ABSTRACT
Research on vaccine approaches that can provide long-term protection against dengue virus infection is needed. Here we describe the construction, immunogenicity, and preliminary information on the protective capacity of recombinant, replication-competent rhesus monkey rhadinovirus (RRV), a persisting herpesvirus. One RRV construct expressed nonstructural protein 5 (NS5), while a second recombinant expressed a soluble variant of the E protein (E85) of dengue virus 2 (DENV2). Four rhesus macaques received a single vaccination with a mixture of both recombinant RRVs and were subsequently challenged 19 weeks later with 1 × 105 PFU of DENV2. During the vaccine phase, plasma of all vaccinated monkeys showed neutralizing activity against DENV2. Cellular immune responses against NS5 were also elicited, as evidenced by major histocompatibility complex class I (MHC-I) tetramer staining in the one vaccinated monkey that was Mamu-A*01 positive. Unlike two of two unvaccinated controls, two of the four vaccinated monkeys showed no detectable viral RNA sequences in plasma after challenge. One of these two monkeys also showed no anamnestic increases in antibody levels following challenge and thus appeared to be protected against the acquisition of DENV2 following high-dose challenge. Continued study will be needed to evaluate the performance of herpesviral and other persisting vectors for achieving long-term protection against dengue virus infection.
IMPORTANCE Continuing studies of vaccine approaches against dengue virus (DENV) infection are warranted, particularly ones that may provide long-term immunity against all four serotypes. Here we investigated whether recombinant rhesus monkey rhadinovirus (RRV) could be used as a vaccine against DENV2 infection in rhesus monkeys. Upon vaccination, all animals generated antibodies capable of neutralizing DENV2. Two of four vaccinated monkeys showed no detectable viral RNA after subsequent high-dose DENV2 challenge at 19 weeks postvaccination. Furthermore, one of these vaccinated monkeys appeared to be protected against the acquisition of DENV2 infection on the basis of undetectable viral loads and the lack of an anamnestic antibody response. These findings underscore the potential utility of recombinant herpesviruses as vaccine vectors.
KEYWORDS: DENV vaccine, herpesviral vector, live-vector vaccines, recombinant vaccine
INTRODUCTION
The mosquito-borne dengue virus (DENV) exists as four different subtypes, denoted DENV1 to DENV4. Together, all four subtypes account for approximately 390 million infections in over 120 countries annually. Globally, DENV has expanded drastically within the last 5 decades. Four billion people are now estimated to be at risk each year due to increased international travel, increases in urban populations, and insufficient mosquito control (1–3).
The majority of primary DENV infections occur without the emergence of symptoms or with very mild symptoms. However, it is estimated that more than 2 million DENV infections annually result in severe clinical outcomes, such as dengue hemorrhagic fever and dengue shock syndrome, which may be fatal (1, 4). One potential risk factor for severe DENV infection is antibody-dependent enhancement (ADE) due to preexisting immunity against heterotypic DENV serotypes. During ADE, cross-reactive yet nonneutralizing antibodies against previously encountered DENV serotypes bind to virus particles and deliver them to cells of the myeloid lineage, thereby enhancing infection (5–8). Infants are also prone to ADE from the passive transfer of maternal antibodies against DENV (9, 10). Due to an increasing fraction of the world's population being at risk of contracting DENV, the potential severity of infection, and the lack of dengue-specific antiviral drugs, the development of optimal DENV vaccines is an important goal.
Currently, the only licensed DENV vaccine is Dengvaxia (chimeric yellow fever-tetravalent dengue vaccine [CYD-TDV]) from Sanofi Pasteur. CYD-TDV is a tetravalent live attenuated vaccine made by replacing the premembrane (prM) and envelope (E) genes of the yellow fever live attenuated vaccine strain with the prM and E genes of DENV serotypes 1 to 4. The vaccine has been approved in only a few countries (11, 12). The efficacy of CYD-TDV is heavily dependent on the serotype, with the poorest protection against DENV2 (13). In addition, longer-term assessments have raised concerns because the 3-year postvaccination risk for hospitalization upon DENV infection was elevated in CYD-TDV recipients under the age of 9 years compared to the placebo group (14).
The Laboratory of Infectious Disease at the National Institute of Allergy and Infectious Disease (NIAID) is pursuing a different approach toward a live attenuated replicating DENV vaccine. Their approach utilizes a mixture of attenuated strains of all four DENV serotypes. The DENV1, DENV3, and DENV4 strains used in this vaccine harbor deletions in the 3′ untranslated region, and the attenuated strain of DENV4 was employed as a backbone for the remaining dengue virus serotype 2 (15). One formulation (TV003) elicited full protection against DENV infection in small-scale testing using a human challenge model (16). Results of human clinical trials have yet to be reported.
