ABSTRACT
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). HTLV-1 cell-to-cell transmission is dependent on the release of infectious virus particles into the virological synapse. The HTLV-1 particle structure is still poorly understood, and previous studies analyzed viruses produced by transformed lymphocytic cell lines chronically infected with HTLV-1, particularly the MT-2 cell line, which harbors truncated proviruses and expresses aberrant forms of the Gag protein. In this study, we demonstrate that the chronically infected SP cell line harbors a relatively low number of proviruses, making it a more promising experimental system for the study of the HTLV-1 particle structure. We first identified the genomic sites of integration and characterized the genetic structure of the gag region in each provirus. We also determined that despite encoding a truncated Gag protein, only the full-length Gag protein was incorporated into virus particles. Cryo-transmission electron microscopy analyses of the purified virus particles revealed three classes of particles based upon capsid core morphology: complete cores, incomplete cores, and particles without distinct electron densities that would correlate with the capsid region of a core structure. Observed cores were generally polygonal, and virus particles were on average 115 nm in diameter. These data corroborate particle morphologies previously observed for MT-2 cells and provide evidence that the known poor infectivity of HTLV-1 particles may correlate with HTLV-1 particle populations containing few virus particles possessing a complete capsid core structure.
IMPORTANCE Studies of retroviral particle core morphology have demonstrated a correlation between capsid core stability and the relative infectivity of the virus. In this study, we used cryo-transmission electron microscopy to demonstrate that HTLV-1 particles produced from a distinct chronically infected cell line are polymorphic in nature, with many particles lacking organized electron densities that would correlate with a complete core structure. These findings have important implications for infectious HTLV-1 spread, particularly in the context of cell-to-cell transmission, a critical step in HTLV-1 transmission and pathogenesis.
KEYWORDS: Gag, core morphology, cryoelectron microscopy, human retrovirus, infectivity, retrovirus assembly
INTRODUCTION
Human T-cell leukemia virus type 1 (HTLV-1) was the first-described human retrovirus and is estimated to have emerged in the human population via multiple events of zoonotic transmission from monkeys to humans (1–3). Some of these transmission events are estimated to have occurred as early as the Paleolithic Stone Age (4, 5). Despite low levels of infectivity and transmissibility, there are likely 10 million to 20 million carriers worldwide, and the virus is endemic in Japan, the Pacific Islands, the Caribbean, Central Africa, and Brazil (6).
HTLV-1 is the etiological agent of adult T-cell leukemia (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (1, 3, 7–9). While cell-to-cell transmission is generally thought to be the main mode of HTLV-1 spread, it was previously established that cell-free transmission can contribute to HTLV-1 infection as well (10, 11). As a retrovirus, HTLV-1 particle assembly is driven by Gag, the structural polyprotein precursor (12–14). Retroviral particle morphogenesis involves an immature stage, in which the Gag polyprotein remains uncleaved, and a mature stage, in which Gag is cleaved by the viral protease after assembly and budding (15, 16). During particle morphogenesis, the immature Gag lattice undergoes a morphological switch that results in mature capsid (CA) core formation, which contains the genomic RNA as well as viral and host proteins needed for virus replication. It was previously established for other retroviruses that the structure and stability of the CA core contribute to virus infectivity (17).
HTLV-1 has been notorious for being difficult to study in cell culture, with deletion-prone molecular clones that possess low levels of protein expression and particle release from both transfected and primary infected cells (18–21). These factors have prohibited a rigorous analysis of how HTLV-1 replicates in cells, including particle assembly and structure. Given these restraints, most HTLV-1 structural studies have relied on the use of transformed lymphocytic cell lines that are chronically infected with HTLV-1, particularly the MT-2 cell line (22). Cryo-electron tomography (cryo-ET) was used to observe the general morphology of authentic HTLV-1 particles produced by MT-2 cells, and it was found that both the particle and core structures are highly polymorphic, exhibiting a generally polygonal structure with evidence of curved, membrane-following facets (23). Importantly, many HTLV-1 particles were found to have incomplete cores, and many others had electron-dense interiors with no visible core structure. These observations suggested that the majority of HTLV-1 particles produced from chronically HTLV-1-infected cells have either an incomplete core or no core structure. However, it is known that MT-2 cells contain eight integrated proviruses, with four of them containing a truncated gag gene, and Northern blot analysis confirmed that irregularly structured mRNAs are expressed (24). Thus, particles with aberrant cores from MT-2 cells could be a result of the incorporation of a truncated Gag protein (25).
In order to further investigate the nature of mature HTLV-1 particles, a panel of T-cell lines chronically infected with HTLV-1 was analyzed for proviral content. In particular, we sought to determine the genomic structure of proviruses within these cells and evaluate the particle morphology of released particles. From these analyses, we identified the SP cell line as a candidate for further investigation of the HTLV-1 particle structure, as it was found to contain a minimal proviral copy number and to contain proviruses with gag sequences having intact CA-encoding regions. Morphological analyses of particles produced from the SP cell line confirmed the variability in HTLV-1 particle structures observed with particles from MT-2 cells, i.e., particles harboring complete cores, incomplete cores, and particles with no organized electron densities indicative of a CA-enclosed core structure. Taken together, these findings indicate that the polymorphic nature of the mature HTLV-1 particle morphology may help explain the low infectivity of cell-free HTLV-1 particles.
RESULTS
Proviral integration sites in chronically HTLV-1-infected cell lines.
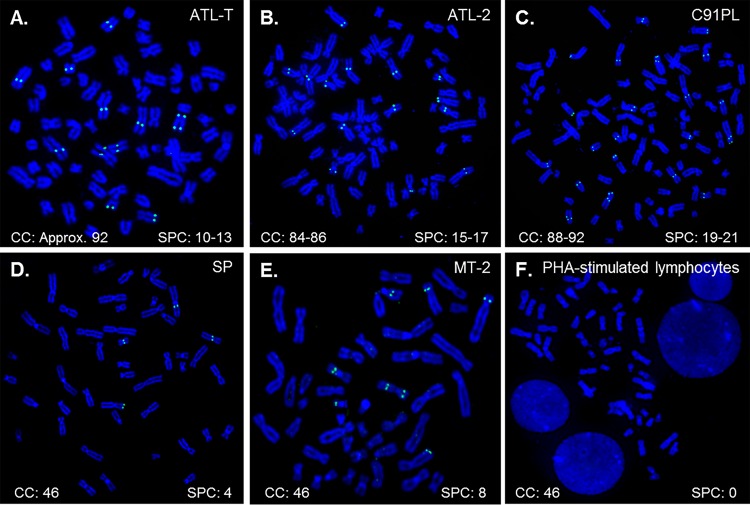
Previous studies of the HTLV-1 particle structure were performed on viruses produced from the MT-2 cell line (23). Eight HTLV-1 proviruses were previously identified in MT-2 cells, and several of these proviruses harbor deletions in the gag gene (24, 26). Previous studies identified a 3.4-kb RNA transcript from the defective proviruses that encodes a myristylated truncated Gag protein that is composed of matrix (MA), a truncated CA protein, a short pX region, and two long terminal repeats (LTRs) (27). This 3.4-kb RNA transcript and the truncated Gag proteins were subsequently found to be packaged into virus particles produced from MT-2 cells (25). Since the MT-2 cell line harbors eight proviruses, a number of which could produce aberrant Gag proteins (24), we wanted to study the structure of HTLV-1 produced by another chronically infected cell line, ideally one in which truncated Gag products were not incorporated into the viral particles. A panel of four chronically HTLV-1-infected cell lines (ATL-T, ATL-2, C91PL, and SP) was probed by fluorescence in situ hybridization (FISH) for HTLV-1 proviral content by using the previously described ACH molecular clone (18). MT-2 cells were used as a positive control for proviral copy numbers. Phytohemagglutinin (PHA)-stimulated lymphocytes were used as a negative control to evaluate off-target binding of the probe to genomic sequences. We discovered a broad range of proviral copy numbers between and even within the different cell lines (Fig. 1). The SP cell line harbored the lowest number of HTLV-1 proviruses, with four consistent signals, whereas the C91PL cell line contained as many as 21 signals. Some of the cell lines (ATL-T, ATL-2, and C91PL) exhibited aneuploidy as well, leading to various proviral counts per cell. Given this, the SP cell line represented the most promising chronically infected cell line for analysis of HTLV-1 particle formation from an authentic provirus.
