Excessive herbivory by native deer can reduce abundance of native vegetation and increase non-native vegetation, but how does the forest recover when deer are excluded or numbers are reduced? The expectation is that understory vegetation will return to a previously diverse condition, but this is not always the case. We found that community-level monitoring captured both change and lack of change in vegetation cover, height, species richness, and response of common species, but failed to capture response of uncommon species. In contrast, monitoring of target species favored by deer indicated increased plant size and reproduction when deer were reduced or excluded. We conclude that measuring individual plants (growth, flowering and reproductive success) provides a sensitive and powerful assessment of forest understory responses to deer management.
Keywords: Deer herbivory, deer management, earthworms, forest understory, invasive species, multiple stressors, plant community
Abstract
Excessive herbivory can have transformative effects on forest understory vegetation, converting diverse communities into depauperate ones, often with increased abundance of non-native plants. White-tailed deer are a problematic herbivore throughout much of eastern North America and alter forest understory community structure. Reducing (by culling) or eliminating (by fencing) deer herbivory is expected to return understory vegetation to a previously diverse condition. We examined this assumption from 1992 to 2006 at Fermilab (Batavia, IL) where a cull reduced white-tailed deer (Odocoileus virginianus) abundance in 1998/1999 by 90 % from 24.6 to 2.5/km2, and at West Point, NY, where we assessed interactive effects of deer, earthworms, and invasive plants using 30 × 30 m paired fenced and open plots in 12 different forests from 2009 to 2012. We recorded not only plant community responses (species presence and cover) within 1 m2 quadrats, but also responses of select individual species (growth, reproduction). At Fermilab, introduced Alliaria petiolata abundance initially increased as deer density increased, but then declined after deer reduction. The understory community responded to the deer cull by increased cover, species richness and height, and community composition changed but was dominated by early successional native forbs. At West Point plant community composition was affected by introduced earthworm density but not deer exclusion. Native plant cover increased and non-native plant cover decreased in fenced plots, thus keeping overall plant cover similar. At both sites native forb cover increased in response to deer reduction, but the anticipated response of understory vegetation failed to materialize at the community level. Deer-favoured forbs (Eurybia divaricata, Maianthemum racemosum, Polygonatum pubescens and Trillium recurvatum) grew taller and flowering probability increased in the absence of deer. Plant community monitoring fails to capture initial and subtle effects of reduced or even cessation of deer browse on browse sensitive species. Measuring responses of individual plants (growth, flowering and reproductive success) provides a more sensitive and powerful assessment of forest understory responses to deer management.
Introduction
Conservation successes should be celebrated, but the acclaim is muted when recovery of a species is so successful that its abundance threatens other species. Adaptable native and introduced wild or feral species (including browsers and grazers such as deer and kangaroos) thrive in landscapes transformed by humans over the past two centuries in Europe, Australia, Japan, New Zealand and North America (Forsyth et al. 2010; Tanentzap et al. 2012; Foster et al. 2014; Howland et al. 2014; Perea et al. 2014; Iijima and Ueno 2016).
The success of white-tailed deer (Odocoileus virginianus) in North America illustrates a conservation success gone awry. Hunted to near extinction by the late 1800s, the species made such a remarkable comeback that large populations quickly created economic and ecological problems (Leopold et al. 1947; Severinghouse and Brown 1956; Halls 1984). Scientists initially focused on selective effects of deer, primarily on valuable timber species (Habeck 1959; Frelich and Lorimer 1985; Horsley et al. 2003). While some browsing can have beneficial effects on plant diversity by reducing the competitive ability of some fast growing species (Leonardsson et al. 2015), large ungulate populations often create simplified and less diverse ecosystems.
In the past decades, ecologists have documented cascading negative impacts of large deer populations (Porter and Underwood 1999; McShea and Rappole 2000; Côté et al. 2004; Griggs et al. 2006). These effects percolate through food webs affecting directly the plant species eaten, and indirectly species such as insects, birds and decomposers, that are dependent upon the diversity of primary producers for their own existence and livelihoods (Wardle and Bardgett 2004; McGraw and Furedi 2005; Martin et al. 2011; Nuttle et al. 2011; Schweitzer et al. 2014; Chips et al. 2015). This is not necessarily a new phenomenon because problems in Europe and North America have been reported for nearly a century or longer (Leopold et al. 1947; Schabel 2001; Ammer et al. 2010).
The complexities and interconnectedness of local and regional ecosystems, as well as the many potential drivers of change (climate, invasive species, trophic downgrading, etc.) (Tylianakis et al. 2008; Estes et al. 2011; Craven et al. 2016), obscure the current and future implications of ungulate impacts. Maintaining ecosystem function and ecosystem services requires high species diversity in multiple trophic groups including primary producers, above-ground insect herbivores and soil decomposers (Soliveres et al. 2016; van der Plas et al. 2016), organisms that are particularly negatively affected by high ungulate browse pressure (Côté et al. 2004).
Around the world foresters and managers have responded to high ungulate browse pressure by culling ungulate populations, or by fencing endangered plant populations and conservation areas. These are stopgap measures that can provide (at least in the case of trees) a temporary reprieve until increased height ensures survival of individuals. No such spatial escape exists for the vast majority of herbaceous plants that represent the staple of deer diets in spring and summer, and that are under particular threat (Williams et al. 2000; Fletcher et al. 2001; McGraw and Furedi 2005; Wiegmann and Waller 2006; Wang and Mopper 2008; Knight et al. 2009a; Frerker et al. 2014).