Several strategies for the development of nonreplicating viral vaccines focus on the DENV E protein, which is the main target for neutralizing antibodies. DENV E consists of a membrane-spanning region and an N-terminal ectodomain. The ectodomain is divided into three domains, namely, envelope domain I (EDI) to EDIII (17, 18). All three EDs play an important role in the evocation and specificity of humoral responses (19, 20). Adjuvanted recombinant EDIII constructs have been shown to generate anti-DENV responses in nonhuman primates (21). Additionally, a Venezuelan equine encephalitis virus replicon vector-based vaccine containing a C-terminally truncated soluble form of the DENV E protein (E85) elicited serotype-specific antibodies capable of neutralizing DENV in rhesus macaques (22).
In this report, we describe the use of a replication-competent, persisting herpesvirus expressing DENV2 E85 and nonstructural protein 5 (NS5) in rhesus monkeys as an experimental vaccine against DENV2.
RESULTS
Expression of DENV2 proteins in recombinant RRV-infected rhesus fibroblasts.
Replication-competent RRVs encoding either a codon-optimized soluble variant of the E protein (E85) or NS5 of DENV2 were generated (Fig. 1). Rhesus fibroblasts (RFs) permissive for lytic RRV replication were infected with recombinant RRVs, and cell lysates and cell culture supernatants were prepared and analyzed for the presence of the desired DENV2 gene products by immunoblotting. DENV2 E85 was readily detected in the RF cell lysate as well as in the cell culture supernatant (Fig. 2A). Similarly, the expression of DENV2 NS5 was detected in the cell lysate of RRV-NS5-infected RF cells (Fig. 2B).
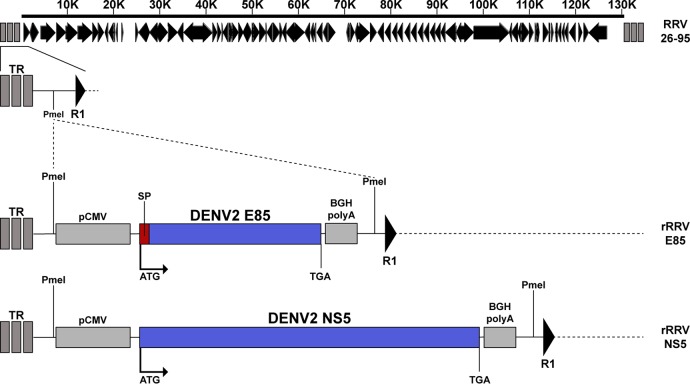
FIG 1.
Positions of DENV2 E85 and NS5 transgene cassettes in the recombinant RRV constructs. The expression cassette for codon-optimized DENV2 E85, preceded by the PrM-derived signal peptide (SP), or codon-optimized NS5 was inserted between the left terminal repeats (TR) and the first open reading frame R1 of RRV. The expression cassette consists of the cytomegalovirus promoter (pCMV), the transgene, and the bovine growth hormone (BGH) poly(A) signal.
FIG 2.
Expression of the desired DENV2 proteins in rhesus fibroblast cultures after infection with RRV-DENV2 recombinants. RF cell lysates were prepared, and cell culture supernatants were harvested. Proteins were separated by SDS-polyacrylamide gel electrophoresis, and the separated proteins were transferred onto a PVDF membrane and subsequently tested with DENV2-specific antibodies. (A) Recombinant RRV-E85 (rRRV-E85) represents either the cell lysate or the cell culture supernatant from RF cells infected with RRV-E85. Blots were probed with an antibody specific for the E protein of DENV2 (4G2). (B) Recombinant RRV-NS5 represents cell lysate from RF cells infected with RRV-NS5. Blots were reacted with an antibody specific for NS5 of DENV2. kDa, atomic mass (in thousands).
Recombinant RRV vaccination phase.
Recombinant RRV was evaluated as a vaccine against DENV2 infection in RRV-negative rhesus macaques (Table 1). A mixture of two recombinant RRVs, one expressing E85 and the other expressing NS5, was given to four rhesus macaques intravenously, whereas two additional monkeys served as unvaccinated controls. Two antigens were included in the vaccine that each test monkey received in order to enhance the chances of seeing protective effects.
TABLE 1.
Characteristics of research animals
| Monkey | Inoculum | Sexa | Age (yr)b | Mamu-A*01 statusc | RRV status PVc,d |
|---|---|---|---|---|---|
| r09084 | rRRV-E85/NS5 | F | 5.33 | − | + |
| r10019 | rRRV-E85/NS5 | F | 4.75 | − | + |
| r12078 | rRRV-E85/NS5 | F | 2.17 | + | + |
| rhBE94 | rRRV-E85/NS5 | M | 5.83 | − | + |
| rh2313 | None | M | 10.75 | + | − |
| r08037 | None | M | 6.42 | − | − |
F, female; M, male.
