from SHIV acquisition for up to 14.5 weeks, compared to 8 weeks with the wild-type antibody (5). Additionally, VRC01-LS also displayed increased localization to gut tissue, a key site for HIV-1 pathogenesis (70). Bournazos et al. (51) demonstrated that the in vivo activity of bnAbs was dependent on the opsonization of virus particles and their removal from circulation. Furthermore, amino acid substitutions to the Fc domain, such as GASDALIE, that have been demonstrated to be superior to wild-type Fc for engagement with activating Fc receptors (71) led to enhanced control of viremia (51). Hence, it is plausible to administer long-lasting and potent engineered therapeutics to HIV-1-infected individuals that may prove superior to HAART, as demonstrated in humanized mice (25), such that in addition to suppressing viral replication, antibodies would directly eliminate HIV-1-infected cells via mechanisms such as ADCML and ADCC. However, a caveat to consider is that during the course of HIV-1 infection, NK cells are phenotypically altered and exhibit reduced cytotoxicity (reviewed in reference 72), thus complicating the utilization of antibody-based therapies for HIV-1 cure.
Elimination of the HIV-1 reservoir represents the current paradigm to the development of a cure. Future human studies are needed to determine quantitatively if antibody therapies can significantly affect the size of the HIV-1 reservoir. In an SHIV study, macaques receiving V3-specific monoclonal antibody PGT121 experienced a reduction in the size of HIV-1 DNA reservoir measured in the PBMCs, lymph nodes, and the gastrointestinal mucosa (18). Additionally, a combination of 3BNC117, 10-1074, and PG16 antibodies in humanized mice resulted in decreased proviral reservoir size measured as total cell-associated HIV-1 DNA (20). A recent report indicated that the coexistence in vivo of at least three autologous broadly neutralizing antibodies targeting the V1/V2, V3, and CD4 binding sites of the envelope protein in an HIV-1-infected individual imparted elite control of HIV-1 infection (21). These in vivo studies support the principle of using bnAbs as therapeutics for reservoir reduction, imperative for the development of a cure for HIV/AIDS.
According to our findings, bnAbs moderately eliminated primary HIV-1-infected CDT cells via ADCML and ADCC in vitro. We found that V1/V2-specific bnAbs PG9 and PG16 and their combinations elicit efficient clearance of infected cells, whereas CD4 binding site antibodies exhibit minimal contribution to these processes. In summary, antibody reactivity to the surface of primary HIV-1-infected CD4 T cells, but not neutralization breadth or potency, correlated with effector functions ADCML and ADCC. Thus, it is recommended that candidate antibodies to be administered as anti-HIV-1 therapies be assessed for antibody binding to HIV-1-infected cells so as to induce maximal elimination of the viral reservoir.
MATERIALS AND METHODS
Study participants and blood processing.
Healthy HIV-1-seronegative human volunteers were recruited for blood specimens. Informed written consent was obtained from each donor according to the guidelines set forth for conduct of ethical research approved by the University of Toronto and St. Michael's Hospital Toronto institutional ethics boards. PBMCs were isolated by Ficoll-Paque (GE Healthcare Bio-Sciences, Sweden) density gradient centrifugation. Cells were frozen in −150°C until further use. For plasma, blood tubes were centrifuged at 1,800 × g for 10 min to pellet cells and undiluted plasma was collected.
Virus production.
Eleven unique HIV-1 strains were used in the study; details are found in Table 1. HIV-1 BaL proviral plasmid was a gift from Alan Cochrane (Department of Molecular Genetics, University of Toronto, Toronto, Canada). All other viruses were obtained from the NIH AIDS Reagent Program (NIH, USA). HIV-1 NL4-3 and BaL were propagated from proviral plasmids transfected into HEK 293T cells (American Type Culture Collection [ATCC], USA) using the FuGENE HD reagent (Promega, USA). Supernatants were harvested 2 days following transfection. HIV-1 NL4-3 supernatant was filtered through a 0.45-μm syringe and frozen at −80°C. HIV-1 BaL supernatants were used to infect U87 CD4+ CCR5+ cells (NIH AIDS Reagent Program, USA) for another 2 days, and viral supernatants were harvested and purified using the Fast-Trap lentivirus purification system (Millipore, USA) as per the manufacturer's protocol and frozen at −80°C until further use. All other viruses were obtained as supernatants from the NIH AIDS Reagent Program and were expanded by infecting purified CD4 T cells from healthy donors. Briefly, CD4 T cells were enriched from PBMCs of healthy donors by negative selection (StemCell Technologies, Canada) and stimulated with 1 μg/ml each of anti-CD3 (clone OKT3) and anti-CD28 (clone 28.2) antibodies (BioLegend, USA) and 50 IU/ml of interleukin 2 (IL-2; Roche, USA) for 3 days prior to infection with each of the viruses. Virus supernatants were harvested 3 to 4 days later, filtered through a 0.45-μm filter, and frozen at −80°C.
