Abstract
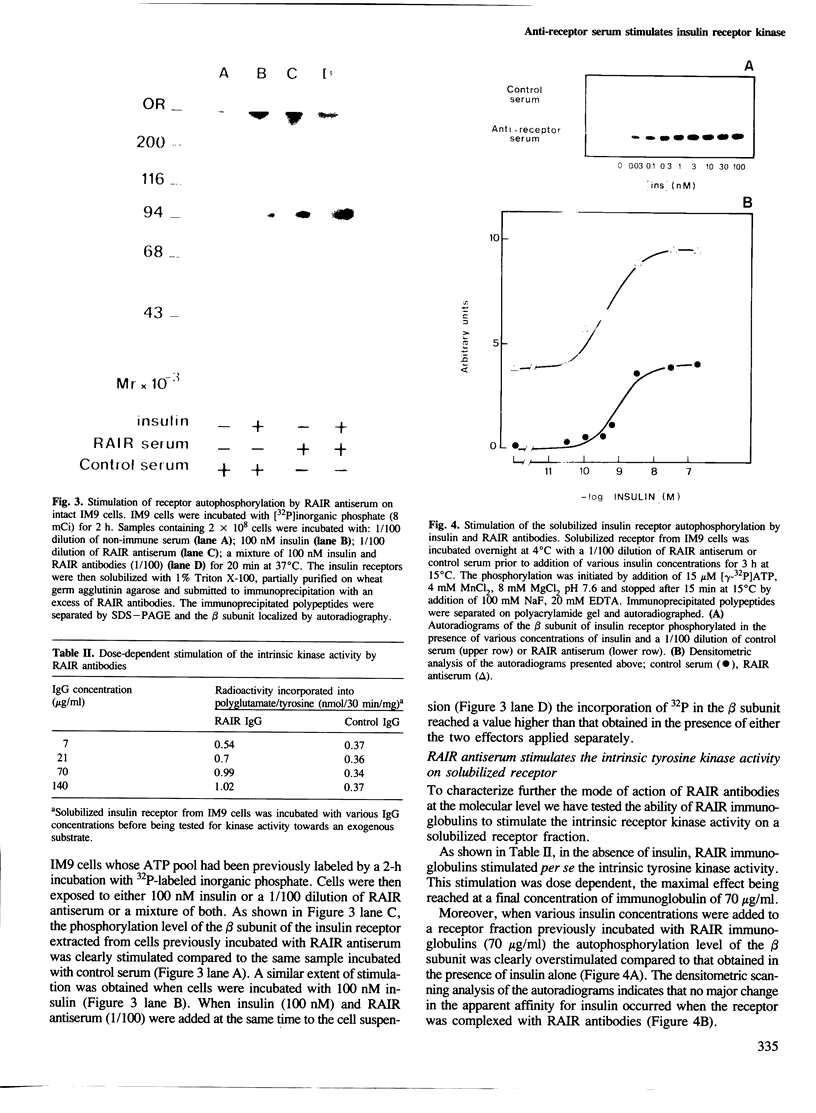
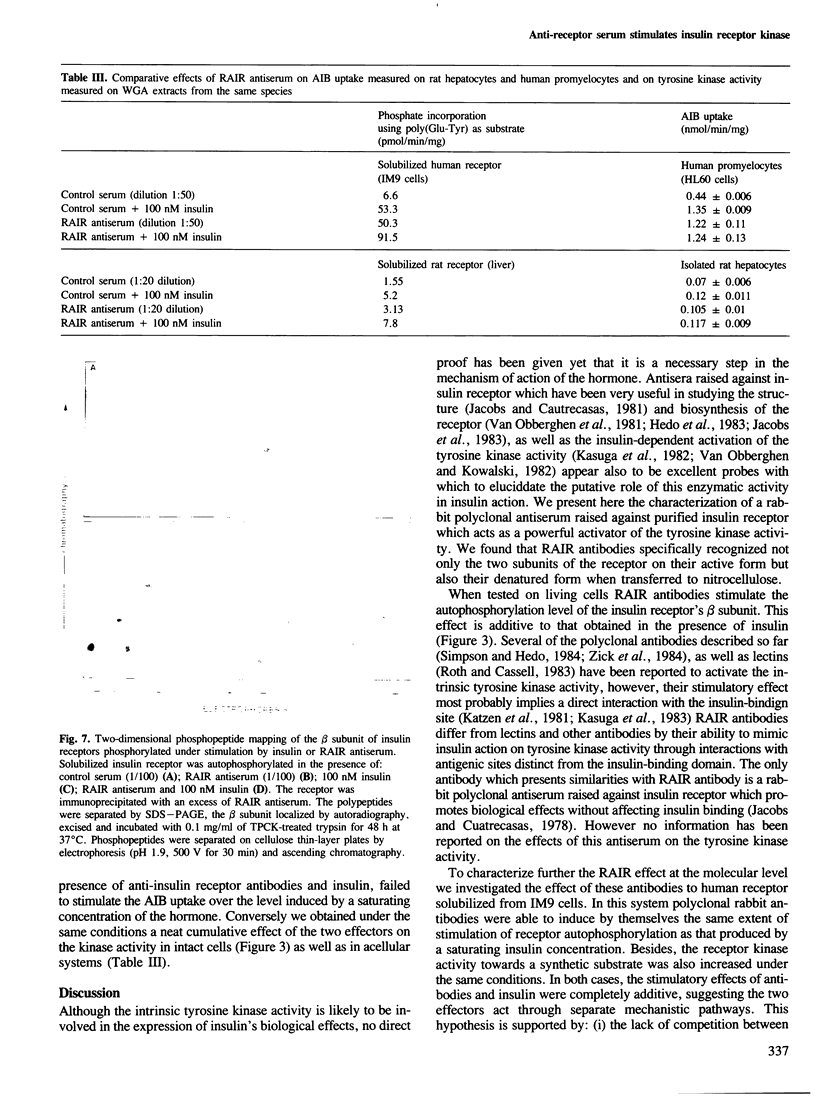
This paper describes the properties of rabbit polyclonal antibodies directed against purified human insulin receptor which strongly stimulate the intrinsic tyrosine kinase activity. The stimulatory effect of the antibodies on the kinase activity was obtained on the insulin receptor autophosphorylation as well as on the kinase activity towards a synthetic substrate. This stimulation is additive to that induced by insulin. Moreover, rabbit antibodies do not impair insulin binding. These data strongly suggest that antibodies and insulin act through separate pathways. This conclusion is reinforced by the differences observed on the phosphopeptide maps of the receptor's beta subunit whose phosphorylation was performed either in the presence of insulin or rabbit antibodies. Interestingly, these polyclonal antibodies can also induce an activation of the receptor autophosphorylation by interacting only with extracellular determinants. The anti-insulin receptor antibodies mimic insulin in their stimulatory effect on amino acid (AIB) uptake, but they have a different effect to that found on the kinase activity; the simultaneous addition of the antiserum and insulin failed to stimulate this amino acid transport over the level induced by a saturating concentration of hormone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Edery M., Ellis L., Standring D., Beaudoin J., Roth R. A., Rutter W. J. Expression of a functional human insulin receptor from a cloned cDNA in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8014–8018. doi: 10.1073/pnas.82.23.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Gazzano H., Kowalski A., Fehlmann M., Van Obberghen E. Two different protein kinase activities are associated with the insulin receptor. Biochem J. 1983 Dec 15;216(3):575–582. doi: 10.1042/bj2160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorescu F., Flier J. S., Kahn C. R. Defect in insulin receptor phosphorylation in erythrocytes and fibroblasts associated with severe insulin resistance. J Biol Chem. 1984 Dec 25;259(24):15003–15006. [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M., Lavau M., Horne J. S., Wardzala L. J. Proposed mechanism for increased insulin-mediated glucose transport in adipose cells from young, obese Zucker rats. Large intracellular pool of glucose transporters. J Biol Chem. 1985 Feb 25;260(4):2197–2201. [PubMed] [Google Scholar]
