Abstract
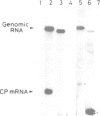
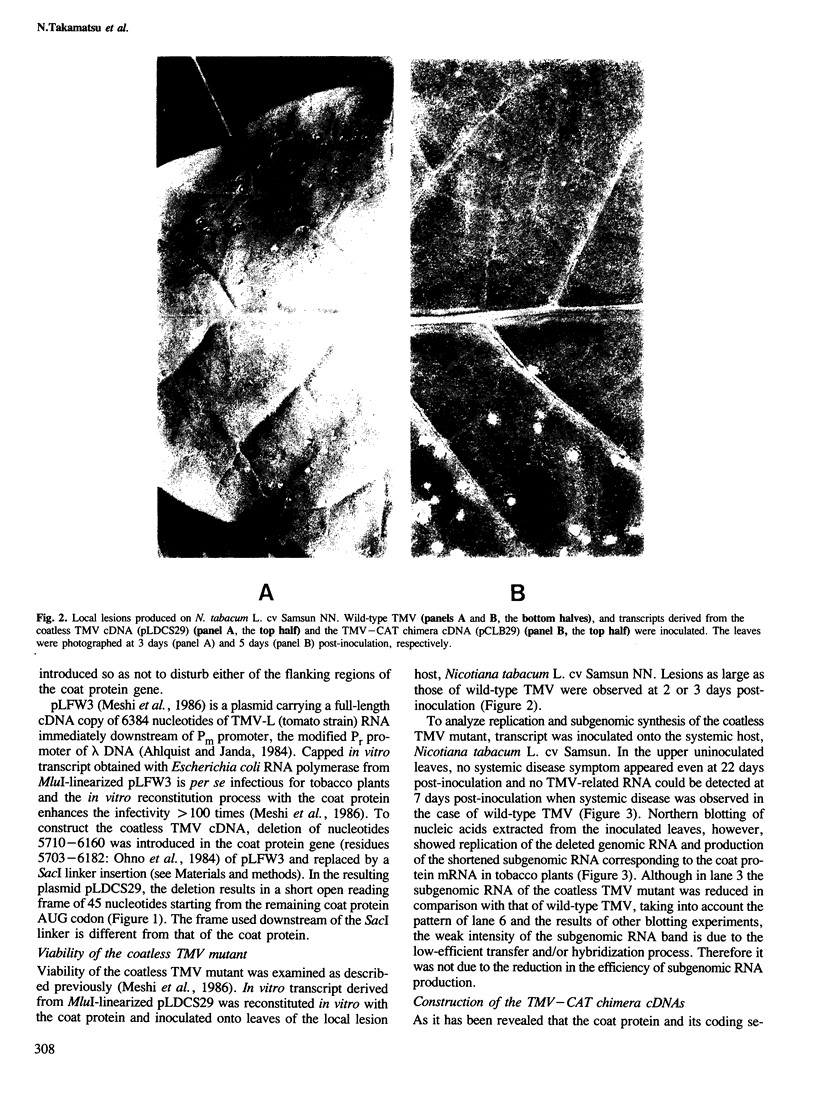
We have constructed three tobacco mosaic virus (TMV) cDNA derivatives by modification of the full-length cDNA clone from which infectious TMV-RNA can be transcribed in vitro. A coatless TMV construct lacks most of the coat protein gene and chimeric TMV constructs retain the bacterial chloramphenicol acetyltransferase (CAT) gene in place of the coat protein gene. When in vitro transcripts from these cDNA derivatives were inoculated on the local lesion tobacco plants, TMV-specific lesions were produced. In the case of the TMV–CAT chimeras, however, the lesions were small compared to those of wild-type TMV and those produced by transcript derived from the coatless construct. Northern blot analysis of RNA extracted from the inoculated leaves of the systemic host plants revealed replication of the derivative genomic RNAs and production of their own subgenomic RNAs corresponding to the coat protein mRNA. The TMV–CAT chimeras produced biologically active CAT in the inoculated leaves of the systemic host. CAT activity increased at least until 2 weeks post-inoculation and was ~0.1 units/mg of tissue at 10 days post-inoculation. Thus, TMV–RNA may be utilized as a new plant expression vector.
Keywords: coatless TMV mutant, complementary DNA expression system, plant RNA vector, TMV–CAT chimera, tobacco mosaic virus
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabekov J. G., Dorokhov YuL Plant virus-specific transport function and resistance of plants to viruses. Adv Virus Res. 1984;29:313–364. doi: 10.1016/s0065-3527(08)60412-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Block M., Schell J., Van Montagu M. Chloroplast transformation by Agrobacterium tumefaciens. EMBO J. 1985 Jun;4(6):1367–1372. doi: 10.1002/j.1460-2075.1985.tb03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth L., Richards K. E. Tobacco mosaic virus: model for structure and function of a simple virus. Adv Virus Res. 1981;26:145–199. doi: 10.1016/s0065-3527(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Aoyagi M., Yamanashi Y., Saito H., Ikawa S., Meshi T., Okada Y. Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J Biochem. 1984 Dec;96(6):1915–1923. doi: 10.1093/oxfordjournals.jbchem.a135026. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y., Nishiguchi M., Kiho Y. Single amino acid substitution in 30K protein of TMV defective in virus transport function. Virology. 1983 Nov;131(1):255–258. doi: 10.1016/0042-6822(83)90551-2. [DOI] [PubMed] [Google Scholar]
- SIEGEL A., ZAITLIN M., SEHGAL O. P. The isolation of defective tobacco mosaic virus strains. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1845–1851. doi: 10.1073/pnas.48.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Meshi T., Okada Y. The initiation site for transcription of the TMV 30-kDa protein messenger RNA. FEBS Lett. 1984 Jul 23;173(1):247–250. doi: 10.1016/0014-5793(84)81056-x. [DOI] [PubMed] [Google Scholar]