Virus infections.
Total CD4 T cells were enriched from PBMCs of healthy HIV-1-negative donors; typical purity was >95%. CD4 T cells were stimulated as described above for 3 days at 4 × 106/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 IU/ml of penicillin, 100 μg/ml of streptomycin, and 2 mM l-glutamine (R-10 medium; all reagents from Wisent, Canada). Synchronous magnetic HIV-1 infections were performed as described previously (73–75) at a multiplicity of infection (MOI) of 0.2. Infections were monitored by intracellular staining of HIV-1 Gag protein using Kc57-fluorescein isothiocyanate (FITC) (Beckman Coulter, USA) and CD4-allophycocyanin (APC) (clone OKT4; BioLegend, USA) antibodies. Typically, 15 to 40% of the CD4 T cells were HIV-1 Gag+ and >50% viable by day four of infection. CD4 T cells within these ranges were used for all assays described here.
HIV-1 envelope-specific antibodies.
All antibodies used in the study were acquired from the NIH AIDS Reagent Program (NIH, USA) and were of the human IgG1 isotype. Antibody characteristics are described in Table 2. Antibodies were diluted to 50 μg/ml in phosphate-buffered saline (PBS; Wisent, Canada) and stored at −80°C until use. Unless otherwise specified, each antibody was tested at 2 μg/ml in respective assays for binding or killing of HIV-1-infected CD4 T cells. Normal human IgG (R&D Systems, USA) was used at equivalent concentrations in each assay as a negative control.
Antibody binding to HIV-1-infected CD4 T cells.
A total of 0.5 × 105 to 1 × 105 mock-infected or HIV-1-infected CD4 T cells were washed and plated into 96-well V-bottom plates in 100 μl of PBS containing 2% FBS. HIV-1 envelope-specific antibodies were added to corresponding wells at 2 μg/ml, and plates were moved to a 4°C fridge for 30 min. Cells were then washed and stained with 10 μg/ml of PE-labeled anti-human IgG Fc antibody (clone HP6017; BioLegend, USA) at 4°C for 30 min. Cells were then washed and permeabilized with BD Cytofix/Cytoperm solution (BD BioSciences, USA) and were eventually stained for intracellular HIV-1 Gag with a 1:100 dilution of Kc57-FITC antibody for 30 min at 4°C. Flow cytometry was performed on a BD FACSCalibur, and data were analyzed on FlowJo (FlowJo, LLC, USA). Antibody binding is reported as the percentage of secondary antibody (PE+) cells within the total infected, HIV-1 Gag+ (FITC+) populations using the following formula: percent antibody binding = [percent anti-human IgG (PE+)/percent total infected cells (FITC+ PE+)] × 100.
ADCML.
Undiluted plasma from blood of four healthy donors was pooled in equal parts. Mock-infected and HIV-1-infected CD4 T cells were washed and resuspended at 1 × 106 cells/ml in pooled plasma and plated in a 96-well U-bottom plate in 100 μl (1 × 105 cells). Antibodies were added at 2 μg/ml to the cells either individually or in combination. For combination experiments, the human IgG isotype control concentration was increased to 6 μg/ml to match the maximal envelope-specific antibody concentrations. Cultures were incubated overnight at 37°C in a 5% CO2 humidified environment. The following day (16 to 18 h later), cells were washed and permeabilized with BD Cytofix/Cytoperm solution and then stained with anti-human CD4-APC (clone OKT4) and Kc57-FITC for 30 min at 4°C. Data were acquired on a BD FACSCalibur flow cytometer, and analysis was performed on FlowJo software. ADCML is calculated as the percentage difference of infected cells (Gag+) in a well from the percentage of infected cells in the no-antibody wells for each respective virus infection using the following equation: percent ADCML = [(percent Gag+ cells in no-antibody wells − percent Gag+ cells in sample)/percent Gag+ cells in no-antibody wells)] × 100. Negative values were assigned as zeroes.