Age at day of vaccination.
+, positive; −, negative.
PV, postvaccination. All monkeys were negative for RRV on the day of vaccination.
To determine the ability of recombinant RRV expressing codon-optimized E85 to elicit antibodies capable of neutralizing DENV2, a flow cytometry-based neutralization assay was performed. Dilutions of plasma samples from the vaccinated animals at two distinct time points were used for analyses. At both time points, plasma of all vaccinated animals showed neutralizing activity against DENV2. Overall, the NEUT50 titers (the plasma dilution at which 50% of the virus was neutralized) ranged from 1:17 to 1:337, 54 days after recombinant RRV administration (Fig. 3). Additional testing at 89 and 129 days post-recombinant RRV administration yielded similar results, with NEUT50 titers of between 1:75 and 1:200 and between 1:52 and 1:153, respectively (Fig. 3 and see Table 3).
FIG 3.

DENV2 plasma neutralization titers prior to challenge. DENV2 neutralization titers in EDTA-treated plasma from four vaccinated research animals were analyzed by using a flow cytometry-based neutralization assay on day 0, day 54, and day 89 after inoculation with the RRV-DENV2 recombinants.
TABLE 3.
DENV2 NEUT50 titers pre- and post-DENV2 challenge
| Group | Monkey | NEUT50 titer on day relative to day of challengea |
|
|---|---|---|---|
| −4 | 37 | ||
| Vaccinated | r09084 | 1:52 | 1:2,591 |
| r10019 | 1:63 | 1:2,682 | |
| r12078 | 1:153 | 1:192 | |
| rhBE94 | 1:112 | 1:3,094 | |
| Control | rh2313 | 1:<5 | 1:311 |
| r08037 | 1:<5 | 1:192 | |
The NEUT50 titer is defined as the highest dilution of plasma that reduced DENV2 infectivity by 50%.
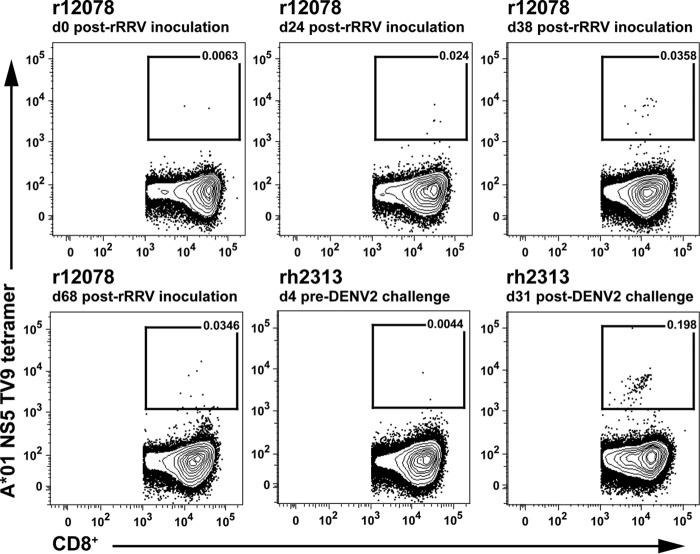
The emergence of DENV2-specific cellular responses upon vaccination was also examined. TV9 MHC-I tetramer staining was used to evaluate the frequency of postvaccination DENV2 NS5-specific CD8+ T cell responses in Mamu-A*01-positive animal r12078. The measurements performed revealed an expansion of DENV2 NS5-specific cellular responses over time, with relatively steady levels 38 to 68 days after immunization (Fig. 4). At day 68 after recombinant RRV administration, 0.0346% of the CD8+ T cells showed NS5-specific staining. In comparison, 0.198% of the CD8+ T cells in Mamu-A*01-positive control animal rh2313 showed positive NS5-specific tetramer staining, 31 days after infection with DENV2 (Fig. 4).
FIG 4.
Frequency of Mamu-A*01 NS5 TV9 tetramer binding T cells. PBMCs were obtained from vaccinated animal r12078 and from DENV2-naive control animal rh2313 at the indicated time points. The frequencies of NS5 TV9 tetramer binding cells in CD3+ CD8+ lymphocytes are shown.
DENV2 challenge phase.
Nineteen weeks into the study, the four immunized and two control animals were challenged subcutaneously with 1 × 105 PFU of a DENV2 challenge stock.