It is widely assumed that reduction or cessation of high deer browse pressure will result in the recovery of understory plant communities but field tests of these assumptions are rare (Tanentzap et al. 2011). When hunting, culling and fencing are used to address excessive deer browse, managers typically, at least in hunting and culling efforts, report the number of deer removed or the resulting deer population density (DeNicola 2008) (but see Wright et al. 2012). However, the extent to which vegetation recovery occurs is less often measured. Furthermore, composition and abundance of understory vegetation are not necessarily linked to changes in deer abundance (Tanentzap et al. 2011).
An important concern is the long-term impact or legacy effects of high deer populations that act in concert with other stressors on local species assemblages and ecosystems. This question has received less attention, although indirect or non-consumptive effects of white-tailed deer, including effects on introduced invasive plants or earthworms, are now being recognized (Knight et al. 2009b; Heckel et al. 2010; Kalisz et al. 2014; Dávalos et al.2015a, b, c). Such perturbations may shift ecoystems into alternative stable states where restoration of a state resembling pre-perturbation conditions may be difficult or impossible (Beisner et al. 2003; Suding et al. 2004; Suding 2011). Persistent and excessive deer herbivory may create such a regime shift by simplifying primary producer communities setting forests on depauperate long-term trajectories (Nuttle et al. 2011, 2014) and affecting ecosystem processes, such as decomposition (Wardle and Bardgett 2004) and microbial community composition, that in turn affect plant growth and potentially net primary productivity (Kardol et al. 2014)
We were particularly interested to assess the response of herbaceous understory vegetation to deer management in the presence of other persistent stressors, such as introduced plants and non-native earthworm invasions. We used a 90 % reduction in the deer herd at Fermilab, IL, USA and an exclosure network at West Point, NY, USA, to test the following hypotheses:
Reduced deer browse pressure will result in recovery of forest understory plant community.
Reduced deer browse pressure will result in recovery of species highly favoured by deer.
Deer browse pressure overwhelms negative effects of other co-occurring stressors, specifically non-native plants and non-native earthworms, on native plant species performance
Methods
Fermilab
Site
U.S. DOE Fermilab National Environmental Research Park (hereafter Fermilab) is located 60 km west of Chicago, in Kane County, IL, USA and contains a mixture of forests, oldfields, restored prairies and active farm lands on 27.5 km2. We selected the largest forest on the site, a 31.6-ha mesic forest (“Big Woods”) dominated by Tilia americana, Fraxinus americana, Quercus macrocarpa and Q. alba, and known for extensive spring displays of Trillium flexipes and T. recurvatum. The forest had been grazed some time prior to establishment of the Research Park. A rapidly expanding deer herd and concomitant decline in Trillium floral displays prompted research to assess deer impacts on understory vegetation. White-tailed deer densities averaged 11/km2 in 1993, and 24.6/km2 in 1998, according to winter aerial surveys. Deer densities were reduced ∼90 % in winter 1998/1999 and were subsequently maintained using sharpshooters at approximately the same density (2.5–6/km2) each year thereafter.
Vegetation data
Community: In 1992 we divided the forest into five 90-m wide units, each with a randomly established permanent transect (420–560 m). In May of even-numbered years (1992–2006) we recorded species presence, and estimated percent cover for each species <1 m tall in 17 cover categories (midpoints: 0.01, 0.2, 0.5, 1, 3, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 98 and 100 %) in 1 m2 quadrats randomly located within contiguous 20 m intervals along each transect (121–129 quadrats each year). Plant species nomenclature and native/non-native origin follows the USDA Plants Database (USDA NRCS 2017). Beginning in 1998 we measured “average” vegetation height to the nearest cm. While this is a quantitative measurement of a qualitative feature, all measurements were made in a consistent manner, and gross differences are assumed to be indicative of true height differences. In 2004 and 2006 we recorded species presence and height in all quadrats, and percent cover of individual species in alternate quadrats (69 and 65, respectively). We summed cover of all species within a quadrat using cover class midpoint values: thus, total cover could exceed 100 % due to layering. We classified each species by origin (native or non-native) and life form (annual and biennial forb combined, hereafter referred to as (bi)annual, perennial forb, graminoid, and woody). Graminoids included all grass-like plants (Poaceae, Cyperaceae and Juncacaeae). Ferns were so infrequent that they were combined with perennial forbs.
Individual species: Beginning in May 1998 and prior to the initial deer cull we collected demographic data for T. recurvatum to supplement frequency and cover data. We recorded stem density, stem height (cm; measured to the junction of the three leaves or to the leaf base), life form (sterile, fertile, one leaf), and presence of browse on each stem within each quadrat.
West Point
Site
US Army Garrison West Point (hereafter West Point) is a 65-km2 site located on the west bank of the Hudson River some 80 km north of New York City, NY, USA. This facility is located in the Hudson Highlands Province, a landscape characterized by steep hills, thin soils and rocky outcrops. We selected 12 upland deciduous forests (1–8 km apart from each other) dominated by oak (Q. rubra and Q. prinus) and/or sugar maple (Acer saccharum); sites differed in land use history, aspect, soil and understory vegetation. Our investigations focused, in part, on the importance of non-native invasive plants, non-native invasive earthworms and their individual, additive or synergistic interactions with white-tailed deer and the resulting impacts on native plant species. We therefore selected 12 sites based on site invasion: 6 sites based on presence and abundance of 3 non-native plant species (Alliaria petiolata, Berberis thunbergii, Microstegium vimineum; two sites each) and six sites that had few or no non-native plant species present (please see Table 1 in Dávalos et al. 2015c). At each site we established two paired 30 m × 30 m plots, 5–50 m apart, with similar overstory and understory composition of which one was randomly selected to be fenced. In early July 2008 we constructed deer-proof 2.6 m high fences (Millennium plastic deer fence, deerBusters.com).