ADCC.
Natural killer (NK) cells were enriched from an HIV-negative healthy donor's PBMCs via negative selection (StemCell Technologies); purity was >95%. Separately, 5 × 104 mock-infected and HIV-1-infected CD4 T cells were washed and plated in 50 μl of R-10 medium. HIV-1 envelope-specific antibodies were added at 4 μg/ml each to corresponding wells, and plates were incubated at room temperature for 5 min. Then, 5 × 104 NK cells (1:1 ratio of CD4 to NK cells) were added to each well in 50 μl of R-10 medium; the final coculture volume was 100 μl, with 2 μg/ml of each antibody. Plates were spun at 400 × g for 2 min and incubated at 37°C in a 5% CO2 humidified incubator for 4 h. Following this period, cells were washed and permeabilized with BD Cytofix/Cytoperm and eventually stained with anti-human CD3-PE (clone HIT3a; BioLegend), anti-human CD4-APC (clone OKT4), LIVE/DEAD violet dye (Invitrogen, Canada), and Kc57-FITC (Beckman Coulter) for 30 min at 4°C. Flow cytometry data were collected on a BD LSRFortessa X-20, and data were analyzed on FlowJo software. ADCC is reported as the percent elimination of HIV-1-infected (Gag+) cells in a sample subtracted from the frequency of HIV-1 Gag+ cells in the no-antibody corresponding wells. Negative values were assigned as zeroes. ADCC was calculated using the following equation: percent ADCC = [(percent Gag+ cells in no-antibody wells − percent Gag+ cells in sample)/percent Gag+ cells in no-antibody wells] × 100.
Combined ADCC and ADCML assays.
Combined ADCC and ADCML assays were performed as the ADCML assays, i.e., overnight coculture in undiluted plasma, and also included NK cells in each well at a 1:1 ratio with 2 × 104 to 5 × 104 CD4 T cells plated per well in a 96-well U-bottom plate. Elimination of HIV-1-infected cells was calculated relative to the no-antibody wells, similar to the methodology employed for ADCML and ADCC assays. Negative values were assigned as zeroes.
Flow cytometry analysis.
All flow cytometry analysis was performed on FlowJo software (FlowJo, LLC, USA). Viable lymphocytes were gated with a forward-scatter (FSC) versus side-scatter (SSC) gate. Mock-infected controls were included in each experiment and were used as the background to set the placement of the gate for intracellular HIV-1 Gag-staining CD4 T cells with the Kc57-FITC-labeled antibody in HIV-1-infected samples. In the antibody binding experiments, the HIV-1-infected CD4 T cell plus human IgG isotype control antibody condition was used to determine the gates for positive signal from secondary antibody staining. For ADCML, ADCC, and ADCML-ADCC assays, we stained cells after fixation and permeabilization with anti-human CD4-APC-labeled antibody (clone OKT4) along with the HIV-1 Gag Kc57-FITC antibody. HIV-1 Gag+ cells were identified as described above with the aid of the mock-infected controls and within the infected-cell populations; we then set gates to distinguish between the two subpopulations expressing and deficient for CD4 expression. In the ADCC and ADCML-ADCC assays, we additionally stained cells with anti-human CD3-PE-labeled antibody (clone HIT3a) following fixation and permeabilization and gated on the CD3-expressing cells (CD4 T cells) first; then we identified the HIV-1-infected Gag+ cells as described above and set gates within the infected-cell population to identify the two subpopulations expressing and deficient for CD4 expression.
Statistical analyses and data presentation.
Two-tailed tests were conducted for all statistical analyses. Nonparametric Wilcoxon matched-pair signed-rank tests were performed throughout the study, comparing the human IgG (isotype) well to each paired sample. Mann-Whitney U tests were performed to compare results from single-antibody experiments to results from experiments conducted with various combinations of antibodies against HIV-1-infected CD4 T cells. Spearman correlations were conducted for regression analysis. Statistical significance was determined as a P value of <0.05. All statistical tests employed are stated in each figure legend. Statistical tests were performed on GraphPad Prism 7 software (GraphPad, USA). Radar graphs of antibody binding to infected CD4 T cells were generated in Microsoft Excel 2011.