FIG 1.
Localization of proviral integration sites in chronically HTLV-1-infected cell lines. (A to D) The proviral HTLV-1 copy numbers in the ATL-T (A), ATL-2 (B), C91PL (C), and SP (D) cell lines were determined by using a fluorescently labeled probe derived from the ACH molecular clone by fluorescence in situ hybridization as described in Materials and Methods. (E) The MT-2 cell line was used as a positive control. (F) Uninfected lymphocytes were used as a negative control. The chromosome count (CC) and signals per cell (SPC) are noted for each cell line, quantified from at least 10 cells for each cell line. Chromosome staining was done by using DAPI.
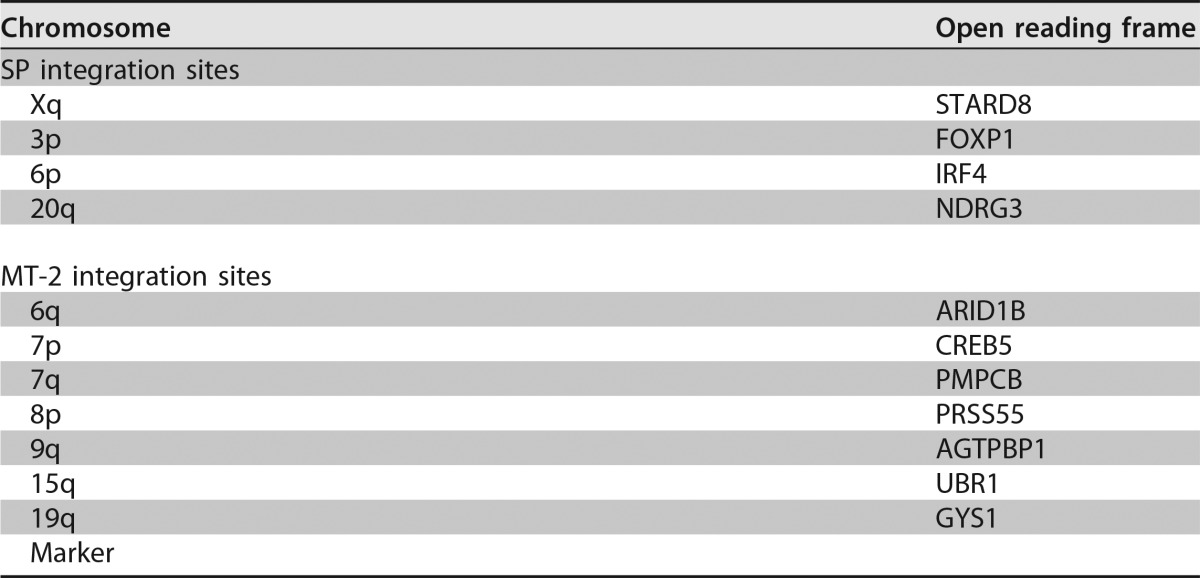
4′,6-Diamidino-2-phenylindole (DAPI) stain inversion was used to determine the approximate chromosomal sites of integration for the proviruses in the MT-2 and SP cell lines (Table 1). The SP cell line had proviruses integrated in the short arms (p) of chromosomes (Chrs) 3 and 6 and in the long arms (q) of Chrs 20 and X. Additionally, we determined that seven of the proviruses harbored within the MT-2 cell lines are located on the short arms of Chrs 7 and 8 and the long arms of Chrs 6, 7, 9, 15, and 19. The integration site of the eighth provirus within MT-2 cells was unable to be reliably determined by using FISH analysis and DAPI stain inversion. The irregularity in the cytogenetic banding of the Chr of integration for this provirus is likely due to translocations as a result of transformation; this Chr is denoted “marker” in Table 1.
TABLE 1.
Genomic integration sites of SP and MT-2 cell proviruses
Next, linker-mediated PCR was performed, followed by Illumina sequencing, in order to determine the genetic sites of integration of the SP and MT-2 proviruses. Sequences were grouped based on sequence similarity and aligned to the human and HTLV-1 genomes by using the NCBI Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Four integration sites for the SP cell line were identified, which correlated with the results of FISH analysis (Fig. 1). These sites were located in the FOXP1 (forkhead box P1) open reading frame in Chr 3p, IRF4 (interferon regulatory factor 4) in Chr 6p, NDRG3 (N-Myc downstream-regulated gene 3) in Chr 20q, and STARD8 (StAR-related lipid transfer domain-containing 8) in Chr Xq. Additionally, seven sites of integration were identified within MT-2 cells that were consistent with the locations identified by FISH (Table 1). Additional sequences with homology to regions on Chr 5 and Chr 2 were found, either of which may correspond to the eighth provirus determined to be integrated within the marker Chr seen with FISH. These results define the genomic sites of integration for HTLV-1 proviruses within SP and MT-2 lymphocytes and indicate the promise of the SP cell line as a useful model system for the study of HTLV-1 particle assembly.
HTLV-1 Gag gene and protein sequences in the SP cell line.
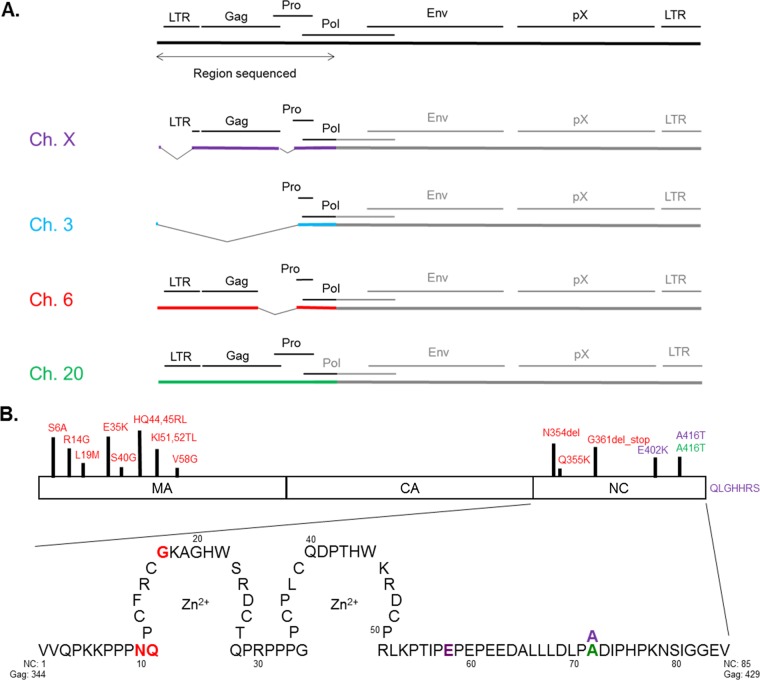
The general proviral structure and nucleotide sequence of the gag genes in the SP cell proviruses were determined via Sanger sequencing (Fig. 2A). Amplification was done with primers designed within the surrounding genomic DNA and in two different locations within the proviral pro and pol genes to account for genetic diversity that may exist. Sequencing was completed with primers spanning the entire 5′ end of the genome (within the 5′ LTR and across Gag) to ensure overlapping reads for validation of results. The HTLV-1 provirus located at Chr 3p was found to have a deletion of the entire 5′ end of the genome, resulting in no gag sequence. Two other proviruses (at Chrs 20q and Xq) were found to harbor a complete, intact gag sequence, and a fourth provirus (at Chr 6p) was predicted to harbor a truncated gag sequence.