In order to evaluate the protective ability of our vaccine regimen against DENV2 infection, plasma samples from all the monkeys were analyzed for the presence of DENV2 by using real-time quantitative reverse transcriptase PCR. Two of the four vaccinated animals, namely, animals r09084 and r12078, showed no sign of viral RNA in plasma over 10 days of postchallenge measurements, whereas DENV2 was readily detectable in all of the remaining animals, including both control animals (Table 2). Control group macaque r08037 had the highest peak viral load, with 5.95 × 106 DENV2 copies/ml at day 10 postchallenge. One vaccinated animal (r10019) showed overall lower viral loads than those in the other infected monkeys and with a peak viral load of 3.36 × 103 DENV2 copies/ml at day 10.
TABLE 2.
Viral loads after DENV2 challenge
| Group | Monkey | DENV2 load (RNA copies/ml) on postchallenge daya: |
|||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 10 | ||
| Vaccinated | r09084 | <20 | <20 | <20 | <20 | <20 | <20 |
| r10019 | <400 | <400 | <400 | 1,120 | <400 | 3,360 | |
| r12078 | <20 | <20 | <20 | <20 | <20 | ND | |
| rhBE94 | 15,900 | 17,700 | 33,300 | 88,400 | 18,200 | <20 | |
| Control | rh2313 | 1,560 | 760 | 730 | 3,230 | 14,300 | 28,600 |
| r08037 | 520 | 610 | <400 | <400 | 4,410 | 5,950,000 | |
Viral loads were quantified via quantitative real-time PCR. Monkeys were challenged 19 weeks after inoculation of recombinant RRV. <20 indicates that the viral load was below the limit of detection of 20 DENV2 RNA copies/ml plasma; <400 indicates that the monkeys tested positive but that the viral load was below the limit of quantitation; ND, not done.
We also evaluated neutralizing antibody titers pre- versus post-DENV2 challenge (Table 3 and Fig. 5). Three of the four vaccinated monkeys showed a marked, anamnestic response in anti-DENV2 neutralization titers following challenge. NEUT50 titers in these animals went from the range of 1:52 to 1:112 prior to challenge to 1:2,591 to 1:3,094 by day 37 postchallenge (Table 3). These titers were much higher than those that were observed in the two control monkeys at this day 37 time point (NEUT50 titers of 1:192 and 1:311). The one vaccinated monkey without a significant increase in neutralizing titers following challenge (r12078) was also one of the monkeys without measurable DENV2 RNA in plasma following challenge. Interestingly, this monkey also had the highest postvaccination neutralizing antibody response (Fig. 3 and Table 3).
FIG 5.
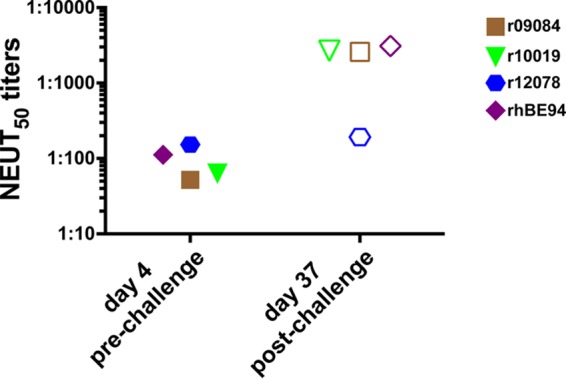
Comparison of DENV2 plasma neutralization titers pre- and post-DENV2 challenge. DENV2 neutralization titers of plasma of the four vaccinated animals were analyzed by using a flow cytometry neutralization assay 4 days prior to and 37 days after DENV2 challenge.
DISCUSSION
Vaccination with persistent, replication-competent, recombinant RRV expressing a soluble variant of the DENV2 E protein (E85) generated neutralizing antibodies against DENV2 in all four vaccinated monkeys (Fig. 3). The NEUT50 titers in these animals were comparable to the titers observed in control monkeys after primary DENV2 infection (Table 3). Overall, the observed NEUT50 titers were higher than those observed in a previous study using a live attenuated virus in rhesus macaques (23). Although showing some variability, the NEUT50 titers in our study remained rather steady until the day of challenge 19 weeks after the single time of vaccine administration. In contrast, a study utilizing an alpha vector-based vaccine containing E85 showed a decline of NEUT50 titers over time after the second immunization (22).
The protective effects of recombinant RRV against high-dose DENV2 challenge were moderate. Two of the four vaccinated monkeys (animals r12078 and r09084) had no detectable viral RNA in plasma during the challenge phase (Table 2); one of these two monkeys exhibited no significant boost in neutralizing titers against DENV2 following challenge, indicating the absence of an anamnestic response (Table 3 and Fig. 5). The lack of an anamnestic response in animal r12078 further supports the claim that it was protected against DENV2 acquisition. Noteworthy, all aviremic monkeys enrolled in a study utilizing CYD-TDV showed anamnestic responses post-DENV challenge (24). Although viral RNA was not detected in the plasma of animal r09084 following challenge, the presence of an anamnestic antibody response indicates that this animal indeed became infected by DENV2 but that the level of replication was below the level of detection with our viral RNA load assay.