Table 1.
Effects of study year, plant species origin (O; native, non-native), earthworm density (E; low, high) and fencing (F; open, fenced) on vegetation cover (%) at 12 sites at West Point, NY from 2009 to 2012 according to linear mixed-model. Model included site, plot within site and quadrat within plot as random factors. Non-significant study factors were dropped from selected models and not included in the table.
| Factor | Cover |
|||
|---|---|---|---|---|
| Estimate* | SE* | χ2 (df = 1)** | P** | |
| Intercept | 0.046 | 0.008 | ||
| Year | 0.002 | 0.001 | ||
| Origin (non-native) | −0.035 | 0.003 | ||
| Earthworm (high) | 4.69E−04 | 0.009 | ||
| Fencing (open) | −0.008 | 0.002 | ||
| O × Y | −0.003 | 0.001 | 7.35 | 0.01 |
| O × E | 0.020 | 0.003 | 59.42 | <0.001 |
| O × F | 0.014 | 0.002 | 33.05 | <0.001 |
Est Estimate; SE estimate standard error.
Estimates and standard errors are reported from the model fitted with restricted maximum likelihood.
Chi-squared statistics and P-values are from likelihood ratio tests with each parameter removed from the maximum likelihood-based model, with all other parameters retained. It was not possible to test the significance of all terms because of higher order interactions.
Vegetation data
Community: We established ten 1 m2 permanent quadrats in a stratified random manner in each fenced and open plot in July 2008 (11 sites) or May 2009 (1 site) (12 sites × 2 plots per site × 10 quadrats per plot = 240 vegetation quadrats). Within each quadrat we recorded species presence, and estimated the percent cover for each species <1 m tall in 17 cover categories (midpoints: 0.01, 0.2, 0.5, 1, 3, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 98 and 100 %). Plant species nomenclature and native/non-native status follows USDA Plants (USDA NRCS 2017). We recorded data twice a year, in mid-May (2009–2012) and late July (2008–2012), and again in May 2016. We estimated vegetation height by measuring the “average” height of vegetation at four locations within each quadrat, and then averaging these heights for each quadrat. We classified each species by origin and life form, and summed cover of individual plant species as described above under Fermilab.
Individual species: We selected three native perennial plant species favoured by deer (Eurybia divaricata, Maianthemum racemosum and Polygonatum pubescens) in 2012 for additional study. We assessed all sites for presence of each species but collected data only at sites where we could find a minimum number of individuals in both open and fenced plots (2 sites for M. racemosum and P. pubescens [25 plants/plot minimum] and 6 sites for E. divaricata [50 plants/plot minimum]).
In mid-June 2012 we randomly located four 2 m × 30 m belt transects in each fenced plot at sites 5 and 8 and recorded stem height (cm, measured from ground to highest leaf node), width (cm) of the widest leaf, and reproductive status of each M. racemosum and P. pubescens. We found very few (or no) plants in belt transects in open plots; therefore we randomly established additional transects near open plots for a total of eight belt transects at site 5 in 2012 (seven transects in 2016) and nine transects at site 8. We repeated these measures in approximately the same locations in late May 2016.
In mid-September 2012 we recorded density, height, and reproductive status of each E. divaricata in the 10 permanent 1 m2 quadrats in each open and fenced plot at the six sites that contained this species. If E. divaricata was not present in the initial quadrat location, we searched for the closest E. divaricata, and then placed the quadrat with the plant in one corner and shifted to maximize number of plants in the re-established quadrat. Thus, we recorded frequency and cover in initial quadrats, and plant vigor in initial and additional quadrats.
Earthworm monitoring
In late July 2008–2011 we randomly selected 5 of the 10 vegetation quadrats within each open and fenced plot, stratified equally across the plot, and sampled earthworms within a 0.25-m2 frame placed 1 m distant from the vegetation quadrat. To ensure equal sampling of plots over time, we rerandomized sample locations each year. We removed and sifted all leaf litter, capturing any earthworms, then firmly placed a metal frame (10 cm × 25 cm × 25 cm) on the ground. We then poured 3.79 L of aqueous mustard solution (15 g L−1; Frontier Natural Products Co-op, Norway, IA) evenly across the frame area, and collected all surfacing earthworms. We preserved specimens in 10 % formalin before transferring to 70 % ethanol, then weighed and identified each individual to species (sexually mature worms) or genus (immature worms). Sites varied in earthworm abundance and species composition, naturally grouping into either ‘high’ (12.4 ± 1.78 individuals and 8.6 ± 1.67 g per 0.25 m2; means ± 1 SE: eight sites) or ‘low’ (1.4 ± 0.37 individuals and 0.88 ± 0.36 g per 0.25 m2; means ± 1 SE: four sites) earthworm abundance based on means from 2008 to 2011 (Dávalos et al. 2015c). We only recorded non-native earthworm species. Common genera included Amynthas, Dendrobaena and Lumbricus.