FIG 2.
Sequence analysis of the gag gene regions of integrated HTLV-1 proviruses identified in the SP cell line. (A) The 5′ end of each HTLV-1 provirus harbored within the SP cell line was sequenced by using Sanger sequencing. The region of the genome analyzed by DNA sequencing (encompassing the 5′ long terminal repeat, gag, pro, and the 5′ end of the pol gene) is indicated below the full-length genome. Chromosomes X, 3, 6, and 20 are labeled Ch. X, Ch. 3, Ch. 6, and Ch. 20, respectively. Each proviral sequence shown is color-coded as indicated. (B) Amino acid variation in Gag proteins among HTLV-1 proviruses in SP cells. Sequencing results were used to infer the effects on the primary structure of the encoded Gag protein in the 4 HTLV-1 proviruses. The matrix (MA), capsid (CA), and nucleocapsid (NC) domains of Gag are identified. A detailed view of the NC amino acid sequence is shown at the bottom, with the zinc finger domains indicated as the regions circling each Zn2+ atom and with amino acid variation color-coded to identify the proviral sequence from which they were observed. The amino acids in NC and Gag are indicated alongside the NC amino acid sequence shown.
Sequencing results were used to predict the amino acid sequences of the Gag proteins expressed by the three proviruses harboring gag and to identify any amino acid changes (Fig. 2B). Sequences were compared against the HTLV-1 reference sequence (RefSeq accession number NC_001436.1) and a codon-optimized gag sequence (28). The provirus harboring a truncated gag sequence on Chr 6 was predicted to have the largest amount of diversity of the three proteins, with significant variation in amino acid sequence, particularly in the MA domain of the protein. The Chr 6 provirus contains a deletion of the C-terminal end of the gag gene within the provirus, resulting in a predicted truncation of the nucleocapsid (NC) portion of the protein. A missing stop codon at the end of NC in the provirus on Chr X is predicted to result in a slightly elongated Gag protein. Only the provirus encoded on Chr 20 is predicted to encode a full-length protein similar to that of the wild type. Nonetheless, proviruses on Chrs 6, 20, and X were found to encode full-length, intact CA domains of the Gag protein, which are responsible for oligomerization and capsid formation, suggesting that SP cells remain a useful tool for analysis of the HTLV-1 capsid core structure.
HTLV-1 particle release in SP cells.
We next sought to determine HTLV-1 gene expression, particle release, and morphology in SP cells. MT-2 cells were used as a point of comparison, as HTLV-1 has been best characterized in this cell line. Additionally, MT-2 cells were used as the source of viral particles in the only previous investigation of general HTLV-1 morphology using cryo-transmission electron microscopy (TEM) and cryo-ET thus far (23). Viral release was quantified by determining HTLV-1 MA protein levels in the viral supernatant. MT-2 and SP cells were grown in culture for 14 days, and an equal volume of the cell-free supernatant was used in an HTLV-1 MA enzyme-linked immunosorbent assay (ELISA). HTLV-1 release by MT-2 cells was found to be 13-fold higher than that in SP cells (Fig. 3A). Cell proliferation over the course of the experiment was found to be only 3-fold higher for MT-2 than for SP cells after 10 days in culture, indicating that the difference in antigen production is more likely the result of lower-level virus particle production from SP cells than from MT-2 cells. We next compared the HTLV-1 Gag expression profiles in lysates and purified viruses by immunoblot analysis, using the day 0 and day 10 time points. We detected a band consistent with truncated NC (t-NC) in the SP lysates. This same band appears to be absent in the purified virus or is at least below the antibody detection limit, suggesting that the truncated protein is excluded from incorporation into the budding particle (Fig. 3B). These results confirm that the SP cell line produces virus particles with full-length Gag molecules and represents a suitable model system to study authentic HTLV-1 particle assembly and structure.
FIG 3.
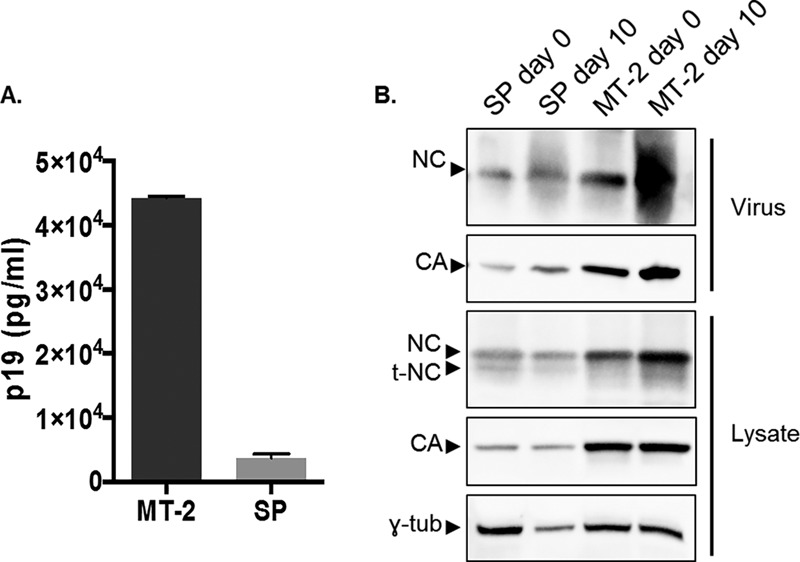
Analysis of HTLV-1 Gag expression in cells and packaged into virus particles. (A) ELISA. HTLV-1 MA (p19) production from MT-2 and SP cells was determined by using a p19-based ELISA as described in Materials and Methods. (B) Immunoblot analysis. HTLV-1 proteins from cell lysates and released into cell culture supernatants of MT-2 and SP cells were analyzed by immunoblot analysis at day 0 or day 10 posttransfection (with a 1:2 split at day 6) as described in Materials and Methods. γ-tub, gamma tubulin. Shown are representative data from 3 independent experiments.
Size and morphology of HTLV-1 produced by SP cells.
Authentic HTLV-1 particles were collected from the supernatant of cells grown for 14 days in culture. Cell debris was removed by low-speed centrifugation and double filtration. Viruses were purified and concentrated by a 3-step ultracentrifugation protocol. Briefly, viruses were concentrated on an Optiprep cushion and inactivated with 2,2′-dipyridyl disulfide (AT-2) (29). Inactivated viruses were purified by ultracentrifugation on a 10 to 30% Optiprep gradient, and the viral band was collected, pelleted, and resuspended for analysis by cryo-TEM.