Animal r12078 had the highest neutralizing titers of the four vaccinated monkeys, and it was the one monkey apparently protected fully against acquisition. These results suggest an approximate neutralization titer that can protect against the acquisition of infection with the DENV2 challenge dose that was used. Although clinical parameters were not monitored in this experiment, the very low viral loads in two of the remaining vaccinated monkeys would likely translate into protective effects against clinical manifestations of disease.
Our data show that the recombinant RRV regimen was able to induce a specific T cell response against NS5. However, the frequency of these T cells was about 1 order of magnitude lower than the frequencies observed in an unvaccinated rhesus monkey infected by DENV2 (Fig. 4). Although NS5 is among the main targets for CD8+ T cells in naturally occurring DENV infections, it may be worth considering additional nonstructural genes as a part of the vaccine regimen. In particular, NS3 has been shown to be the most immunodominant target of cellular responses (25, 26).
Unfortunately, no plasma samples were collected beyond day 10 in the study described here. Plasma sample collection dates and the duration of collection were chosen based on a thorough meta-analysis of available data on DENV infections in nonhuman primates (27). However, the overall kinetics of viremia in all infected animals differed from the expected outcome. The reasons for this are not clear.
Given that this is the first attempt at using a recombinant herpesvirus vector to protect against DENV in an animal model, we are encouraged by these early findings. A variety of variations in vaccine design can be envisioned to increase the potency and breadth of immune responses in future experiments.
MATERIALS AND METHODS
Cell culture.
Rhesus fibroblast (RF) cells were cultured and maintained in Dulbecco's modified Eagle medium (DMEM; Life Technologies) supplemented with 20% fetal bovine serum (FBS; Life Technologies) and Primocin (InvivoGen). Vero cells (ATCC CCL-81) were cultured and maintained in 199 medium with Earle's balanced salt solution (EBSS; GE Healthcare Life Sciences) supplemented with 5% FBS (Life Technologies) and a 1% penicillin-streptomycin-amphotericin B solution (Millipore).
Generation of dengue virus 2 E85 and NS5 polynucleotides.
A pUC plasmid containing the sequence for a codon-optimized soluble variant of the DENV2 New Guinea C strain (DENV2_NGC) (GenBank accession number AF038403.1) E protein (E85) was made to order (GenScript). E85 is made up of 85% of a C-terminally truncated soluble form of the DENV2 E protein. It contains the E protein signal peptide (SP) derived from the last 18 residues of the premembrane protein (PrM), E domains I to III, the first alpha helix (H1), and part of the conserved sequence (CS). It lacks the second alpha helix (H2) and both membrane-spanning regions. Similarly, a pUC plasmid containing the sequence for codon-optimized NS5 of DENV2 was made (GenScript).
Generation of recombinant rhesus monkey rhadinovirus.
Recombinant RRVs expressing either codon-optimized E85 or codon-optimized NS5 were made by overlapping cosmid transfection as previously described (28). Recombinant RRV-infected RF culture supernatants were harvested and spun down twice at 2,000 × g for 5 min to remove any cell debris, and resulting virus titers in the culture supernatants of infected rhesus fibroblast cells were measured via quantitative real-time PCR using an RRV latency-associated nuclear antigen (LANA)-specific primer set. Quantification was performed by using the TaqMan Fast Virus 1-Step master mix (Life Technologies) in a real-time PCR thermocycler (Applied Biosystems 7500 fast and 7500 real-time PCR system) with forward primer ACCGCCTGTTGCGTGTTA, reverse primer CAATCGCCAACGCCTCAA, and the reporter FAM (6-carboxyfluorescein)-CAGGCCCCATCCCC.
Preparation of dengue virus 2 challenge virus.
Vero cells were seeded at a density of 1 × 106 cells in a T-175 flask. The following day, the cells were incubated with DENV2_NGC (GenBank accession number AF038403.1) at a multiplicity of infection (MOI) of 0.01 at 37°C for 1 h. Afterwards, the cells were kept in serum-free HyClone 199 medium (GE Healthcare Life Sciences) supplemented with 1% l-glutamine (Life Technologies) and a 1% penicillin-streptomycin-amphotericin B solution (Millipore). The supernatant was harvested at 6 days postinoculation, spun down at 800 × g, aliquoted, and subsequently analyzed with a plaque assay.
Research animals.
