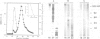Abstract
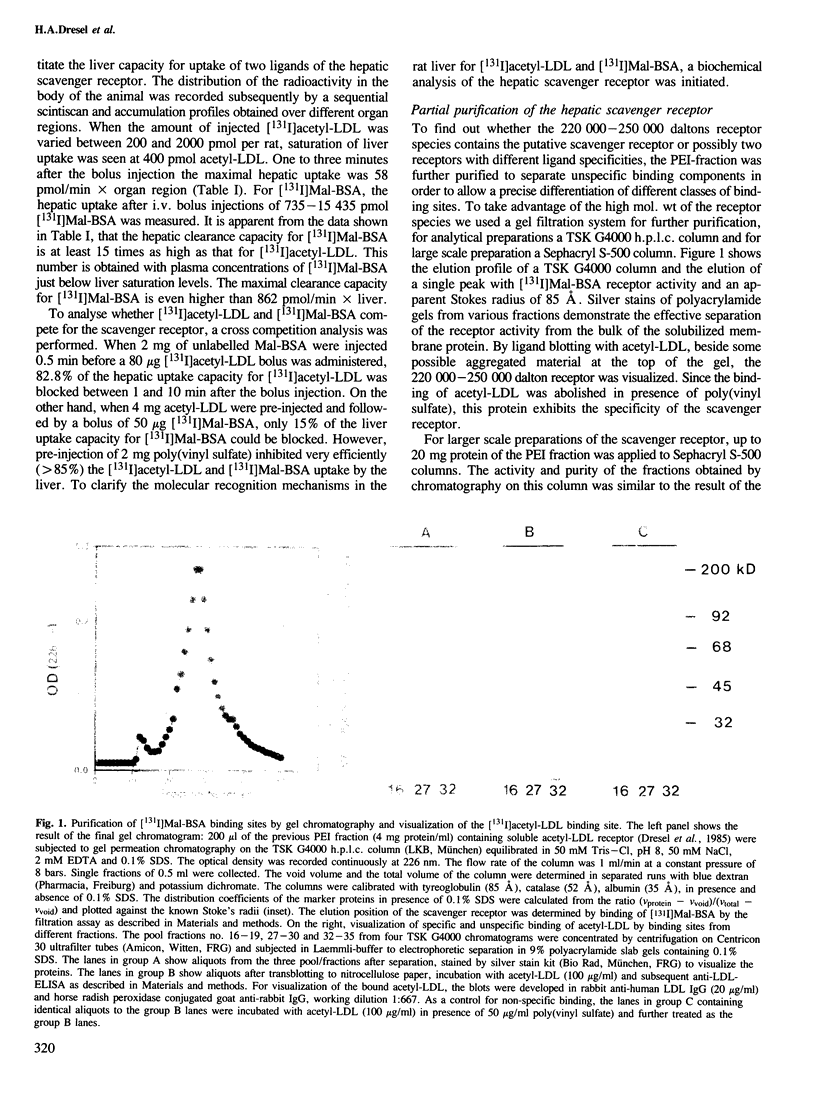
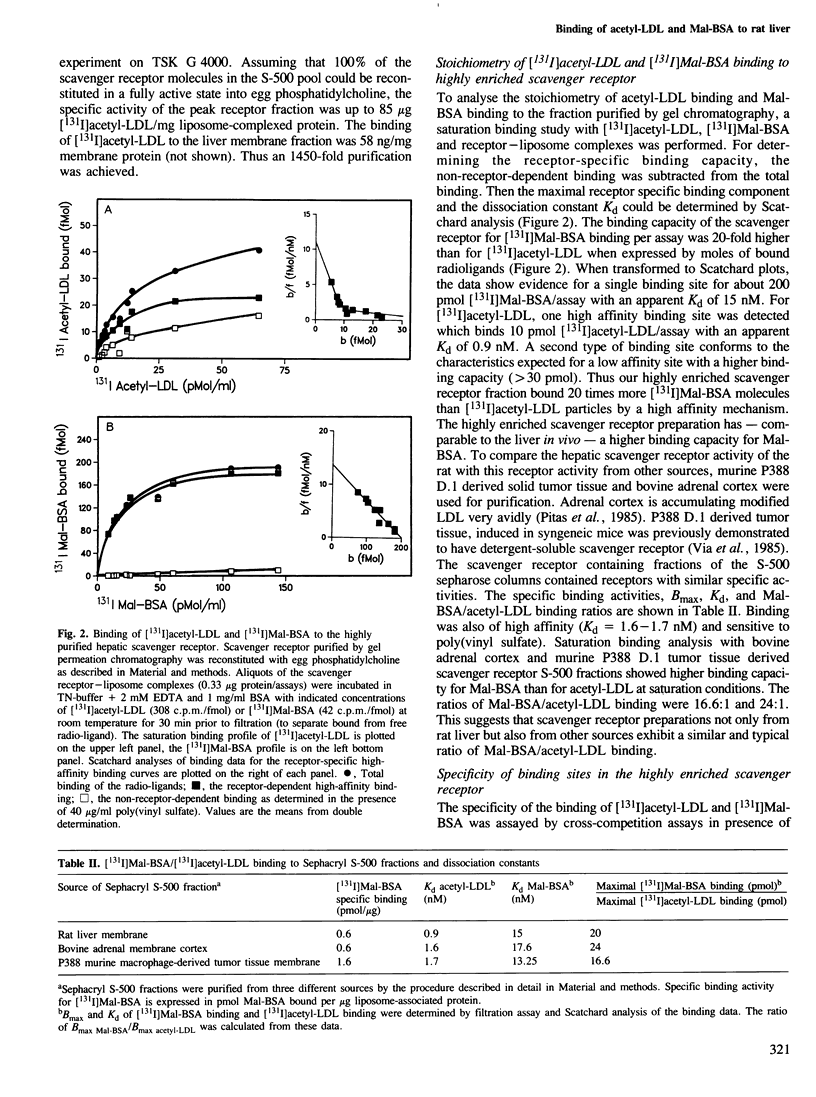
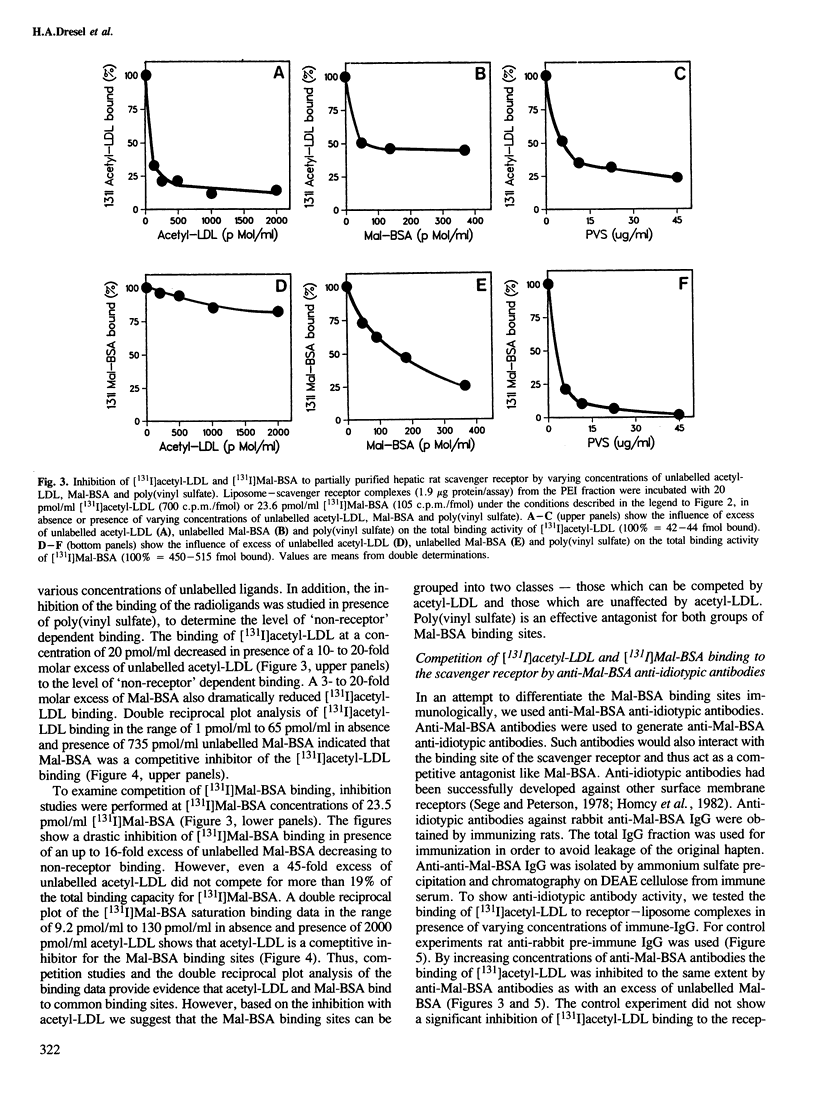
The liver is the major organ involved in clearance of acetylated low density lipoprotein (acetyl-LDL) and maleylated serum albumin (Mal-BSA). Quantitative analysis of the hepatic uptake by sequential scintigraphy in rats shows that the hepatic uptake capacity for Mal-BSA is at least 15 times larger than for acetyl-LDL particles. A membrane-associated M approximately 250,000 daltons hepatic receptor for acetyl-LDL and Mal-BSA was 1450-fold purified from total membrane by Triton X-114 solubilization, chromatography on polyethylenimine cellulose and gel filtration. This receptor incorporated into liposomes displayed a saturable binding of [131I]Mal-BSA with a dissociation constant Kd = 15 nM and to [131I]acetyl-LDL with a dissociation constant Kd = 0.9 nM. The binding of both ligands was sensitive to poly(vinyl sulfate). The purified scavenger receptor system has a binding capacity for [131I]Mal-BSA 20 times larger than for [131I]acetyl-LDL. This is similar to the maximal removal capacity of the rat liver for both ligands in vivo. Binding