As expected based on results from the ELISAs and immunoblot analyses, HTLV-1 purification from SP cell culture supernatants was challenging given the relatively low level of virus particle release from these cells. Besides the lower level of virus particle release, the cell culture supernatants contained large amounts of contaminating vesicles, even after double filtration. This may be a consequence of the interleukin-2 (IL-2) supplementation required to grow and maintain this cell line, as it was previously reported that lymphocytic cell lines grown in the presence of IL-2 produce high levels of vesicles (30). Standard gradient purification (23) failed to remove these contaminating vesicles. To solve this problem, we used a CD45 depletion protocol (31) between the AT-2 inactivation and gradient ultracentrifugation steps. Briefly, the concentrated and inactivated viral supernatant was incubated with ferromagnetic beads decorated with anti-CD45 antibodies. Contaminating vesicles produced by lymphocytic cells under IL-2 complementation are CD45+, while retroviruses are CD45−, as CD45 is excluded from retroviral budding sites (32, 33). After incubation, the supernatant was passed through a magnetic column, where the beads were retained along with the CD45+ vesicles, while the viruses flowed through. The CD45-depleted viral supernatant was then purified by gradient ultracentrifugation and pelleting, as described previously (23). The purified virus was loaded onto an electron microscopy (EM) grid and imaged by cryo-TEM.
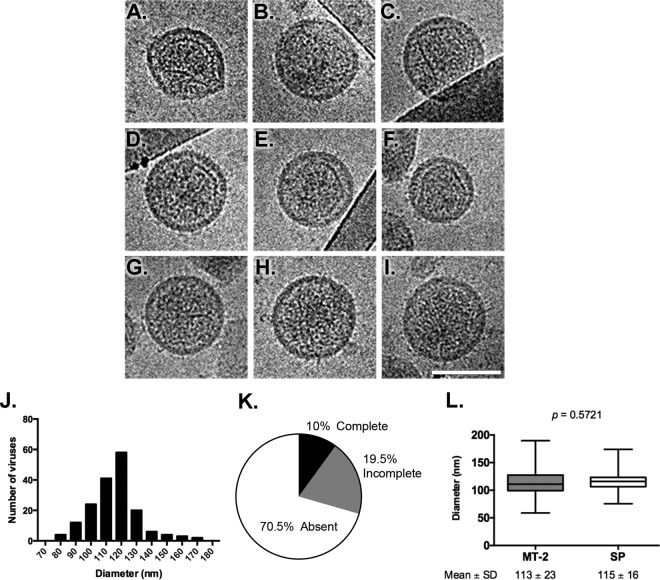
HTLV-1 particles produced from SP cells had an average diameter of 115 ± 16 nm and a broad size distribution, ranging from 76 to 175 nm (Fig. 4J). Particles presented loosely polygonal viral cores, with straight facets and angular vertices (Fig. 4A to F) but also curved lattices that follow the viral membrane curvature (Fig. 4A and F). In one instance, a tubular core was seen (Fig. 4B). Three basic core morphologies are seen: complete cores (Fig. 4A to C), incomplete cores (Fig. 4D to F), and particles with internal density but no organized core structure (Fig. 4G to I), which were seen in 10%, 19.5%, and 70.5% of the particles, respectively (Fig. 4K). Interestingly, HTLV-1 particles produced from SP cells recapitulated the results described previously for HTLV-1 particles produced from MT-2 cells (Fig. 4L) (23), indicating that polymorphic cores are characteristic of HTLV-1 produced from chronically infected cell lines and not an artifact of the MT-2 cell line.
FIG 4.
Size and morphology of HTLV-1 particles produced by SP cells. (A to I) Analysis of HTLV-1 particles by using cryo-transmission electron microscopy. SP cells were grown for 14 days, and HTLV-1 particles were purified and concentrated from the cell culture supernatants. Representative examples of the types of particles observed are shown. (J) Analysis of the size distribution of virus particles from three independent experiments. Virus particle diameters were determined by the averaging of two perpendicular measurements. (K) Distribution of HTLV-1 core morphology from SP cells. The proportions of HTLV-1 particles having complete, incomplete, or a lack of electron density indicative of a CA core structure are depicted in a pie chart. (L) Comparisons of sizes of HTLV-1 particles produced from either MT-2 or SP cells. The number of virus particles analyzed for MT-2 cells was 1,074 (mean diameter, 113 ± 23 nm), and the number analyzed for SP cells was 174 (mean diameter, 115 ± 16 nm). No significant differences between virus particles produced from MT-2 cells and those produced from SP cells were observed (P = 0.5721). The MT-2 virus particle diameters were previously determined (23).
DISCUSSION
Recent technological advances in microscopy and computation have allowed many advances in the detailed study of retroviral morphology (34–37). Nonetheless, the majority of studies have utilized human immunodeficiency virus type 1 (HIV-1) as a model (35, 36, 38–40). However, although retroviruses have structurally conserved Gag proteins and similar replication strategies, there is great morphological variability among different immature and mature retrovirus particles (including core morphology). HIV-1, considered the prototypical lentivirus, has conical cores (41), whereas Rous sarcoma virus (RSV), an alpharetrovirus, and murine leukemia virus (MuLV), a gammaretrovirus, have polygonal cores (42, 43). Recently, the structure of prototype foamy virus was described by using cryo-TEM and was revealed to have a polygonal mature core surrounded by an intermediate and organized layer of electron density (44). The intractability of HTLV-1 molecular clones and low levels of virus production from primary infected cells have thus far prevented a thorough analysis of mature HTLV-1 particles, which may provide insights into structure-function relationships. Particles produced by chronically infected cell lines, particularly the MT-2 cell line, represent a means to bypass these limitations. By using this system, it was demonstrated previously that HTLV-1 has a loosely polygonal core, with flat facets and angular vertices similar to those of RSV and MuLV but also presenting curved surfaces and an unexpected prevalence of particles with incomplete cores (23). However, as MT-2 cells are known to harbor proviruses predicted to encode abnormal proteins, it was necessary to validate these results with HTLV-1 particles derived from another source in which abnormal Gag proteins were not incorporated into budding particles.
The SP cell line was derived from an activated primary peripheral blood lymphocyte (PBL) culture isolated from a 46-year-old female ATL patient (45). Although initially described as containing a single integrated HTLV-1 provirus, our study shows that the SP cell line actually harbors four proviruses. This discrepancy may be explained by the more sophisticated techniques used in the present study as well as by the accumulation of proviruses within the genome during culture. The hypothesis that proviruses accumulate within the genome during culture is supported by the observation that the C91PL cell line was shown previously to harbor a much lower copy number of proviral genes than seen here (46) and by other studies of the MT-2 cell line, which reported as many as 12 proviruses within the cell genome (26). The agreement between data from our FISH and sequencing analyses regarding the number and location of HTLV-1 proviruses for both SP and MT-2 cells supports the conclusion that the SP cell line analyzed in this study harbors four integrated proviruses.
It is important to note that there is evidence to suggest that passaging of cells may result in an alteration of proviral content. A recent study explored three different independently passaged MT-2 cell lines and found that all three exhibited different proviral contents, ranging from 6 to 12 proviruses (26). Additionally, general sites of integration within chromosomes were identified for the different cell lines, at least five of which agree with the data presented here. It is likely that a core subset of proviruses is characteristic of all MT-2 cell lines and that additional proviruses may be unique to different passages and lots, further supporting the hypothesis that HTLV-1 proviruses accumulate within the genomes of infected lymphocytes during cell culture.
Of the four genes in which proviruses were identified within SP cells, two (NDRG3 and IRF4) were previously associated with ATL and HTLV-1 infection. One study found that the expression of NDRG3 was upregulated by HTLV-1 p30 (47). Likewise, IRF4 was found to be constitutively expressed in ATL as opposed to nonlymphocytic HTLV-1 infection (48). A number of studies have linked activating mutations and high levels of expression of IRF4 with HTLV-1 infection, with IRF4 expression resulting in reduced expression levels of genes previously shown to be dysregulated in ATL cases (48–53). As proviral integration was found in the 5′ ends of both of these genes, it is possible that integration may alter gene expression and thus may have contributed to transformation or ATL.