Six rhesus macaques of Indian origin (Macaca mulatta) were utilized for this study, all of which were housed at the Wisconsin National Primate Research Center (WNPRC). Animals were cared for in accordance with the guidelines of the Weatherall Report (30) under a protocol approved by the University of Wisconsin Graduate School Animal Care and Use Committee. All animals were under anesthesia during recombinant RRV vaccinations and DENV2 challenge.
Recombinant RRV vaccination.
Recombinant RRV stocks expressing codon-optimized DENV2 E85 or NS5 were diluted to a total of 1 × 109 genome copies/ml in 1× phosphate-buffered saline (PBS). One milliliter of each dilution was administered to four rhesus macaques intravenously. Two animals served as unvaccinated controls.
DENV2 challenge.
The DENV2 challenge stock was diluted to a total of 1 × 105 PFU/ml in PBS, 1 ml of which was administered to all six rhesus macaques subcutaneously.
In vitro expression of DENV2 proteins.
A total of 250,000 RF cells were seeded into each well of a six-well plate. The following day, cells were infected with 50 μl of a stock of recombinant RRV (1 × 109 genome copies/ml) expressing codon-optimized DENV2 E85 or NS5. Cells were kept in culture 6 days before harvesting of the cells or cell culture supernatants when cells exhibited advanced cytopathic effect.
Antibodies and reagents.
The following antibodies were used in this study: mouse anti-DENV2-E (4G2; Millipore), rabbit anti-DENV2-NS5 (Thermo Fisher Scientific), a goat anti-mouse–horseradish peroxidase (HRP) conjugate (SouthernBiotech), a goat anti-rabbit-HRP conjugate (SouthernBiotech), a rat anti-mouse-IgG2a-allophycocyanin (APC) conjugate (BioLegend), a mouse anti-CD3-peridinin chlorophyll protein (PerCP)-Cy5.5-conjugate (BD Bioscience), a mouse anti-CD8-Brilliant Violet 421 (BV421) conjugate (BioLegend), a mouse anti-CD14-Brilliant Violet 510 (BV510) conjugate (BioLegend), a mouse anti-CD16-BV510 conjugate (BioLegend), a mouse anti-CD20-BV510 conjugate (BioLegend), and a mouse anti-granzyme B-phycoerythrin (PE) conjugate (Life Technologies). In addition, the Live/Dead Fixable Aqua Dead Cell Stain kit (Life Technologies) was used in this study. The MHC-I A*01/NS5 TV9 tetramer conjugated to APC was produced at the NIH Tetramer Core Facility (task order 20448).
Immunoblotting.
Cells were harvested, resuspended, and lysed with an NP-40-based lysis buffer containing a protease inhibitor (Roche). After centrifugation, supernatants were transferred into new tubes, and their protein levels were measured and normalized by using a bicinchoninic acid protein assay kit (Pierce). Each lysate was mixed with an equal volume of 5× nonreducing sample buffer (Thermo Fisher Scientific) or 2× sodium dodecyl sulfate (SDS) Laemmli sample buffer (Sigma-Aldrich). Additionally, cell culture supernatants were harvested and mixed with an equal volume of 5× nonreducing sample buffer. Subsequently, samples were incubated at 97°C for 5 min and separated by SDS-polyacrylamide gel electrophoresis (PAGE) under either reducing or nonreducing conditions. The proteins from the gels were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) and subjected to immunoblotting. Briefly, the membranes were blocked with 1× PBS containing 5% skim milk and incubated with the appropriate primary antibody overnight. Three wash steps with PBS containing Tween were followed by incubation with the appropriate HRP-conjugated secondary antibodies. After a final wash, specific signals were detected with a LAS4000 Minisystem (GE Healthcare Systems) by using the SuperSignal West Pico chemiluminescent substrate (Pierce).
Flow cytometry neutralization assay.
Plasma samples from each research animal were serially diluted, and 110 μl of each dilution was added to a 1.5-ml Eppendorf tube together with 110 μl of a 1:5 dilution of the DENV2 challenge stock (2 × 104 PFU/ml). The samples were placed into a cell culture incubator at 37°C for 1 h. After incubation, 100 μl of each sample was added to a 100% confluent Vero cell layer in 24-well plates. The plates were incubated at 37°C for 1 h and gently agitated every 15 min. Afterwards, the wells were aspirated, and the cells were washed with 500 μl of cell culture medium. Finally, 400 μl of fresh cell culture medium was added to each well, and the cells were incubated at 37°C for 24 h. Subsequently, cell culture medium was aspirated, and the cells were detached via trypsinization and distributed into 96-well cluster tubes. The cells were then centrifuged at 530 × g for 10 min, and the medium was decanted. The cells contained in approximately 100 μl residual liquid were vortexed and fixated with 250 μl of a 2% paraformaldehyde solution at 4°C for 15 min. Afterwards, cells were washed twice with 500 μl of a saponin-based permeabilization buffer. Following aspiration of permeabilization buffer and subsequent vortexing, 100 μl of a 1:1,000 dilution of a mouse anti-DENV2 envelope antibody (4G2) was added to the cluster tubes, and the tubes were incubated at room temperature for 1 h. After an additional wash step, 100 μl of a 1:200 dilution of an anti-mouse IgG2a-APC-conjugated antibody was added each tube, which was then incubated in the dark at room temperature for 1 h. Finally, cells were washed, centrifuged, vortexed, and analyzed by flow cytometry. The NEUT50 titer is defined as the highest dilution of plasma that reduced binding of the dengue virus-infected Vero cells by 50% and was calculated by using a sigmoidal model and nonlinear regression analysis (GraphPad Prism 7).
