Abstract
It has been suggested that the infectious agents of scrapie and Creutzfeldt-Jakob disease (CJD) are 'prions' constituted by a protease resistant glycopeptide, PrP. To analyze the role of PrP in CJD infectivity we re-evaluated the biochemical characteristics of infectivity. First, when the infectious agent is not aggregated, infectivity is exquisitely sensitive to proteinase K treatment, and therefore a proteinase-K-resistant molecule (e.g. PrP) is unlikely to contain information essential for agent replication. Second, removal of sugar residues from Gp34 (the major precursor of the proteolyzed PrP band) failed to reduce infectivity. Third, one-half of the PrP peptides could be separated from significant infectivity using nondenaturing conditions with practical quantitative recovery of infectivity. These studies suggest that PrP in itself is unlikely to be the replicating component of the infectious agent. We suggest that these as yet undefined agents may consist of core protein and nucleic acid that are incompletely assembled in, and protected by, cell membranes. This hypothesis would explain the absence of conventional viral particles in these diseases, account for observed membrane pathology including altered behavior of endogenous membrane proteins, and would be consistent with the replication and transforming properties of CJD that indicate there is an agent specific nucleic acid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T., Cramp W. A., Haig D. A., Clarke M. C. Does the agent of scrapie replicate without nucleic acid? Nature. 1967 May 20;214(5090):764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Meyer R. K., Prusiner S. B. Scrapie PrP 27-30 is a sialoglycoprotein. J Virol. 1985 Feb;53(2):596–606. doi: 10.1128/jvi.53.2.596-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J. M. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Requirement of a protein component for scrapie infectivity. Intervirology. 1980;14(3-4):213–216. doi: 10.1159/000149185. [DOI] [PubMed] [Google Scholar]