Statistical Analyses
We evaluated effect of deer reduction on understory plant communities at Fermilab by comparing cover, species richness and vegetation height values pre (before 1998) and post deer reduction [a fixed factor with two levels (before and after): GLMMs with study year as a random factor to account for temporal correlation within years]. Samples within Fermilab (all at one site) were considered independent. At both Fermilab and West Point we classified each species by origin (native or non-native) and life-form (annual and biennial forb, perennial forb, graminoid [Poaceae, Cyperaceae and Juncacaeae], ferns and fern allies, and woody). Also for both studies, we transformed cover values (arcsine squared-root) and species richness (log) in order to meet model assumptions.
West Point analyses are based on maximum species cover between May and July for each sampling year (2009–2012). We used Linear Mixed Models (LMM) to evaluate effects of year, site invasion (sites selected based on presence and abundance of three non-native plant species), fencing (fenced or open), earthworm density (low or high based on earthworm monitoring results) and their interactions on total cover, species richness and diversity indexes. We dropped non-significant factors from the final model. We tested second level interactions only, excluding the site invasion × earthworm density interaction as we only had one site dominated by non-native vegetation with low earthworm density. We applied a second set of LMM models to evaluate effects of year, fencing, earthworm density, plant species origin, and their interactions on total cover and cover by life form (different models for each life form). We did not evaluate site invasion in models that separated species cover by lifeform as site invasion and plant species origin are not independent: Sites classified as “invaded” will, by definition, have higher cover of non-natives. All models included site, plot within site and quadrat within plot as random factors in order to reflect the hierarchical nature of the data. Including random factors allowed us to control for possible pseudoreplication while preserving the spatial variation contained in data collected among quadrats within the same plot (Millar and Anderson 2004; Schank and Koehnle 2009).
To evaluate significance of explanatory factors, we started with a full model and then compared it with a model without the tested factor via a log likelihood test. All chi-square statistics and associated P-values in the results section refer to log likelihood test results. We checked assumptions of all models at each step of the model procedure. We fitted all mixed models with package lme4 (Bates et al. 2014) in statistical package R (R Core Team 2016).
We assessed plant community composition at both Fermilab and West Point with non-metric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PerMANOVA) including site and plot within site as strata (Anderson 2001). We fitted NMDS and PerMANOVA with the metaMDS and adonis functions, respectively, in R package vegan (Oksanen et al. 2012). We used Bray-Curtis distance matrices, based on log-transformed species abundance data for both procedures. We fitted our study factors [deer exclusion (West Point) or reduction (Fermilab), and earthworm abundance and site invasion (at West Point only)] to the ordination (NMDS) using the envirofit function, which provides a goodness of fit and p-value via a permutation procedure. We only included species with maximum cover higher than 0.01 % and present at least 10 times over the study period.
We conducted separate analyses for May 2016 data and did not evaluate temporal effects because we excluded two sites due to fence damage. We evaluated effects of study factors on percent cover with ANOVA using median vegetation cover across quadrats within a plot (N = 10) as response.
We used Linear Mixed Models to evaluate effects on individual species cover and height and Generalized Linear Mixed Models with binomial errors to evaluate effects on species frequency or flowering probability. Trillium recurvatum models at Fermilab evaluated changes before and after deer reduction (a fixed factor with two levels [before and after]) and included sampling year as a random factor to account for temporal correlation within years. Models for West Point evaluated the effects of fencing, earthworm density and site invasion and included site and plot within site as random factors.