ACKNOWLEDGMENTS
We thank Catia Perciani for assistance with illustrations used for generating figures as well as proofreading the manuscript. We also thank Robert Reinhard for informed discussions.
Biosafety level 3 laboratory space was provided by the Combined Containment Level 3 Unit at the University of Toronto.
Funding for this study was provided by Canadian Institutes of Health Research (CIHR) grant HIG-132040, Canadian HIV Cure Enterprise Team grant HIG-133050, and Ontario HIV Treatment Network (OHTN) AHRC grant G769. S.M. received the Queen Elizabeth Graduate Scholarship in Science and Technology (QEII-GSST) and the CIHR Doctoral awards and gratefully acknowledges their financial support.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun TW, Murray D, Justement JS, Blazkova J, Hallahan CW, Fankuchen O, Gittens K, Benko E, Kovacs C, Moir S, Fauci AS. 2014. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci U S A 111:13151–13156. doi: 10.1073/pnas.1414148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73:4009–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, Todd JP, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. 2014. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 6:243ra288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolis DM, Koup RA, Ferrari G. 2017. HIV antibodies for treatment of HIV infection. Immunol Rev 275:313–323. doi: 10.1111/imr.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O'Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE. 2015. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O'Brien C, Weiland D, Robles A, Kummerle T, Wyen C, Levin R, Witmer-Pack M, Eren K, Ignacio C, Kiss S, West AP Jr, Mouquet H, Zingman BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Klein F. 2017. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med 23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP Jr, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC. 2016. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O'Dell SJ, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, Chun TW. 2016. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, Oliveira T, Lorenzi JC, Parrish EH, Learn GH, West AP Jr, Bjorkman PJ, Schlesinger SJ, Seaman MS, Czartoski J, McElrath MJ, Pfeifer N, Hahn BH, Caskey M, Nussenzweig MC. 2016. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, Donau OK, Plishka RJ, Buckler-White A, Seaman MS, Lifson JD, Koup RA, Fauci AS, Nussenzweig MC, Martin MA. 2017. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Buning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freund NT, Wang H, Scharf L, Nogueira L, Horwitz JA, Bar-On Y, Golijanin J, Sievers SA, Sok D, Cai H, Cesar Lorenzi JC, Halper-Stromberg A, Toth I, Piechocka-Trocha A, Gristick HB, van Gils MJ, Sanders RW, Wang LX, Seaman MS, Burton DR, Gazumyan A, Walker BD, West AP Jr, Bjorkman PJ, Nussenzweig MC. 2017. Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller. Sci Transl Med 9:eaal2144. doi: 10.1126/scitranslmed.aal2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 157:2168–2173. [PubMed] [Google Scholar]
- 24.Caskey M, Klein F, Nussenzweig MC. 2016. Broadly neutralizing antibodies for HIV-1 prevention or immunotherapy. N Engl J Med 375:2019–2021. doi: 10.1056/NEJMp1613362. [DOI] [PubMed] [Google Scholar]
- 25.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon H, Macke J, West AP Jr, Foley B, Bjorkman PJ, Korber B, Yusim K. 2015. CATNAP: a tool to compile, analyze and tally neutralizing antibody panels. Nucleic Acids Res 43:W213–W219. doi: 10.1093/nar/gkv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diskin R, Scheid JF, Marcovecchio PM, West AP Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calarese DA, Lee HK, Huang CY, Best MD, Astronomo RD, Stanfield RL, Katinger H, Burton DR, Wong CH, Wilson IA. 2005. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci U S A 102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol 76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Xu X, Bishop A, Jones IM. 2005. Reintroduction of the 2G12 epitope in an HIV-1 clade C gp120. AIDS 19:833–835. doi: 10.1097/01.aids.0000168980.74713.9e. [DOI] [PubMed] [Google Scholar]