- Czub M., Braig H. R., Diringer H. Pathogenesis of scrapie: study of the temporal development of clinical symptoms, of infectivity titres and scrapie-associated fibrils in brains of hamsters infected intraperitoneally. J Gen Virol. 1986 Sep;67(Pt 9):2005–2009. doi: 10.1099/0022-1317-67-9-2005. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Minor J. E., Hegarty J. D., Bhavanandan V. P. Interaction of sialoglycoproteins with wheat germ agglutinin-sepharose of varying ratio of lectin to Sepharose. Use for the purification of mucin glycoproteins from membrane extracts. J Biol Chem. 1986 Jun 15;261(17):7755–7761. [PubMed] [Google Scholar]
- Gibbons R. A., Hunter G. D. Nature of the scrapie agent. Nature. 1967 Sep 2;215(5105):1041–1043. doi: 10.1038/2151041a0. [DOI] [PubMed] [Google Scholar]
- Griffith J. S. Self-replication and scrapie. Nature. 1967 Sep 2;215(5105):1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Hunter G. D., Kimberlin R. H., Collis S., Millson G. C. Viral and non-viral properties of the scrapie agent. Ann Clin Res. 1973 Oct;5(5):262–267. [PubMed] [Google Scholar]
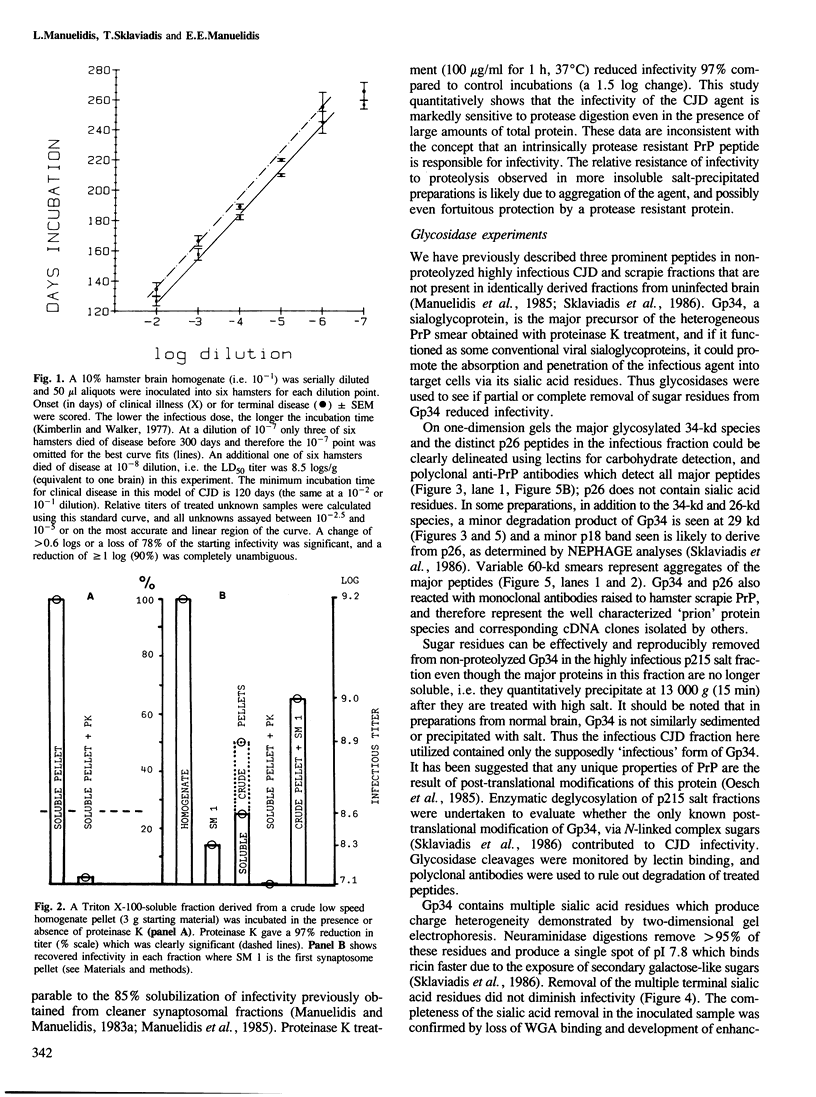
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Lax A. J., Millson G. C., Manning E. J. Involvement of protein in scrapie agent infectivity. Res Vet Sci. 1983 Mar;34(2):155–158. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacz E. J., Manuelidis L. Interspecies transmission of Creutzfeldt-Jakob disease to Syrian hamsters with reference to clinical syndromes and strains of agent. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3432–3436. doi: 10.1073/pnas.75.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Manuelidis E. E. Recent developments in scrapie and Creutzfeldt-Jakob disease. Prog Med Virol. 1986;33:78–98. [PubMed] [Google Scholar]