Staining of MHC-I tetramer-positive CD8+ T cells.
Peripheral blood mononuclear cells (PMBCs) of the research animals were isolated by using Ficoll density gradient centrifugation. Up to 8 × 105 cells in a volume of 100 μl per flow cytometry tube were incubated with 25 μl of a master mix containing the fluorochrome-conjugated MHC-I A*01/NS5 TV9 tetramer at 37°C for 45 min in the dark. Subsequently, 50 μl of a master mix containing fluorochrome-labeled monoclonal antibodies (MAbs) against the surface molecule CD8 was added to each tube. In order to create a “dump gate,” cells were also stained with anti-CD14, anti-CD16, and anti-CD20 antibodies labeled with appropriate fluorochromes as well as an amine reactive dye. The samples were then incubated at room temperature for 20 min in the dark. Afterwards, cells were washed with a PBS-based wash buffer containing 0.1% bovine serum albumin (BSA) and 0.45 g/liter NaN3 and pelleted at 530 × g, the supernatant was decanted, and cells contained in the residual liquid were vortexed and fixated with 250 μl of a 2% paraformaldehyde solution at 4°C for 20 min in the dark. Subsequently, cells were treated with 500 μl of a saponin-based permeabilization buffer, vortexed, and incubated at room temperature for 10 min in the dark. After an additional wash step, 50 μl of a master mix containing fluorochrome-labeled MAbs against CD3 and granzyme B was added to each tube, and the tubes were incubated at room temperature for 30 min in the dark. Finally, cells were washed, pelleted, vortexed, and analyzed by flow cytometry.
DENV2 load measurement.
RNA was extracted from frozen, EDTA-anticoagulated plasma by using the QIAamp circulating nucleic acid kit according to the manufacturer's protocols (Qiagen). Extractions were performed by using 1 ml of the sample and eluted in 80 μl (2 × 40 μl) for maximum nucleic acid recovery. The isolated viral RNA was reverse transcribed in triplicate reaction mixtures containing 20 μl of the eluted RNA each and amplified by using TaqMan Fast Virus 1-Step master mix (Life Technologies) in a real-time PCR thermocycler (Applied Biosystems 7500 fast and 7500 real-time PCR system). The reverse transcription reaction was performed at 50°C for 30 min. An activation temperature of 95°C for 10 min was followed by 40 amplification cycles of 95°C for 15 s and 60°C for 1 min. A double-quenched (ZEN/Iowa Black FQ) FAM dye qPCR probe was used (500 nM primers and 250 nM probe; IDT Technologies). The probe and primer sequences were modified to match the DENV2_NGC sequence (GenBank accession number AF038403.1). The oligonucleotide sequences are as follows: forward primer CAGGCTATGGCACTGTCACGAT, reverse primer CCATTTGCAGCAACACCATCTC, and the reporter FAM-CTCTCCGAGAACGGGCCTCGACTTCAA (29). Quality control was performed at several levels, and every RNA extraction batch contained a positive plasma sample and an uninfected plasma sample. Quantitation took into account the extraction and elution volumes. Standard curves were generated in every run, using 3-fold serial dilutions of commercially available total DENV2 RNA standards (Amplirun; Vircell).
ACKNOWLEDGMENTS
This work was supported by funding from the Wallace H. Coulter Center for Translational Research at the University of Miami (to D.I.W.) and by National Institutes of Health grant AI063928 (to R.C.D.).
We thank Wendy Newton, Nancy Schultz-Darken, and Kimberly Weisgrau at the Wisconsin National Primate Research Center for facilitating the care, handling, and sampling of the monkeys and the processing of the blood samples and Teresa Giret for the MHC typing of the animals. We also thank Shara N. Pantry for critical reading of the manuscript.