Results
Fermilab
Community
Cover and vegetation height
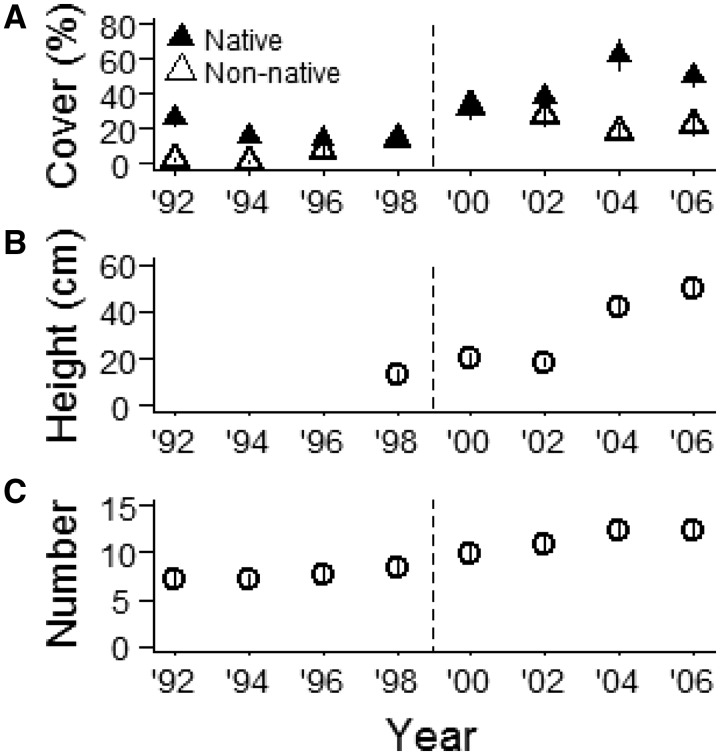
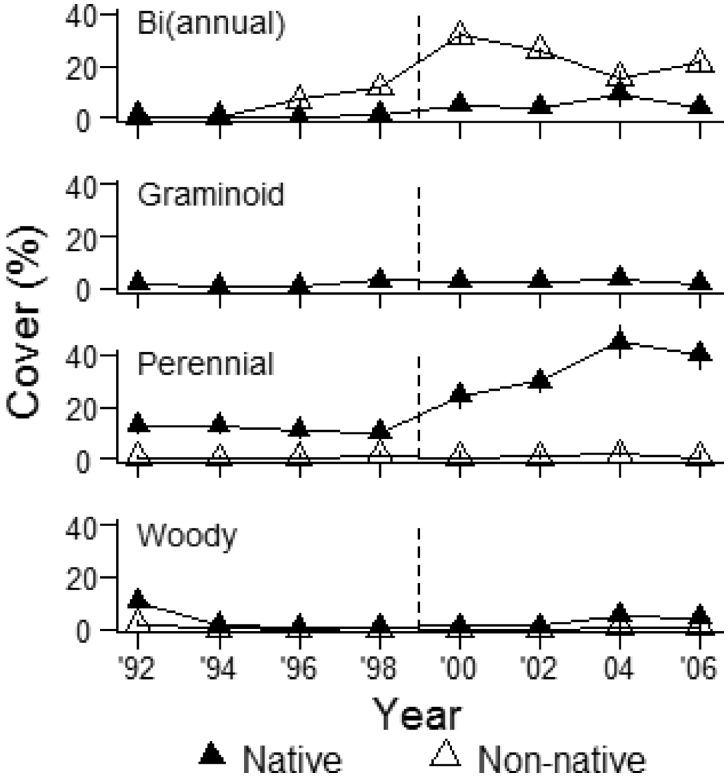
Total cover increased significantly after deer reduction, and cover of native plants increased at a significantly faster rate than cover of non-native plants (significant year × origin interaction; Est ± 1SE: 4.8 ± 2.3, t = 2.07, χ2 = 4.3, df = 1, P = 0.04; Fig. 1A). The majority of native cover was contributed by perennial forbs, which increased significantly after deer reduction (Fig. 2; significant year × origin × deer reduction interaction; Est ± 1SE: 0.005 ± 0.0006, t = 6.8, χ2 = 46.3, df = 1, P < 0.001). Cover of non-native perennial forbs did not vary over time. Cover of non-native (bi)annuals was significantly higher than native (bi)annuals. While native (bi)annual cover slightly increased after deer reduction, non-native (bi)annual cover peaked the year after deer reduction and then steadily decreased over time (Fig. 2; significant deer reduction × year interaction; χ2 = 17.18, df = 1, P < 0.001). Over 99 % of non-native (bi)annual cover was contributed by a single species, A. petiolata. We only encountered native graminoid species and their cover was similar before and after deer reduction (Fig. 2; P > 0.05). Native woody cover was higher than non-native woody cover, and increased after deer reduction while non-native woody cover remained stable (Fig. 2; significant year × origin × deer reduction interaction; Est ± 1SE: 0.004 ± 0.0004, t = 9.9, χ2 = 96.2, df = 1, P < 0.001). Vegetation height significantly increased after deer reduction, but we observed a lagged response (Fig. 1B; significant deer reduction × year interaction; χ2 = 9.9, df = 1, P = 0.002).
Figure 1.
Native and non-native vegetation cover (%) (A), vegetation height (B) and number of species (C) in spring 1992–2006 at Fermilab, IL (N = 65–130 one-m2 quadrats). Dotted line indicates deer reduction from 24.6 to 2.5 deer per km2 in 1998/1999. Data represent means ± 1SE.
Figure 2.
Native and non-native vegetation cover (%) according to life form in spring 1992–2006 at Fermilab, IL (N = 65–130 one-m2 quadrats). Dotted line indicates deer reduction from 24.6 to 2.5 deer per km2 in 1998/1999. Data represent means ± 1SE.
Species richness and diversity indexes
Over the 14-year study period we recorded a total of 153 species (13 graminoids, 21 (bi)annuals, 2 ferns, 82 perennial forbs, and 35 woody species), with 77–102 species recorded/year. The majority of species (86 %) were native. Species richness from 1992 to 2006 averaged 9.1 ± 0.15 species per 1 m2 quadrat and significantly increased from an average of 7.0 species per quadrat in 1992 to 12.2 species per quadrat in 2006 (+74 %; Fig. 1C). We found a significant effect of deer reduction and a significant interaction between deer reduction and study year (Est ± 1SE: 0.04 ± 0.015, t = 2.67, χ2 = 7.1, df = 1, P = 0.008).
Diversity (Shannon index) per quadrat significantly increased from 1.8 ± 0.06 in 1992 to 2.4 ± 0.06 in 2006 and was significantly higher after deer reduction (significant deer reduction × year interaction; χ2 = 6.9, df = 1, P = 0.008). Similarly evenness (Pielou’s index) significantly increased from 0.87 ± 0.02 in 1992 to 0.95 ± 0.02 in 2006 and was significantly higher after deer reduction (significant deer reduction effect; Est ± 1SE: 0.07 ± 0.01, t = 7.04, χ2 = 20, df = 1, P < 0.001).
Community composition
PerMANOVA results indicated that species composition differed according to study year (F1,884 = 10.21, R2 = 0.05; P = 0.01), deer reduction (F1,884 = 56.18, R2 = 0.05; P = 0.01) and the interaction between year and deer reduction (F1,884=17.3, R2=0.02; P=0.01). Study factors, although significant, explained a small percentage of the variation (indicated by R2 values). NMDS ordination indicated a difference in vegetation composition before and after deer reduction (Fig. 3). Both study year and deer reduction had a significant goodness of fit (R2=0.23, P < 0.001 and R2=0.1, P < 0.001, respectively). Changes were driven by the six most abundant species: non-native A. petiolata and five native early-successional perennial forbs (Circaea lutetiana, Geum canadense, Laportea canadense, Polygonum virginianum and Viola sororia). Collectively, these six species contributed over 50 % of total cover following deer reduction.