- Manuelidis L., Manuelidis E. E. Search for specific DNAs in Creutzfeldt-Jakob infectious brain fractions using "nick translation". Virology. 1981 Mar;109(2):435–443. doi: 10.1016/0042-6822(81)90515-8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Millson G. C., Hunter G. D., Kimberlin R. H. The physico-chemical nature of the scrapie agent. Front Biol. 1976;44:243–266. [PubMed] [Google Scholar]
- Multhaup G., Diringer H., Hilmert H., Prinz H., Heukeshoven J., Beyreuther K. The protein component of scrapie-associated fibrils is a glycosylated low molecular weight protein. EMBO J. 1985 Jun;4(6):1495–1501. doi: 10.1002/j.1460-2075.1985.tb03808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Oleszak E. L., Manuelidis L., Manuelidis E. E. In vitro transformation elicited by Creutzfeldt-Jakob-infected brain material. J Neuropathol Exp Neurol. 1986 Sep;45(5):489–502. doi: 10.1097/00005072-198609000-00001. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Groth D. F., Bowman K. A., Mock N. I., Cochran S. P., Masiarz F. R. Scrapie agent contains a hydrophobic protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6675–6679. doi: 10.1073/pnas.78.11.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Rohrschneider J. M., Diggelmann H., Ogura H., Friis R. R., Bauer H. Defective cleavage of a precursor polypeptide in a temperature-sensitive mutant of avian sarcoma virus. Virology. 1976 Nov;75(1):177–187. doi: 10.1016/0042-6822(76)90016-7. [DOI] [PubMed] [Google Scholar]
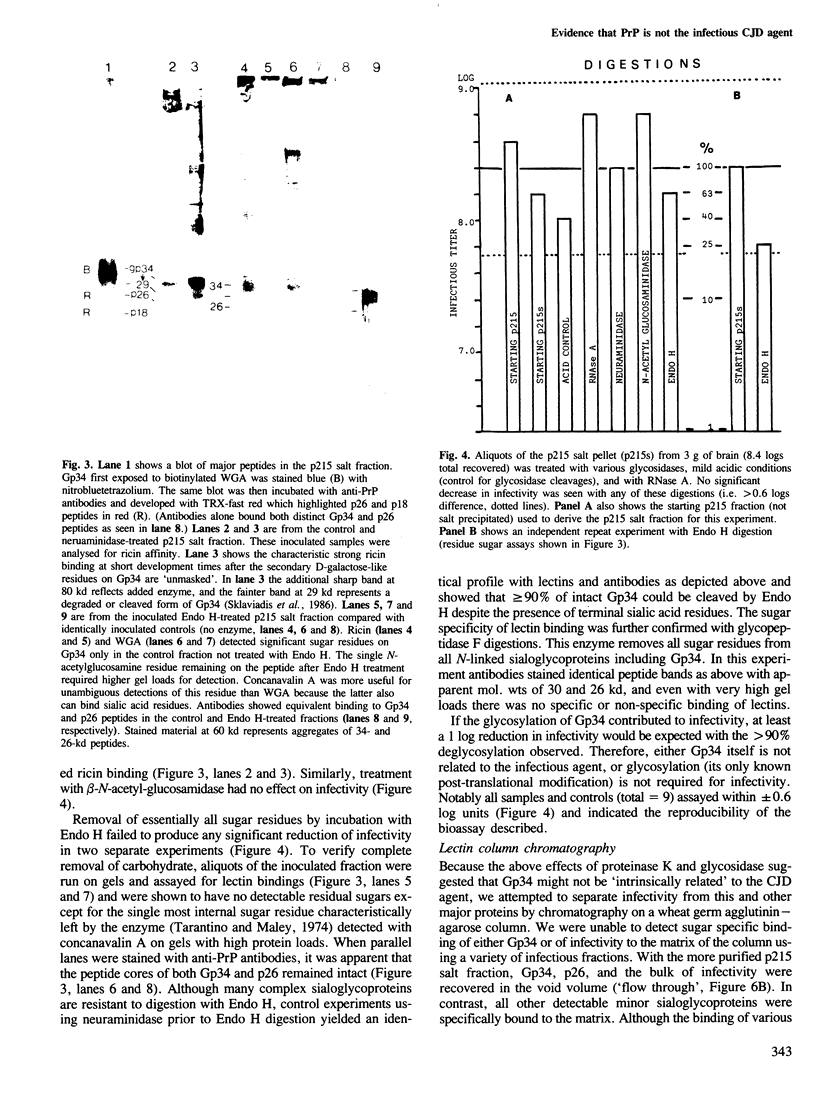
- Sklaviadis T., Manuelidis L., Manuelidis E. E. Characterization of major peptides in Creutzfeldt-Jakob disease and scrapie. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6146–6150. doi: 10.1073/pnas.83.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Zachary J. F., Knupp C. J., Wong P. K. Noninflammatory spongiform polioencephalomyelopathy caused by a neurotropic temperature-sensitive mutant of Moloney murine leukemia virus TB. Am J Pathol. 1986 Sep;124(3):457–468. [PMC free article] [PubMed] [Google Scholar]