Our genomic analyses predicted that the HTLV-1 provirus integrated on Chr 6 of SP cells encodes a truncated Gag protein, which would lead to a shorter NC protein after maturation. Immunoblot analysis confirmed that this truncated NC protein is being expressed in SP cells; however, it seems that this truncated form of Gag is not incorporated into the budding virus particle, as the shorter NC protein cannot be seen in the viruses purified from the SP cell-free supernatant (Fig. 3B). It is possible that the incorporation of Gag into the assembling virus particle is dependent on the viral RNA-Gag interaction that happens through the NC region in Gag, which may be disrupted in the truncated protein. The provirus on Chr 6 presented a high level of sequence diversity in NC: a deletion of an asparagine residue at position 354 of Gag (corresponding to position 10 of NC), a glutamine-to-lysine mutation at position 355 of Gag (position 11 of NC), and a deletion starting at position 361 of Gag (position 17 of NC). These mutations result in the loss of the majority (81%) of the NC protein and, specifically, the loss of both zinc fingers required for viral RNA binding (Fig. 2B) (54). It is thought that the viral RNA acts as a scaffolding element during retrovirus particle assembly (55–57), and so the loss of a specific viral RNA interaction might exclude a Gag protein with a truncated nucleocapsid domain from assembling into particles. Additionally, the high level of sequence diversity observed in the MA region of this provirus may lead to the loss of motifs that are essential for virus assembly and the incorporation of Gag into viral particles (58). This further highlights the utility of studying proviral genomic and particle structures in different chronically infected cell lines, as new insights into protein dynamics may be elucidated.
HTLV-1 particles produced by SP cells have the same size (Fig. 4L) and general morphology as those of viruses produced by MT-2 cells. This corroborates previously reported findings and supports the conclusion that HTLV-1 forms particles with polymorphic cores during replication, indicating that this is not an artifact of the system with which they were initially characterized. An intact core and optimal uncoating kinetics are necessary to protect the viral RNA and host the reverse transcription events after entry into a new target cell (59, 60). Thus, it is reasonable to assume that a particle with an incomplete or absent core could exhibit reduced infectivity. HTLV-1 is notorious for its low rates of infectivity among retroviruses, and the results here help provide an explanation for this observation. There is no evidence in the literature that the morphology of cell-free particles is distinct from that of cell-associated particles involved in cell-to-cell transmission. Therefore, we believe that the particle distribution observed in the present study would also be observed in the context of cell-to-cell transmission.
HTLV-1 particles produced from MT-2 cells included particles (∼10%) that had a turret structure composed of stacked layers of density present in about 10% of the observed virus particles by using three-dimensional (3D) maps derived from cryo-ET (23). Particles with associated turret structures were also observed for viruses produced by SP cells in cryo-TEM two-dimensional (2D) pictures but were observed relatively infrequently, possibly due to the differences between imaging methods.
Our understanding of retrovirus assembly, release, and maturation has advanced significantly in recent years thanks to developments in imaging technology, structural biology, as well as cell and molecular biology. This increase in basic knowledge has already led to the development of novel virus inhibitors designed to target various aspects of virus assembly and maturation (61). The HTLV-transformed SP cell line investigated in this study will help to characterize the mature HTLV-1 particle structure and its relationship to HTLV-1 particle infectivity.
MATERIALS AND METHODS
Cells and genomic DNA preparation.
SP cells were cultured in RPMI (Invitrogen, Carlsbad, CA, USA) containing 20% HyClone FetalClone III (FC3) from Thermo Scientific (Waltham, MA) and 1% penicillin-streptomycin from Life Technologies (Grand Island, NY, USA) and supplemented with human IL-2 (Roche Diagnostics, Indianapolis, IN, USA) at a concentration of 10 U/ml (45). MT-2, ATL-T, ATL-2, and C91PL cell lines were maintained in RPMI containing 10% FC3 and 1% penicillin-streptomycin. The MT-2 cell line was obtained from the NIH AIDS Reagent Program (catalog number 237 and lot number 110133), and early-passage cells were used for analyses. SP and C91PL cells were a kind gift from Cynthia Pise-Masison (National Cancer Institute, NIH, Bethesda, MD), and ATL-T and ATL-2 cells were a generous gift from Masao Matsuoka (Kyoto University, Kyoto, Japan) and Chou-Zen Giam (Uniform Services University of the Health Sciences, Bethesda, MD) (2, 45, 62). Genomic DNA was extracted and purified by using a Roche DNA isolation kit (Roche Diagnostics, Indianapolis, IN, USA).
Fluorescence in situ hybridization analysis.
To arrest cells in metaphase, 60 μl of colcemid (10 μg/ml; Irvine Scientific, Irvine, CA, USA) was added per 10 ml of cell culture medium, and the mixture was allowed to incubate for 2 h at room temperature. Cells were harvested and incubated in a 0.075 M KCl solution at 37°C for 16 min. After hypotonic incubation, cells were prefixed with methanol-acetic acid fixative and chilled at −20°C for 30 to 60 min. Cells were resuspended in fresh fixative before being fixed to a glass slide and dried. Slides were pretreated with RNase A (5 Prime, Gaithersburg, MD, USA) to degrade excess viral RNA in the cytoplasm and prevent off-target binding of the probe. The ACH HTLV-1 clone (18) was used as the FISH probe and was labeled using Green-500 dUTP (Enzo Life Science, Farmingdale, NY, USA) by nick translation (nick translation kit; Abbott Molecular, Des Plaines, IL, USA). The probe was hybridized at 37°C for 24 h in a humidified chamber. As a negative control, the ACH probe was hybridized to uninfected PHA-stimulated blood lymphocytes to check for nonspecific binding. Cells were washed in 2× saline sodium citrate buffer (150 mM NaCl, 15 mM trisodium citrate) at 72°C for 10 s and counterstained with DAPI (Life Technologies). Signals were visualized on an Olympus BX61 microscope workstation (Applied Spectral Imaging, Edingen-Neckarhausen, Germany), and images were captured by using an interferometer-based cooled charge-coupled-device (CCD) camera (Applied Spectral Imaging, Edingen-Neckarhausen, Germany) and FISHView ASI software. Image coloring was inverted, and DAPI banding patterns were used to identify the Chr on which proviruses were integrated. A total of 10 cells from each cell line were analyzed for provirus numbers and integration locations.
Linker-mediated PCR analysis.
Modified Splinkerette PCR was performed as previously reported (63) for MT-2 cells and SP cells. Adaptor oligonucleotides were prepared with standard desalting purification. For the first round of nested PCR, primers Splink R1 (5′-CGA AGA GTA ACC GTT GCT AGG AGA GAC C-3′) and LTR3 R1 (5′-TAC CGG CGA CTC CGT TGG CT-3′) were used. For the second round of nested PCR, primers Splink R2+A (5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG GTG GCT GAA TGA GAC TGG TGT CGA C-3′) and LTR3 R2+A (5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GCC AGC GAC AGC CCA TCC TAT AGC-3′) were used, which contain adaptor sequences for indexing during sequencing. Final products (20 μl) were run on a 2.5% Tris-acetate-EDTA (TAE) gel to confirm the presence of distinct DNA bands. The remaining products were purified by using a Promega Wizard SV Gel and PCR Clean-Up kit (Promega Corp., Madison, WI, USA). For each sample, 100 ng was submitted to the University of Minnesota Genomics Center for library preparation and sequencing on an Illumina HiSeq 2500 system (Illumina, San Diego, CA, USA). Sequences were analyzed for homology to the human (taxonomy ID [taxid] 9606) and HTLV-1 (taxid 11908) genomes by using BLAST from the National Library of Medicine (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences that aligned to both genomes and that contained the Splinkerette adaptor sequence at the 5′ end were selected.