Figure 3.
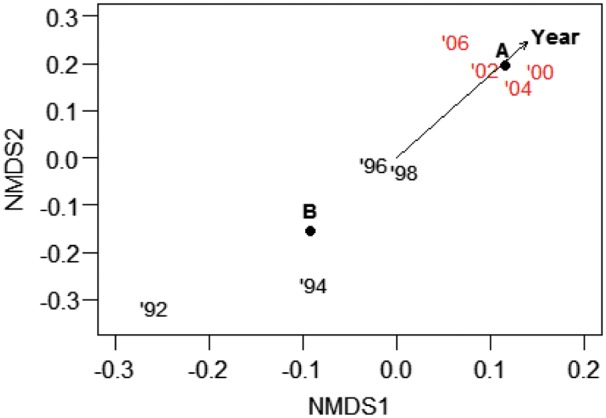
Non-metric multidimensional scaling (NMDS) of the understory vegetation layer in spring 1992–2006 at Fermilab, IL (N = 65-130 one-m2 quadrats). Red year numbers indicate monitoring conducted after deer reduction from 24.6 to 2.5 deer/km2 in 1998/9. Year, a continuous factor, is represented by a vector (arrow) and deer reduction, a categorical factor indicating before (B) or after (A) the deer cull, by its compositional centroid. Points are jittered to allow visualization.
Individual species
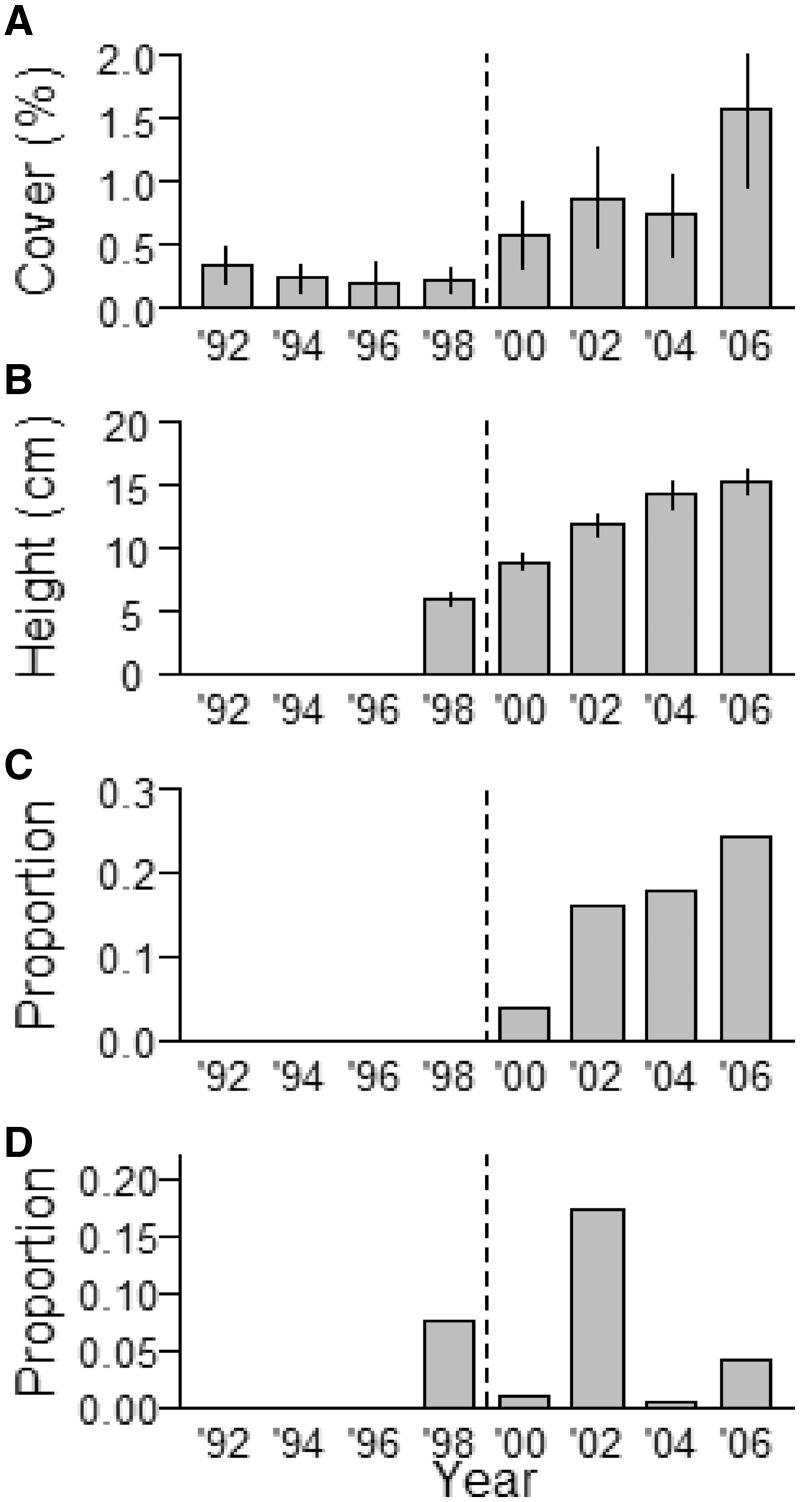
Trillium recurvatum cover significantly increased (Est ± 1SE: 0.02 ± 0.007, t = 3.3, χ2=8.4, df = 1, P = 0.004) after deer reduction from 0.25 % (average 1992–1998) to 0.93 % (average 2000–2006), reaching maximum cover in 2006 (1.6 %; Fig. 4A). Frequency of quadrats with T. recurvatum increased from 26.4 % in 1992 to 33.6 % in 2006, but the increase was not significant (z = 1.5, P = 0.13). We measured a total of 1142 T. recurvatum individual stems over an 8-year period (1998–2006). After deer reduction in 1998/1999, stems became significantly taller (Fig. 4B, Est ± 1SE: −7.9 ± 3.5, t=−2.2, χ2=4.5, df = 1, P = 0.03) and had significantly higher flowering probability (Fig. 4C; Est ± 1SE: 0.4 ± 0.5, z = 7.33, P < 0.001). The proportion of browsed individuals varied dramatically from year to year, but we found no significant effect of deer reduction (Fig. 4D;z = 0.75, P = 0.45).