studies with Mal-BSA, acetyl-LDL and anti-idiotypic receptor antibodies as competitors for [131I]Mal-BSA and [131I]acetyl-LDL binding demonstrate that [131I]Mal-BSA and [131I]acetyl-LDL compete for a common binding site. However, not all of the Mal-BSA binding sites are capable of interacting with acetyl-LDL.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Dresel H. A., Friedrich E., Via D. P., Schettler G., Sinn H. Characterization of binding sites for acetylated low density lipoprotein in the rat liver in vivo and in vitro. EMBO J. 1985 May;4(5):1157–1162. doi: 10.1002/j.1460-2075.1985.tb03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel H. A., Otto I., Weigel H., Schettler G., Via D. P. A simple and rapid anti-ligand enzyme immunoassay for visualization of low-density lipoprotein membrane receptors. Biochim Biophys Acta. 1984 Oct 4;795(3):452–457. doi: 10.1016/0005-2760(84)90172-3. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Hoff H. F., Ho Y. K., Basu S. K., Brown M. S. Stimulation of cholesteryl ester synthesis in macrophages by extracts of atherosclerotic human aortas and complexes of albumin/cholesteryl esters. Arteriosclerosis. 1981 May-Jun;1(3):210–226. doi: 10.1161/01.atv.1.3.210. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Rasmussen R. R., Olch C. L., Fogelman A. M. Two distinct receptors account for recognition of maleyl-albumin in human monocytes during differentiation in vitro. J Clin Invest. 1986 Mar;77(3):681–689. doi: 10.1172/JCI112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homcy C. J., Rockson S. G., Haber E. An antiidiotypic antibody that recognizes the beta-adrenergic receptor. J Clin Invest. 1982 May;69(5):1147–1154. doi: 10.1172/JCI110550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Pitas R. E., Boyles J., Mahley R. W., Bissell D. M. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol. 1985 Jan;100(1):103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Schneider W. J., Goldstein J. L., Brown M. S. Partial purification and characterization of the low density lipoprotein receptor from bovine adrenal cortex. J Biol Chem. 1980 Dec 10;255(23):11442–11447. [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via D. P., Dresel H. A., Cheng S. L., Gotto A. M., Jr Murine macrophage tumors are a source of a 260,000-dalton acetyl-low density lipoprotein receptor. J Biol Chem. 1985 Jun 25;260(12):7379–7386. [PubMed] [Google Scholar]