Sequencing of proviral gag genes.
By using primers specific for the genomic sites of integration (5′-GGC CTT GGT GAT TGA TAT TGC TAC-3′ for Chr 3, 5′-GCA CAA CTG CCT GCG AGA AAC-3′ for Chr 6, 5′-CAC TTC CCA ATC CAA ATG CTT CC-3′ for Chr 20, and 5′-TTT CTG TCC TCT GTC CAC ACT TG-3′ for Chr X) in combination with primers in the pro (5′-GAT GTT GGG TCT TGG TTA GGA AGG-3′) and pol (5′-GTA GGG TTC GAT ATG GCC TGC-3′) regions of the proviral genome, the 5′ regions of the SP cell proviruses were sequenced via Sanger sequencing (Functional Bio, Madison, WI, USA). The orientation of the provirus within the genome was determined by using the genomic sequence and Ensembl (GRCh38.5), which was used to inform sequencing primer design. Sequences were aligned to an HTLV-1 reference genome (RefSeq accession number NC_001436.1). The effects of the identified mutations on protein coding were evaluated by comparing in silico translation alignments of sequences to those of a codon-optimized gag construct (28) and the translated HTLV-1 reference genome.
Immunoblot analysis of Gag production and incorporation into viruses.
SP and MT-2 cells were maintained in cell culture for 10 days, with a 1:2 split on day 6. The cell densities were 5 × 105 cells/ml for both SP and MT-2 cell lines on day 0 and 1.9 × 105 cells/ml and 5.7 × 105 cells/ml for the SP and MT-2 cell lines on day 10, respectively. Cells were lysed in 1% Triton X-100 in phosphate-buffered saline (PBS) supplemented with a commercially available cocktail of protease inhibitors (protease inhibitor cocktail; Sigma-Aldrich, St. Louis, MO, USA). The total protein content of cell lysates was assessed by using the bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL, USA), and 40 μg of protein was loaded per lane. Cell culture supernatants were harvested and spun at 2,000 × g for 5 min to remove cells and double filtered through 0.45- and 0.22-μm syringe filters. This filtrate (4 ml) was then overlaid on an 8% Optiprep–STE (100 mM NaCl, 10 mM Tris, 1 mM EDTA) cushion (1 ml) and centrifuged at 150,000 × g for 1.5 h at 4°C in a SW55 Ti rotor (Beckman Coulter, Brea, CA, USA). An equal volume of pelleted virus (25 μl) was loaded per SDS-PAGE lane. Sample buffer (3× Laemmli buffer) was added to the lysate and viral samples, which were resolved by SDS-PAGE and subjected to immunoblotting with a nitrocellulose membrane using the following antibodies: mouse anti-HTLV p24 (Santa Cruz, Dallas, TX, USA), mouse anti-HTLV p19 (ZeptoMetrix, Buffalo, NY, USA), rabbit polyclonal anti-HTLV p15 (a kind gift from Shawn Hill, National Cancer Institute, Frederick, MD), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Thermo Fisher, Rockford, IL, USA), horseradish peroxidase-conjugated donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA), and a Precision Protein StrepTactin-HRP conjugate (Bio-Rad, Hercules, CA, USA) for molecular weight ladder detection. Tubulin detection with mouse antitubulin (GTU-88; Sigma-Aldrich, St. Louis, MO, USA) was used as a cellular loading control. Images were captured and analyzed by using the Li-Cor C-DiGit blot scanner (Li-Cor, Lincoln, NE, USA).
Concentration, inactivation, and purification of virus particles for cryo-transmission electron microscopy.
Viral particles were purified and concentrated by using 3-step ultracentrifugation combined with CD45 depletion (31). SP and MT-2 cells were grown in culture for 14 days, splitting 1:2 twice a week and increasing the culture volume accordingly. Cellular debris was removed by low-speed centrifugation and double filtering, first through a 0.45-μm syringe filter and then through a 0.22-μm syringe filter. The cell-free supernatant was overlaid onto an 8% Optiprep-STE cushion and centrifuged at 150,000 × g for 1.5 h at 4°C on a type 50.2 Ti rotor (Beckman Coulter, Brea, CA, USA). Pelleted virus particles were resuspended in 2.5 ml of STE buffer and inactivated by treatment with 100 μM AT-2 (Sigma-Aldrich, St. Louis, MO, USA) (29) for 30 min at 37°C. For analysis of virus particles by cryo-TEM, contaminating vesicles were removed following inactivation by incubation with CD45 MicroBeads and passage through a liquid chromatography (LC) magnetic column (Miltenyi Biotec, San Diego, CA, USA). CD45-depleted virus was loaded onto a 10 to 30% Optiprep-STE gradient and centrifuged at 250,000 × g for 3 h at 4°C on an SW55 Ti rotor (Beckman Coulter, Brea, CA, USA). The virus particle band was collected by side puncture using a tuberculin syringe. Virus particles were concentrated, and excess Optiprep was removed by dilution in STE, followed by a final centrifugation step at 200,000 × g for 1 h at 4°C on an SW55 Ti rotor (Beckman Coulter, Brea, CA, USA). Virus particles were resuspended in 15 μl of STE for cryo-TEM.
Grid preparation and cryo-TEM of viruses.
Lacey Formvar- and carbon-coated 300-mesh EM grids (EMS, Hatfield, PA, USA) were glow discharged and loaded onto an FEI MarkIII Vitrobot system (FEI Company, Hillsboro, OR, USA). A 4-μl volume of purified, inactivated, and concentrated virus particles was loaded onto a grid (carbon side) and manually blotted before being plunge frozen in ultracooled liquid ethane. Grids were transferred to and imaged on a Tecnai F30 field emission gun (FEG) transmission electron microscope (FEI Company, Hillsboro, OR, USA) operating at 300 kV. Images were taken at a ×39,000 nominal magnification at an electron dose of ∼25 electrons/Å2 by using a Gatan 4k-by-4k CCD camera (Gatan Inc., Pleasanton, CA, USA). Images were binned by a factor of 2. Defocus values ranged from 4 to 8 μm. Virus particles were measured by using ImageJ software. Two perpendicular diameters were measured and averaged for each virus particle (64).
HTLV-1 MA ELISA.
HTLV-1 MA protein levels in cell-free supernatants were determined after cells were maintained in culture for 14 days by using an HTLV-1 p19-based ELISA (ZeptoMetrix, Buffalo, NY, USA) according to the manufacturer's instructions. The total numbers of cells for both the SP and MT-2 cell lines were determined at day 0 and day 10.
Accession number(s).
The gag sequences from the proviruses integrated within chromosomes 20, X, and 6 have been deposited in GenBank under accession numbers MF116105, MF116106, and MF116107, respectively.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health grant R01 GM098550. Morgan E. Meissner has been supported by NIH grant T32 AI083196 (Institute for Molecular Virology Training Program). The cytogenetic analyses were performed in the Cytogenomics Shared Resource at the University of Minnesota with support from comprehensive Masonic Cancer Center NIH grant P30 CA077598.
We thank Shawn Hill, care of David Derse, for the anti-NC polyclonal antibody; Cynthia Pise-Masison for the SP and C91PL cells; and Masao Matsuoka and Chou-Zen Giam the ATL-T and ATL-2 cells. Cryo-TEM was carried out at the Characterization Facility, University of Minnesota. We thank Jessica L. Martin for critical reviews of the manuscript.
