Abstract
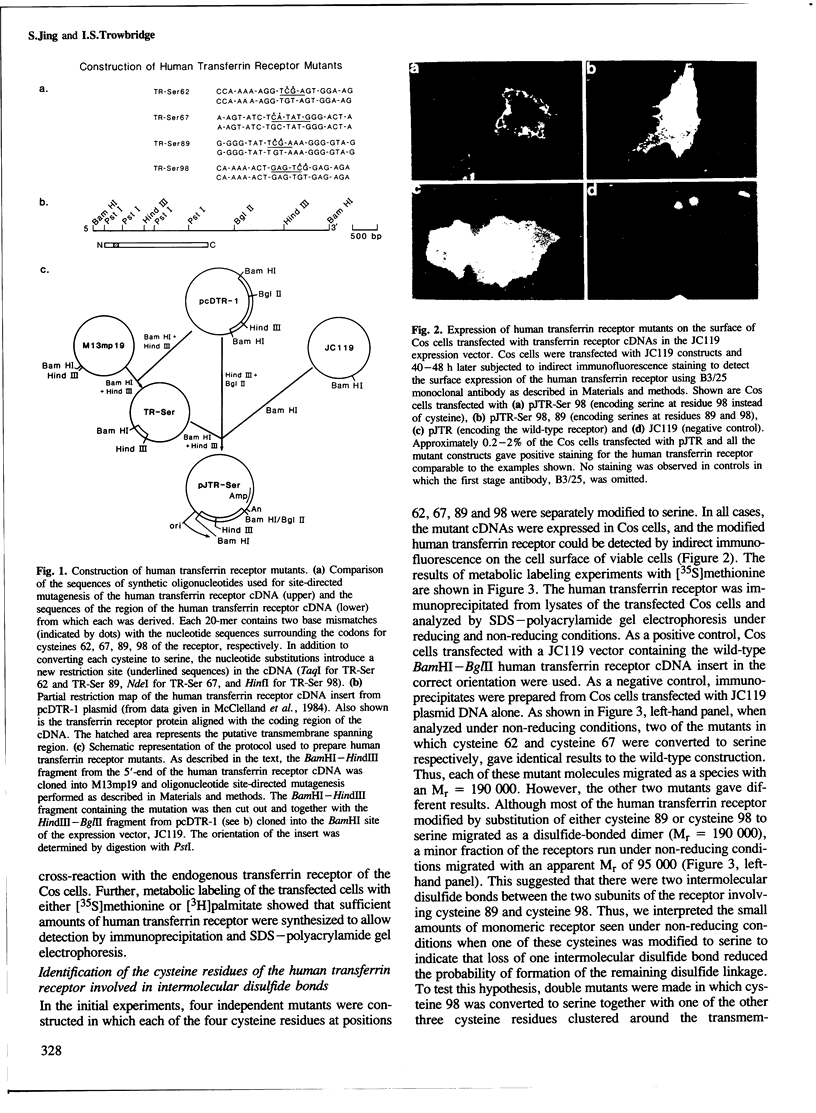
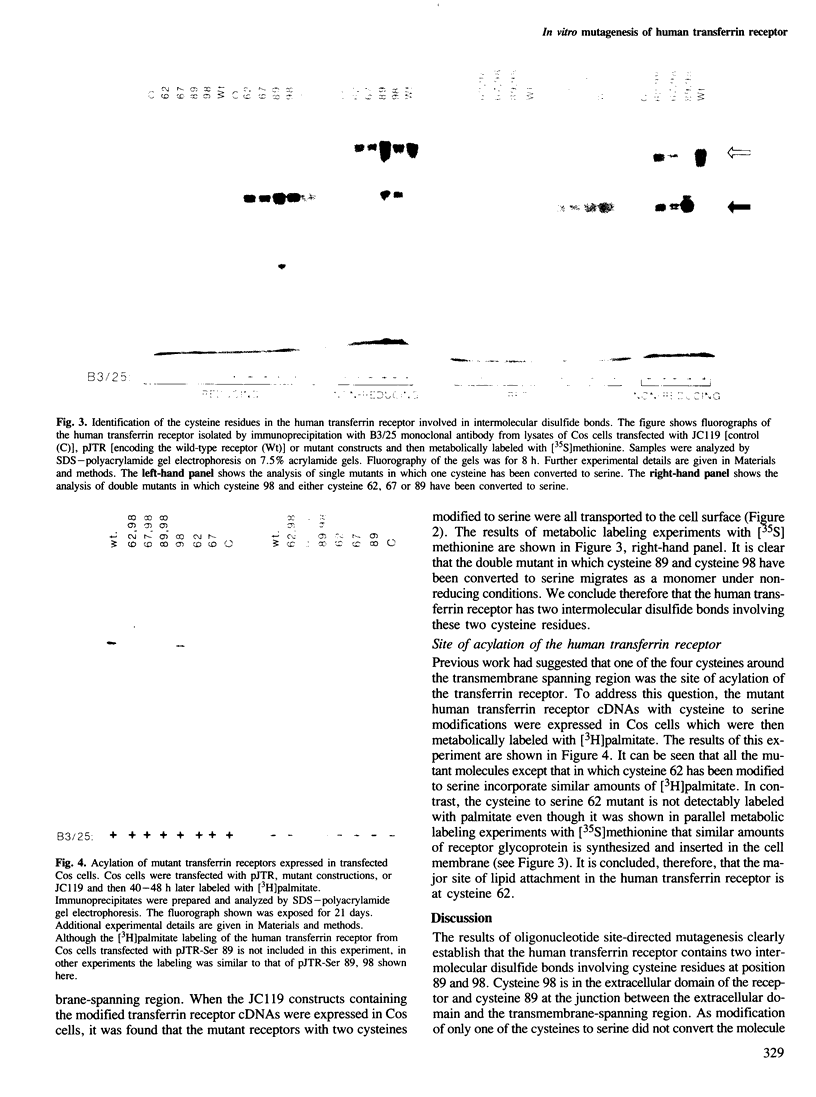
Structural studies of the human transferrin receptor have shown that the molecule is a disulfide-bonded dimer consisting of two identical subunits (Mr = 95,000) which are post-translationally modified by the addition of a fatty acyl moiety. Oligonucleotide site-directed mutagenesis has been used to obtain mutant molecules in which each of the four cysteines, residues 62, 67, 89 and 98, clustered within or adjacent to the membrane-spanning region were modified to serine. By first preparing mutants with only one of these cysteine residues modified to serine and then obtaining additional mutants in which different combinations of two cysteine residues were modified, we have shown that both cysteine 89 and cysteine 98, which are located in the extracellular domain of the receptor, are involved in intermolecular disulfide bonds. Further, we have identified cysteine 62 as the major site of acylation. Each of the mutant molecules is synthesized and transported to the cell surface when the modified human transferrin receptor cDNAs are transiently expressed in simian Cos cells. It should therefore now be possible to design experiments to determine whether these modified receptors bind transferrin normally and mediate iron uptake.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Lodish H. F. How receptors bring proteins and particles into cells. Sci Am. 1984 May;250(5):52–58. doi: 10.1038/scientificamerican0584-52. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Krangel M. S., Strominger J. L. Cysteines in the transmembrane region of major histocompatibility complex antigens are fatty acylated via thioester bonds. J Biol Chem. 1984 Jun 10;259(11):7230–7238. [PubMed] [Google Scholar]