- 32.Pancera M, Wyatt R. 2005. Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage. Virology 332:145–156. doi: 10.1016/j.virol.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Doores KJ, Burton DR. 2010. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol 84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Q, Yu R, Qin X. 2010. The good and evil of complement activation in HIV-1 infection. Cell Mol Immunol 7:334–340. doi: 10.1038/cmi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol 78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levesque K, Finzi A, Binette J, Cohen EA. 2004. Role of CD4 receptor down-regulation during HIV-1 infection. Curr HIV Res 2:51–59. doi: 10.2174/1570162043485086. [DOI] [PubMed] [Google Scholar]
- 37.Wildum S, Schindler M, Munch J, Kirchhoff F. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol 80:8047–8059. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen BK, Gandhi RT, Baltimore D. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol 70:6044–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bournazos S, Ravetch JV. 2017. Anti-retroviral antibody FcgammaR-mediated effector functions. Immunol Rev 275:285–295. doi: 10.1111/imr.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham TN, Lukhele S, Dallaire F, Perron G, Cohen EA. 2016. Enhancing virion tethering by BST2 sensitizes productively and latently HIV-infected T cells to ADCC mediated by broadly neutralizing antibodies. Sci Rep 6:37225. doi: 10.1038/srep37225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raska M, Czernekova L, Moldoveanu Z, Zachova K, Elliott MC, Novak Z, Hall S, Hoelscher M, Maboko L, Brown R, Smith PD, Mestecky J, Novak J. 2014. Differential glycosylation of envelope gp120 is associated with differential recognition of HIV-1 by virus-specific antibodies and cell infection. AIDS Res Ther 11:23. doi: 10.1186/1742-6405-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noel N, Lambotte O, Mouquet H, Schwartz O. 2016. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, Fouts TR, Gallo RC, DeVico AL, Lewis GK. 2013. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 110:E69–E78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doores KJ. 2015. The HIV glycan shield as a target for broadly neutralizing antibodies. FEBS J 282:4679–4691. doi: 10.1111/febs.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pritchard LK, Harvey DJ, Bonomelli C, Crispin M, Doores KJ. 2015. Cell- and protein-directed glycosylation of native cleaved HIV-1 envelope. J Virol 89:8932–8944. doi: 10.1128/JVI.01190-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Y, Guttman M, Williams JA, Verkerke H, Alvarado D, Hu SL, Lee KK. 2016. Changes in structure and antigenicity of HIV-1 Env trimers resulting from removal of a conserved CD4 binding site-proximal glycan. J Virol 90:9224–9236. doi: 10.1128/JVI.01116-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linde ME, Colquhoun DR, Ubaida Mohien C, Kole T, Aquino V, Cotter R, Edwards N, Hildreth JE, Graham DR. 2013. The conserved set of host proteins incorporated into HIV-1 virions suggests a common egress pathway in multiple cell types. J Proteome Res 12:2045–2054. doi: 10.1021/pr300918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iordanskiy S, Santos S, Bukrinsky M. 2013. Nature, nurture and HIV: the effect of producer cell on viral physiology. Virology 443:208–213. doi: 10.1016/j.virol.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC. 2016. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol 80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 53.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Gunthard HF. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 54.Armbruster C, Stiegler GM, Vcelar BA, Jager W, Koller U, Jilch R, Ammann CG, Pruenster M, Stoiber H, Katinger HW. 2004. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother 54:915–920. doi: 10.1093/jac/dkh428. [DOI] [PubMed] [Google Scholar]
- 55.Mehandru S, Vcelar B, Wrin T, Stiegler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, Carrington M, Petropoulos C, Katinger H, Markowitz M. 2007. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol 81:11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landi A, Iannucci V, Nuffel AV, Meuwissen P, Verhasselt B. 2011. One protein to rule them all: modulation of cell surface receptors and molecules by HIV Nef. Curr HIV Res 9:496–504. doi: 10.2174/157016211798842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haller C, Muller B, Fritz JV, Lamas-Murua M, Stolp B, Pujol F, Keppler OT, Fackler OT. 2014. HIV-1 Nef and Vpu are functionally redundant broad-spectrum modulators of cell surface receptors, including tetraspanins. J Virol 88:14241–14257. doi: 10.1128/JVI.02333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pham TN, Lukhele S, Hajjar F, Routy JP, Cohen EA. 2014. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 11:15. doi: 10.1186/1742-4690-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Bredow B, Arias JF, Heyer LN, Moldt B, Le K, Robinson JE, Zolla-Pazner S, Burton DR, Evans DT. 2016. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol 90:6127–6139. doi: 10.1128/JVI.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan W, Bazick J, Sodroski J. 2006. Characterization of the multiple conformational states of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker. J Virol 80:6725–6737. doi: 10.1128/JVI.00118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alsahafi N, Ding S, Richard J, Markle T, Brassard N, Walker B, Lewis GK, Kaufmann DE, Brockman MA, Finzi A. 30 December 2015. Nef proteins from HIV-1 elite controllers are inefficient at preventing ADCC. J Virol doi: 10.1128/JVI.02973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richard J, Prevost J, von Bredow B, Ding S, Brassard N, Medjahed H, Coutu M, Melillo B, Bibollet-Ruche F, Hahn BH, Kaufmann DE, Smith AB III, Sodroski J, Sauter D, Kirchhoff F, Gee K, Neil SJ, Evans DT, Finzi A. 22 March 2017. BST-2 expression modulates small CD4 mimetic sensitization of HIV-1-infected cells to ADCC. J Virol doi: 10.1128/JVI.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saifuddin M, Hedayati T, Atkinson JP, Holguin MH, Parker CJ, Spear GT. 1997. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J Gen Virol 78(Part 8):1907–1911. [DOI] [PubMed] [Google Scholar]
- 65.Saifuddin M, Parker CJ, Peeples ME, Gorny MK, Zolla-Pazner S, Ghassemi M, Rooney IA, Atkinson JP, Spear GT. 1995. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med 182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmitz J, Zimmer JP, Kluxen B, Aries S, Bogel M, Gigli I, Schmitz H. 1995. Antibody-dependent complement-mediated cytotoxicity in sera from patients with HIV-1 infection is controlled by CD55 and CD59. J Clin Invest 96:1520–1526. doi: 10.1172/JCI118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang K, Lan J, Shepherd N, Hu N, Xing Y, Byrd D, Amet T, Jewell C, Gupta S, Kounga C, Gao J, Yu Q. 2015. Blockage of CD59 function restores activities of neutralizing and nonneutralizing antibodies in triggering antibody-dependent complement-mediated lysis of HIV-1 virions and provirus-activated latently infected cells. J Virol 89:9393–9406. doi: 10.1128/JVI.01614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lan J, Yang K, Byrd D, Hu N, Amet T, Shepherd N, Desai M, Gao J, Gupta S, Sun Y, Yu Q. 2014. Provirus activation plus CD59 blockage triggers antibody-dependent complement-mediated lysis of latently HIV-1-infected cells. J Immunol 193:3577–3589. doi: 10.4049/jimmunol.1303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunn BM, Alter G. 2016. Modulating antibody functionality in infectious disease and vaccination. Trends Mol Med 22:969–982. doi: 10.1016/j.molmed.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD, Todd JP, Penzak SR, Saunders KO, Nason MC, Haase AT, Rao SS, Blumberg RS, Mascola JR, Nabel GJ. 2014. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed AA, Keremane SR, Vielmetter J, Bjorkman PJ. 2016. Structural characterization of GASDALIE Fc bound to the activating Fc receptor FcgammaRIIIa. J Struct Biol 194:78–89. doi: 10.1016/j.jsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scully E, Alter G. 2016. NK cells in HIV disease. Curr HIV/AIDS Rep 13:85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones RB, Garrison KE, Mujib S, Mihajlovic V, Aidarus N, Hunter DV, Martin E, John VM, Zhan W, Faruk NF, Gyenes G, Sheppard NC, Priumboom-Brees IM, Goodwin DA, Chen L, Rieger M, Muscat-King S, Loudon PT, Stanley C, Holditch SJ, Wong JC, Clayton K, Duan E, Song H, Xu Y, Sengupta D, Tandon R, Sacha JB, Brockman MA, Benko E, Kovacs C, Nixon DF, Ostrowski MA. 2012. HERV-K-specific T cells eliminate diverse HIV-1/2 and SIV primary isolates. J Clin Invest 122:4473–4489. doi: 10.1172/JCI64560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski M. 2012. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J Immunol 188:3745–3756. [DOI] [PubMed] [Google Scholar]
- 75.Sacha JB, Watkins DI. 2010. Synchronous infection of SIV and HIV in vitro for virology, immunology and vaccine-related studies. Nat Protoc 5:239–246. doi: 10.1038/nprot.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]