REFERENCES
- 1.Yoshida M, Miyoshi I, Hinuma Y. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A 79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popovic M, Sarin PS, Robert-Gurroff M, Kalyanaraman VS, Mann D, Minowada J, Gallo RC. 1983. Isolation and transmission of human retrovirus (human T-cell leukemia virus). Science 219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 3.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paiva A, Casseb J. 2015. Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev Inst Med Trop Sao Paulo 57:1–13. doi: 10.1590/S0036-46652015000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonoda S, Li HC, Tajima K. 2011. Ethnoepidemiology of HTLV-1 related diseases: ethnic determinants of HTLV-1 susceptibility and its worldwide dispersal. Cancer Sci 102:295–301. doi: 10.1111/j.1349-7006.2010.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de Thé G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–410. [DOI] [PubMed] [Google Scholar]
- 8.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031–1032. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 10.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. 2008. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med 14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 11.Fan N, Gavalchin J, Paul B, Wells KH, Lane MJ, Poiesz BJ. 1992. Infection of peripheral blood mononuclear cells and cell lines by cell-free human T-cell lymphoma/leukemia virus type I. J Clin Microbiol 30:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamble TR, Yoo S, Vajdos FF, von Schwedler UK, Worthylake DK, Wang H, McCutcheon JP, Sundquist WI, Hill CP. 1997. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278:849–853. doi: 10.1126/science.278.5339.849. [DOI] [PubMed] [Google Scholar]
- 13.Rayne F, Bouamr F, Lalanne J, Mamoun RZ. 2001. The NH2-terminal domain of the human T-cell leukemia virus type 1 capsid protein is involved in particle formation. J Virol 75:5277–5287. doi: 10.1128/JVI.75.11.5277-5287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ako-Adjei D, Johnson MC, Vogt VM. 2005. The retroviral capsid domain dictates virion size, morphology, and coassembly of gag into virus-like particles. J Virol 79:13463–13472. doi: 10.1128/JVI.79.21.13463-13472.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin JL, Maldonado JO, Mueller JD, Zhang W, Mansky LM. 2016. Molecular studies of HTLV-1 replication: an update. Viruses 8:31. doi: 10.3390/v8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konvalinka J, Kräusslich HG, Müller B. 2015. Retroviral proteases and their roles in virion maturation. Virology 479–480:403–417. doi: 10.1016/j.virol.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Steven AC, Heymann JB, Cheng N, Trus BL, Conway JF. 2005. Virus maturation: dynamics and mechanism of a stabilizing structural transition that leads to infectivity. Curr Opin Struct Biol 15:227–236. doi: 10.1016/j.sbi.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimata JT, Wong FH, Wang JJ, Ratner L. 1994. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology 204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 19.Ohsugi T, Kumasaka T, Urano T. 2004. Construction of a full-length human T cell leukemia virus type I genome from MT-2 cells containing multiple defective proviruses using overlapping polymerase chain reaction. Anal Biochem 329:281–288. doi: 10.1016/j.ab.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Derse D, Hill SA, Lloyd PA, Chung H, Morse BA. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J Virol 75:8461–8468. doi: 10.1128/JVI.75.18.8461-8468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demontis MA, Sadiq MT, Golz S, Taylor GP. 2015. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. J Med Virol 87:2130–2134. doi: 10.1002/jmv.24264. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi I, Kubonishi I, Yoshimoto S, Shiraishi Y. 1981. A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gan 72:978–981. [PubMed] [Google Scholar]
- 23.Cao S, Maldonado JO, Grigsby IF, Mansky LM, Zhang W. 2015. Analysis of human T-cell leukemia virus type 1 particles by using cryo-electron tomography. J Virol 89:2430–2435. doi: 10.1128/JVI.02358-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi N, Konishi H, Sabe H, Shigesada K, Noma T, Honjo T, Hatanaka M. 1984. Genomic structure of HTLV (human T-cell leukemia virus): detection of defective genome and its amplification in MT-2 cells. EMBO J 3:1339–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morozov VA, Weiss RA. 1999. Two types of HTLV-1 particles are released from MT-2 cells. Virology 255:279–284. doi: 10.1006/viro.1998.9578. [DOI] [PubMed] [Google Scholar]
- 26.Hashikura Y, Umeki K, Umekita K, Nomura H, Yamamoto I, Hasegawa H, Yanagihara K, Okayama A. 2016. The diversity of the structure and genomic integration sites of HTLV-1 provirus in MT-2 cell lines. Hum Cell 29:122–129. doi: 10.1007/s13577-016-0136-8. [DOI] [PubMed] [Google Scholar]
- 27.Iino T, Takeuchi K, Nam SH, Siomi H, Sabe H, Kobayashi N, Hatanaka M. 1986. Structural analysis of p28 adult T-cell leukaemia-associated antigen. J Gen Virol 67(Part 7):1373–1379. [DOI] [PubMed] [Google Scholar]
- 28.Martin JL, Cao S, Maldonado JO, Zhang W, Mansky LM. 2016. Distinct particle morphologies revealed through comparative parallel analyses of retrovirus-like particles. J Virol 90:8074–8084. doi: 10.1128/JVI.00666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol 72:7992–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coren LV, Shatzer T, Ott DE. 2008. CD45 immunoaffinity depletion of vesicles from Jurkat T cells demonstrates that exosomes contain CD45: no evidence for a distinct exosome/HIV-1 budding pathway. Retrovirology 5:64. doi: 10.1186/1742-4690-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott DE. 2009. Purification of HIV-1 virions by subtilisin digestion or CD45 immunoaffinity depletion for biochemical studies. Methods Mol Biol 485:15–25. doi: 10.1007/978-1-59745-170-3_2. [DOI] [PubMed] [Google Scholar]
- 32.Esser MT, Graham DR, Coren LV, Trubey CM, Bess JW, Arthur LO, Ott DE, Lifson JD. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J Virol 75:6173–6182. doi: 10.1128/JVI.75.13.6173-6182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen DG, Booth A, Gould SJ, Hildreth JE. 2003. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem 278:52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- 34.Kühlbrandt W. 2014. Cryo-EM enters a new era. eLife 3:e03678. doi: 10.7554/eLife.03678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattei S, Glass B, Hagen WJ, Kräusslich HG, Briggs JA. 2016. The structure and flexibility of conical HIV-1 capsids determined within intact virions. Science 354:1434–1437. doi: 10.1126/science.aah4972. [DOI] [PubMed] [Google Scholar]
- 36.Schur FK, Obr M, Hagen WJ, Wan W, Jakobi AJ, Kirkpatrick JM, Sachse C, Kräusslich HG, Briggs JA. 2016. An atomic model of HIV-1 capsid-SP1 reveals structures regulating assembly and maturation. Science 353:506–508. doi: 10.1126/science.aaf9620. [DOI] [PubMed] [Google Scholar]