- Lesley J. F., Schulte R. J. Inhibition of cell growth by monoclonal anti-transferrin receptor antibodies. Mol Cell Biol. 1985 Aug;5(8):1814–1821. doi: 10.1128/mcb.5.8.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J. F., Schulte R. J. Selection of cell lines resistant to anti-transferrin receptor antibody: evidence for a mutation in transferrin receptor. Mol Cell Biol. 1984 Sep;4(9):1675–1681. doi: 10.1128/mcb.4.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Schulte R., Trotter J. Expression of transferrin receptor on murine hematopoietic progenitors. Cell Immunol. 1984 Jan;83(1):14–25. doi: 10.1016/0008-8749(84)90220-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland A., Kühn L. C., Ruddle F. H. The human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cDNA sequence. Cell. 1984 Dec;39(2 Pt 1):267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Marshall J., Hayman M. J. Identification and characterization of the chicken transferrin receptor. Biochem J. 1985 Dec 15;232(3):735–741. doi: 10.1042/bj2320735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearne P. A., Pietersz G. A., Goding J. W. cDNA cloning of the murine transferrin receptor: sequence of trans-membrane and adjacent regions. J Immunol. 1985 May;134(5):3474–3479. [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Schulte R. Murine cell surface transferrin receptor: studies with an anti-receptor monoclonal antibody. J Cell Physiol. 1982 Sep;112(3):403–410. doi: 10.1002/jcp.1041120314. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Newman R. A., Domingo D. L., Sauvage C. Transferrin receptors: structure and function. Biochem Pharmacol. 1984 Mar 15;33(6):925–932. doi: 10.1016/0006-2952(84)90447-7. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]