Figure 4.
Trillium recurvatum percent cover (A), height (B) (cm; mean ± 1 SE), proportion of flowering individuals (C) and proportion of browsed individuals (D) in spring 1998–2006 at Fermilab, IL (N = 121–128 one-m2 quadrats). No individuals flowered in 1998 (C). Dotted line indicates deer reduction from 24.6 to 2.5 deer per km2 treatment in 1998/1999. We measured cover from 1992 on and other parameters from 1998.
West Point
Community
Cover and vegetation height
Total cover (averaged across all plots and treatments) significantly increased from 46 % (2009) to 51 % (2012; year effect ± SE: 0.001 ± 0.0004; χ2=6.88; df = 1; P=0.008), but did not differ between open and fenced plots, or between sites with high and low earthworm density. Total cover was significantly higher at sites dominated by non-native vegetation than at sites dominated by native vegetation (site invasion effect ± SE: −0.032 ± 0.01; χ2=7.2; df = 1, P = 0.007).
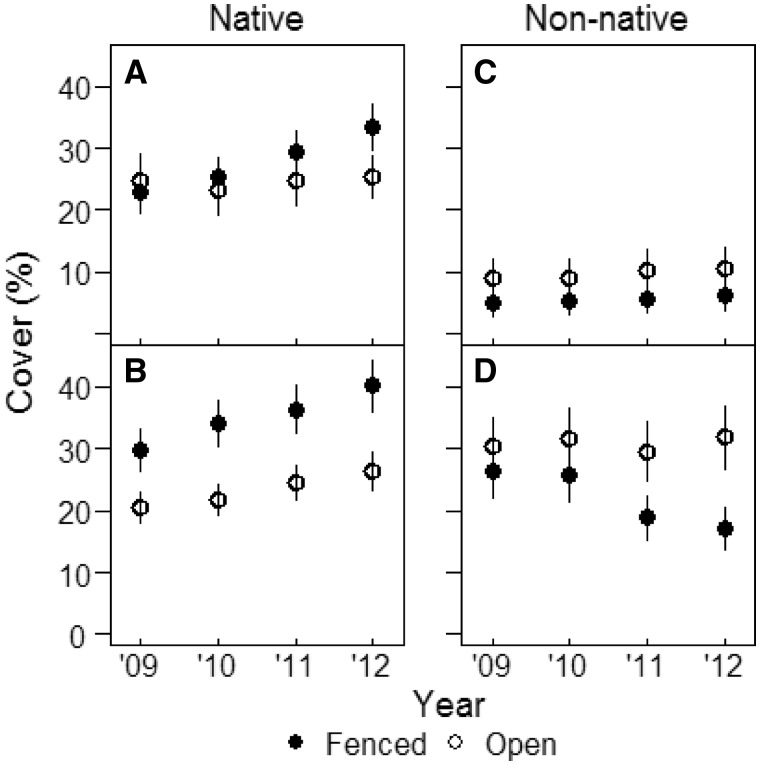
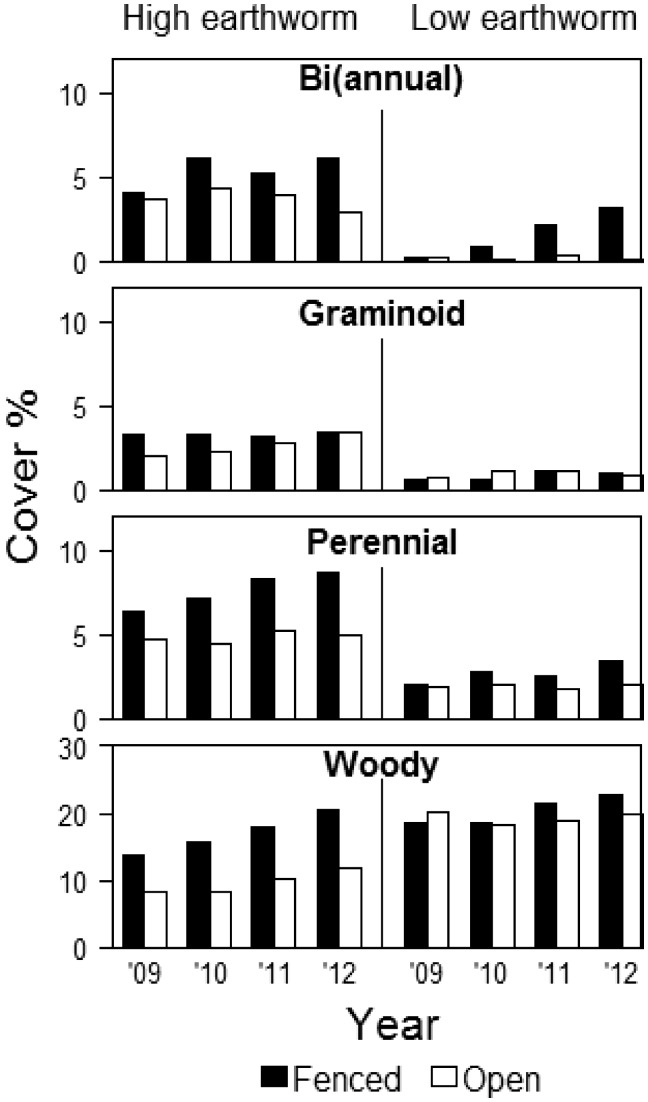
Proportion of cover contributed by native vs non-native plant species shifted over the study period, influenced by both fencing and non-native earthworm abundance. Native plant species cover significantly increased from 2009 to 2012, whereas non-native plant species cover remained stable (significant origin × year interaction; Table 1). Five years after fencing, native cover was significantly higher in fenced than open plots whereas non-native cover was significantly higher in open than fenced plots (significant origin × fencing interaction; Table 1). Non-native cover was significantly higher at high earthworm density sites than at low earthworm density sites, but native cover was similar between low and high earthworm sites (significant origin × earthworm interaction; Fig. 5; Table 1).