- 37.Schur FK, Dick RA, Hagen WJ, Vogt VM, Briggs JA. 2015. The structure of immature virus-like Rous sarcoma virus Gag particles reveals a structural role for the p10 domain in assembly. J Virol 89:10294–10302. doi: 10.1128/JVI.01502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganser-Pornillos BK, von Schwedler UK, Stray KM, Aiken C, Sundquist WI. 2004. Assembly properties of the human immunodeficiency virus type 1 CA protein. J Virol 78:2545–2552. doi: 10.1128/JVI.78.5.2545-2552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogue IB, Hoppe A, Ono A. 2009. Quantitative fluorescence resonance energy transfer microscopy analysis of the human immunodeficiency virus type 1 Gag-Gag interaction: relative contributions of the CA and NC domains and membrane binding. J Virol 83:7322–7336. doi: 10.1128/JVI.02545-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lingappa JR, Reed JC, Tanaka M, Chutiraka K, Robinson BA. 2014. How HIV-1 Gag assembles in cells: putting together pieces of the puzzle. Virus Res 193:89–107. doi: 10.1016/j.virusres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goto T, Ashina T, Fujiyoshi Y, Kume N, Yamagishi H, Nakai M. 1994. Projection structures of human immunodeficiency virus type 1 (HIV-1) observed with high resolution electron cryo-microscopy. J Electron Microsc (Tokyo) 43:16–19. [PubMed] [Google Scholar]
- 42.Butan C, Winkler DC, Heymann JB, Craven RC, Steven AC. 2008. RSV capsid polymorphism correlates with polymerization efficiency and envelope glycoprotein content: implications that nucleation controls morphogenesis. J Mol Biol 376:1168–1181. doi: 10.1016/j.jmb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeager M, Wilson-Kubalek EM, Weiner SG, Brown PO, Rein A. 1998. Supramolecular organization of immature and mature murine leukemia virus revealed by electron cryo-microscopy: implications for retroviral assembly mechanisms. Proc Natl Acad Sci U S A 95:7299–7304. doi: 10.1073/pnas.95.13.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Effantin G, Estrozi LF, Aschman N, Renesto P, Stanke N, Lindemann D, Schoehn G, Weissenhorn W. 2016. Cryo-electron microscopy structure of the native prototype foamy virus glycoprotein and virus architecture. PLoS Pathog 12:e1005721. doi: 10.1371/journal.ppat.1005721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe T, Dezzutti C, Guenthner PC, Lam L, Hodge T, Lairmore MD, Lal RB, Folks TM. 1995. Characterization of a HTLV-I-infected cell line derived from a patient with adult T-cell leukemia with stable co-expression of CD4 and CD8. Leuk Res 19:621–628. doi: 10.1016/0145-2126(95)00030-R. [DOI] [PubMed] [Google Scholar]
- 46.Zucker-Franklin D, Pancake BA, Najfeld V. 2003. Localization of HTLV-I tax proviral DNA in mononuclear cells. Blood Cells Mol Dis 31:1–6. doi: 10.1016/S1079-9796(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 47.Taylor JM, Ghorbel S, Nicot C. 2009. Genome wide analysis of human genes transcriptionally and post-transcriptionally regulated by the HTLV-I protein p30. BMC Genomics 10:311. doi: 10.1186/1471-2164-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mamane Y, Sharma S, Grandvaux N, Hernandez E, Hiscott J. 2002. IRF-4 activities in HTLV-I-induced T cell leukemogenesis. J Interferon Cytokine Res 22:135–143. doi: 10.1089/107999002753452746. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki S, Zhou Y, Refaat A, Takasaki I, Koizumi K, Yamaoka S, Tabuchi Y, Saiki I, Sakurai H. 2010. Human T cell lymphotropic virus 1 manipulates interferon regulatory signals by controlling the TAK1-IRF3 and IRF4 pathways. J Biol Chem 285:4441–4446. doi: 10.1074/jbc.M109.031476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamane Y, Grandvaux N, Hernandez E, Sharma S, Innocente SA, Lee JM, Azimi N, Lin R, Hiscott J. 2002. Repression of IRF-4 target genes in human T cell leukemia virus-1 infection. Oncogene 21:6751–6765. doi: 10.1038/sj.onc.1205843. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Toomey NL, Diaz LA, Walker G, Ramos JC, Barber GN, Ning S. 2011. Oncogenic IRFs provide a survival advantage for Epstein-Barr virus- or human T-cell leukemia virus type 1-transformed cells through induction of BIC expression. J Virol 85:8328–8337. doi: 10.1128/JVI.00570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Refaat A, Zhou Y, Suzuki S, Takasaki I, Koizumi K, Yamaoka S, Tabuchi Y, Saiki I, Sakurai H. 2011. Distinct roles of transforming growth factor-beta-activated kinase 1 (TAK1)-c-Rel and interferon regulatory factor 4 (IRF4) pathways in human T cell lymphotropic virus 1-transformed T helper 17 cells producing interleukin-9. J Biol Chem 286:21092–21099. doi: 10.1074/jbc.M110.200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, Ishii R, Muto S, Kotani S, Watatani Y, Takeda J, Sanada M, Tanaka H, Suzuki H, Sato Y, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Iwanaga M, Ma G, Nosaka K, Hishizawa M, Itonaga H, Imaizumi Y, Munakata W, Ogasawara H, Sato T, Sasai K, Muramoto K, Penova M, Kawaguchi T, Nakamura H, Hama N, Shide K, Kubuki Y, Hidaka T, Kameda T, Nakamaki T, Ishiyama K, Miyawaki S, Yoon SS, Tobinai K, Miyazaki Y, Takaori-Kondo A, Matsuda F, et al. . 2015. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 54.Qualley DF, Stewart-Maynard KM, Wang F, Mitra M, Gorelick RJ, Rouzina I, Williams MC, Musier-Forsyth K. 2010. C-terminal domain modulates the nucleic acid chaperone activity of human T-cell leukemia virus type 1 nucleocapsid protein via an electrostatic mechanism. J Biol Chem 285:295–307. doi: 10.1074/jbc.M109.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cimarelli A, Sandin S, Höglund S, Luban J. 2000. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J Virol 74:3046–3057. doi: 10.1128/JVI.74.7.3046-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell S, Rein A. 1999. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J Virol 73:2270–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Rocquigny H, El Meshri SE, Richert L, Didier P, Darlix JL, Mély Y. 2014. Role of the nucleocapsid region in HIV-1 Gag assembly as investigated by quantitative fluorescence-based microscopy. Virus Res 193:78–88. doi: 10.1016/j.virusres.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Maldonado JO, Martin JL, Mueller JD, Zhang W, Mansky LM. 2014. New insights into retroviral Gag-Gag and Gag-membrane interactions. Front Microbiol 5:302. doi: 10.3389/fmicb.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forshey BM, von Schwedler U, Sundquist WI, Aiken C. 2002. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J Virol 76:5667–5677. doi: 10.1128/JVI.76.11.5667-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hulme AE, Kelley Z, Okocha EA, Hope TJ. 2015. Identification of capsid mutations that alter the rate of HIV-1 uncoating in infected cells. J Virol 89:643–651. doi: 10.1128/JVI.03043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spearman P. 2016. HIV-1 Gag as an antiviral target: development of assembly and maturation inhibitors. Curr Top Med Chem 16:1154–1166. doi: 10.2174/1568026615666150902102143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagasaki M, Zhang J, Morikawa S, Harada T, Nabika T, Tanaka Y. 2005. Human leukocyte antigen-class II-negative long-term cultured human T-cell leukemia virus type-I-infected T-cell lines with progressed cytological properties significantly induce superantigen-dependent normal T-cell proliferation. Pathol Int 55:264–272. doi: 10.1111/j.1440-1827.2005.01823.x. [DOI] [PubMed] [Google Scholar]
- 63.Uren AG, Mikkers H, Kool J, van der Weyden L, Lund AH, Wilson CH, Rance R, Jonkers J, van Lohuizen M, Berns A, Adams DJ. 2009. A high-throughput splinkerette-PCR method for the isolation and sequencing of retroviral insertion sites. Nat Protoc 4:789–798. doi: 10.1038/nprot.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grigsby IF, Zhang W, Johnson JL, Fogarty KH, Chen Y, Rawson JM, Crosby AJ, Mueller JD, Mansky LM. 2010. Biophysical analysis of HTLV-1 particles reveals novel insights into particle morphology and Gag stoichiometry. Retrovirology 7:75. doi: 10.1186/1742-4690-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]