Abstract
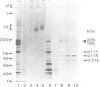
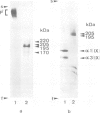
Here we describe the isolation and identification of a major cartilage glycoprotein which is co-extracted along with typical hyaline cartilage components such as collagen types II and IX from chicken embryo sternum. In polyacrylamide gel electrophoresis it migrates as a high molecular mass protein (greater than 10(6) daltons) which on reduction gives rise to a prominent doublet at 205/195 kd and minor bands at 220 and 170 kd. The intact molecule sediments as a 13S component in rate zonal centrifugation, indicative of a highly extended conformation in solution. In these properties it closely resembles myotendinous antigen, a glycoprotein recently detected in a number of embryonic tissues, including cartilage. This identity was confirmed by immunoblotting using a monoclonal antibody (M1) specific for myotendinous antigen. Electron micrographs of the rotary shadowed molecule revealed an unusual six-armed structure, indistinguishable in form and dimensions from hexabrachion, a recently discovered contaminant of cellular fibronectin preparations. These structures could be decorated with the M1-antibody, demonstrating that hexabrachion is myotendinous antigen. This extended, potentially multivalent molecule could provide an ideal substrate to connect widely spaced components in a highly hydrated tissue such as cartilage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruckner P., Mayne R., Tuderman L. p-HMW-collagen, a minor collagen obtained from chick embryo cartilage without proteolytic treatment of the tissue. Eur J Biochem. 1983 Nov 2;136(2):333–339. doi: 10.1111/j.1432-1033.1983.tb07746.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Vaughan L., Winterhalter K. H. Type IX collagen from sternal cartilage of chicken embryo contains covalently bound glycosaminoglycans. Proc Natl Acad Sci U S A. 1985 May;82(9):2608–2612. doi: 10.1073/pnas.82.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Laurie G. W., Cannon F. B., Martin G. R., Kleinman H. K. In vitro regulation of cartilage matrix assembly by a Mr 54,000 collagen-binding protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5126–5130. doi: 10.1073/pnas.83.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chiquet M., Fambrough D. M. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol. 1984 Jun;98(6):1926–1936. doi: 10.1083/jcb.98.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Fambrough D. M. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol. 1984 Jun;98(6):1937–1946. doi: 10.1083/jcb.98.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Carrell N., McDonagh J. Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J Cell Biol. 1981 Dec;91(3 Pt 1):673–678. doi: 10.1083/jcb.91.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Inglesias J. L. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature. 1984 Sep 20;311(5983):267–269. doi: 10.1038/311267a0. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Crossin K. L., Edelman G. M. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt A. T., Kleinman H. K., Pennypacker J. P., Martin G. R. Identification of an adhesion factor for chondrocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):385–388. doi: 10.1073/pnas.77.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt A. T., Varner H. H., Silver M. H., Dessau W., Wilkes C. M., Martin G. R. The isolation and partial characterization of chondronectin, an attachment factor for chondrocytes. J Biol Chem. 1982 Mar 10;257(5):2330–2334. [PubMed] [Google Scholar]
- Huber S., van der Rest M., Bruckner P., Rodriguez E., Winterhalter K. H., Vaughan L. Identification of the type IX collagen polypeptide chains. The alpha 2(IX) polypeptide carries the chondroitin sulfate chain(s). J Biol Chem. 1986 May 5;261(13):5965–5968. [PubMed] [Google Scholar]
- Kielty C. M., Kwan A. P., Holmes D. F., Schor S. L., Grant M. E. Type X collagen, a product of hypertrophic chondrocytes. Biochem J. 1985 Apr 15;227(2):545–554. doi: 10.1042/bj2270545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller-Glauser W., Humbel B., Glatt M., Sträuli P., Winterhalter K. H., Bruckner P. On the role of type IX collagen in the extracellular matrix of cartilage: type IX collagen is localized to intersections of collagen fibrils. J Cell Biol. 1986 May;102(5):1931–1939. doi: 10.1083/jcb.102.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. A short chain (pro)collagen from aged endochondral chondrocytes. Biochemical characterization. J Biol Chem. 1983 Aug 10;258(15):9504–9509. [PubMed] [Google Scholar]
- Shotton D., Burke B., Branton D. The shape of spectrin molecules from human erythrocyte membranes. Biochim Biophys Acta. 1978 Sep 26;536(1):313–317. doi: 10.1016/0005-2795(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner H. H., Furthmayr H., Nilsson B., Fietzek P. P., Osborne J. C., Jr, De Luca S., Martin G. R., Hewitt A. T. Chondronectin: physical and chemical properties. Arch Biochem Biophys. 1985 Dec;243(2):579–585. doi: 10.1016/0003-9861(85)90535-1. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Winterhalter K. H., Bruckner P. Proteoglycan Lt from chicken embryo sternum identified as type IX collagen. J Biol Chem. 1985 Apr 25;260(8):4758–4763. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui N., Benya P. D., Nimni M. E. Identification of a large interrupted helical domain of disulfide-bonded cartilage collagen. J Biol Chem. 1984 Nov 25;259(22):14175–14179. [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of a precursor form of M collagen from embryonic chicken cartilage. Eur J Biochem. 1984 Feb 1;138(3):629–633. doi: 10.1111/j.1432-1033.1984.tb07961.x. [DOI] [PubMed] [Google Scholar]