G.F.B., D.M.M., M.R., D.I.W., and R.C.D. planned the experiments; G.F.B., D.M.M., M.J.R, A.D., V.K.B., L.G.-N., and E.G.R. conducted the experiments; G.F.B., D.M.M., M.R., Y.C.S., D.I.W., and R.C.D. analyzed the data; and G.F.B. and R.C.D. wrote the manuscript. We declare no conflict of interest.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. 2012. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler DJ. 2006. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 277:3–16; discussion 16–22, 71–73, 251–253. [DOI] [PubMed] [Google Scholar]
- 4.Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 5.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flipse J, Wilschut J, Smit JM. 2013. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic 14:25–35. doi: 10.1111/tra.12012. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB, O'Rourke EJ. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 9.Halstead SB. 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467. doi: 10.1016/S0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 10.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 11.Chambers TJ, Nestorowicz A, Mason PW, Rice CM. 1999. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 73:3095–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanofi Pasteur. 2016. First dengue vaccine approved in more than 10 countries. Sanofi Pasteur, Lyon, France: http://www.sanofipasteur.com/en/articles/first_dengue_vaccine_approved_in_more_than_10_countries.aspx. [Google Scholar]
- 13.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 14.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortes M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M, CYD-TDV Dengue Vaccine Working Group. 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead SS. 2016. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev Vaccines 15:509–517. doi: 10.1586/14760584.2016.1115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP. 2016. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 8:330ra36. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 17.Modis Y, Ogata S, Clements D, Harrison SC. 2005. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 79:1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roehrig JT. 2003. Antigenic structure of flavivirus proteins. Adv Virus Res 59:141–175. doi: 10.1016/S0065-3527(03)59005-4. [DOI] [PubMed] [Google Scholar]
- 19.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, de Silva AM Jr. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messer WB, de Alwis R, Yount BL, Royal SR, Huynh JP, Smith SA, Crowe JE Jr, Doranz BJ, Kahle KM, Pfaff JM, White LJ, Sariol CA, de Silva AM, Baric RS. 2014. Dengue virus envelope protein domain I/II hinge determines long-lived serotype-specific dengue immunity. Proc Natl Acad Sci U S A 111:1939–1944. doi: 10.1073/pnas.1317350111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Hermida L, Bernardo L, Martin J, Alvarez M, Prado I, Lopez C, Sierra BDLC, Martinez R, Rodriguez R, Zulueta A, Perez AB, Lazo L, Rosario D, Guillen G, Guzman MG. 2006. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 24:3165–3171. doi: 10.1016/j.vaccine.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 22.White LJ, Sariol CA, Mattocks MD, Wahala WMPB, Yingsiwaphat V, Collier ML, Whitley J, Mikkelsen R, Rodriguez IV, Martinez MI, de Silva A, Johnston RE. 2013. An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J Virol 87:3409–3424. doi: 10.1128/JVI.02298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaney JE Jr, Matro JM, Murphy BR, Whitehead SS. 2005. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol 79:5516–5528. doi: 10.1128/JVI.79.9.5516-5528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirakhoo F, Pugachev K, Zhang Z, Myers G, Levenbook I, Draper K, Lang J, Ocran S, Mitchell F, Parsons M, Brown N, Brandler S, Fournier C, Barrere B, Rizvi F, Travassos A, Nichols R, Trent D, Monath T. 2004. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J Virol 78:4761–4775. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appanna R, Huat TL, See LL, Tan PL, Vadivelu J, Devi S. 2007. Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in Malaysia. Clin Vaccine Immunol 14:969–977. doi: 10.1128/CVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons CP, Dong T, Chau NV, Dung NT, Chau TN, Thao LTT, Dung NT, Hien TT, Rowland-Jones S, Farrar J. 2005. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J Virol 79:5665–5675. doi: 10.1128/JVI.79.9.5665-5675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Althouse BM, Durbin AP, Hanley KA, Halstead SB, Weaver SC, Cummings DA. 2014. Viral kinetics of primary dengue virus infection in non-human primates: a systematic review and individual pooled analysis. Virology 452–453:237–246. doi: 10.1016/j.virol.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilello JP, Morgan JS, Damania B, Lang SM, Desrosiers RC. 2006. A genetic system for rhesus monkey rhadinovirus: use of recombinant virus to quantitate antibody-mediated neutralization. J Virol 80:1549–1562. doi: 10.1128/JVI.80.3.1549-1562.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson BW, Russell BJ, Lanciotti RS. 2005. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol 43:4977–4983. doi: 10.1128/JCM.43.10.4977-4983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weatherall D. December 2006. The use of non-human primates in research. Academy of Medical Sciences, London, United Kingdom: https://royalsociety.org/~/media/Royal_Society_Content/policy/publications/2006/Weatherall-Report.pdf. [Google Scholar]