- Hedo J. A., Kahn C. R., Hayashi M., Yamada K. M., Kasuga M. Biosynthesis and glycosylation of the insulin receptor. Evidence for a single polypeptide precursor of the two major subunits. J Biol Chem. 1983 Aug 25;258(16):10020–10026. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptor: structure and function. Endocr Rev. 1981 Summer;2(3):251–263. doi: 10.1210/edrv-2-3-251. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Cuatrecasas P. Monensin blocks the maturation of receptors for insulin and somatomedin C: identification of receptor precursors. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1228–1231. doi: 10.1073/pnas.80.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Sasaki N., Kahn C. R., Nissley S. P., Rechler M. M. Antireceptor antibodies as probes of insulinlike growth factor receptor structure. J Clin Invest. 1983 Oct;72(4):1459–1469. doi: 10.1172/JCI111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen H. M., Vicario P. P., Mumford R. A., Green B. G. Evidence that the insulin-like activities of concanavalin A and insulin are mediated by a common insulin receptor linked effector system. Biochemistry. 1981 Sep 29;20(20):5800–5809. doi: 10.1021/bi00523a024. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Guillouzo A., Freychet P. Ultrastructual and biochemical studies of isolated adult rat hepatocytes prepared under hypoxic conditions. Cryopreservation of hepatocytes. Exp Cell Res. 1976 Mar 15;98(2):382–395. doi: 10.1016/0014-4827(76)90448-1. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Maddux B. A., Goldfine I. D. Regulation of insulin receptor kinase activity by insulin mimickers and an insulin antagonist. Biochem Biophys Res Commun. 1983 Aug 30;115(1):245–252. doi: 10.1016/0006-291x(83)90996-8. [DOI] [PubMed] [Google Scholar]
- Shia M. A., Pilch P. F. The beta subunit of the insulin receptor is an insulin-activated protein kinase. Biochemistry. 1983 Feb 15;22(4):717–721. doi: 10.1021/bi00273a001. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Stadtmauer L. A., Rosen O. M. Phosphorylation of exogenous substrates by the insulin receptor-associated protein kinase. J Biol Chem. 1983 Jun 10;258(11):6682–6685. [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Fujita-Yamaguchi Y., Larner J. Insulin-like effect of trypsin on the phosphorylation of rat adipocyte insulin receptor. J Biol Chem. 1983 Dec 25;258(24):14749–14752. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Kowalski A. Phosphorylation of the hepatic insulin receptor: stimulating effect of insulin on intact cells and in a cell-free system. FEBS Lett. 1982 Jul 5;143(2):179–182. doi: 10.1016/0014-5793(82)80094-x. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. Receptor-mediated phosphorylation of the hepatic insulin receptor: evidence that the Mr 95,000 receptor subunit is its own kinase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):945–949. doi: 10.1073/pnas.80.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Yu K. T., Werth D. K., Pastan I. H., Czech M. P. src kinase catalyzes the phosphorylation and activation of the insulin receptor kinase. J Biol Chem. 1985 May 10;260(9):5838–5846. [PubMed] [Google Scholar]
- Zick Y., Rees-Jones R. W., Taylor S. I., Gorden P., Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984 Apr 10;259(7):4396–4400. [PubMed] [Google Scholar]