Figure 5.
Native (left) and non-native (right) vegetation cover (%) in open and fenced plots (N = 10 one-m2 quadrats per plot) at sites with low (A and C) and high (B and D) earthworm density at West Point, NY from 2009 to 2012. Data represent means ± 1SE (N = 4 and 8 sites with low and high earthworm density, respectively).
Analyses of native cover by life form indicated significant interactions between year, fencing, plant species origin and earthworm density (Table 2). Over the 4 years, averaged across all plots and treatments, native (bi)annual cover (3.31 %) was significantly higher than non-native (bi)annual cover (1.02 %), and native (bi)annual cover tended to be higher at high earthworm sites (3.02 %) than at low earthworm sites (0.50 %; Fig. 6). Native graminoid cover (2 % averaged across all years and treatments) was significantly lower than non-native graminoid cover (9 % on average per quadrat). Both native and non-native graminoid cover were significantly higher at high earthworm density sites than at low earthworm density sites (Fig. 6). We found significant interactions between all study factors (Table 2). Over time, native graminoid cover remained stable in open and fenced plots, whereas non-native graminoid cover decreased in fenced plots (from 9 to 3 %).
Table 2.
Model results for the effects of study year (Y), plant species origin (O; native, non-native), earthworm density (E; low, high) and fencing (F; open, fenced) on vegetation cover (%) according to life form at 12 sites at West Point, NY from 2009 to 2012. We ran independent linear mixed models for each life form. All models included site, plot within site and quadrat within plot as random factors. Non-significant study factors were dropped from selected models and not included in the table.
| Factor | Intercept | Year | Origin (non-native) | Earthworm (low) | Fencing (open) | O × Y | O × E | O × F | Y × F | |
|---|---|---|---|---|---|---|---|---|---|---|
| (Bi)Annuals | ||||||||||
| Est* | 0.006 | −0.002 | ||||||||
| SE | 0.002 | 0.001 | ||||||||
| χ2** | 15.16 | |||||||||
| P | <0.001 | |||||||||
| Graminoids | ||||||||||
| Est | 3.85 | −0.44 | 9.47 | −2.12 | −2.34 | −1.36 | −10.91 | 5.61 | 1.29 | |
| SE | 3.87 | 0.51 | 1.36 | 6.36 | 2.15 | 0.59 | 1.39 | 1.31 | 0.59 | |
| χ2 | 5.37 | 82.5 | 121.47 | 4.85 | ||||||
| P | 0.02 | <0.001 | <0.001 | 0.03 | ||||||
| Perennials | ||||||||||
| Est | 0.022 | −0.021 | −0.01 | −0.004 | 0.009 | 0.004 | ||||
| SE | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | ||||
| χ2 | 109.51 | 24.82 | ||||||||
| P | <0.001 | <0.001 | ||||||||
| Woody | ||||||||||
| Est | 0.027 | 0.001 | −0.015 | 0.011 | −0.006 | −0.013 | 0.009 | |||
| SE | 0.005 | 0.0005 | 0.002 | 0.008 | 0.002 | 0.002 | 0.002 | |||
| χ2 | 5.22 | 34.29 | 18.61 | |||||||
| P | 0.02 | <0.001 | <0.001 | |||||||
Est Estimate; SE estimate standard error.
Estimates and standard errors are reported from the model fitted with restricted maximum likelihood.
Chi-squared statistics and P-values are from likelihood ratio tests with each parameter removed from the maximum likelihood-based model, with all other parameters retained. It was not possible to test the significance of all terms because of higher order interactions. All chi-square tests have 1 degree of freedom.
Figure 6.
Native vegetation cover (%) according to life form and earthworm density (high or low) in open and fenced plots (N = 10 one-m2 quadrats per plot) in May–July 2009–2012 at 12 sites at West Point, NY. Data represent means.
Perennial native cover (5 % averaged across all years and treatments) was significantly higher than non-native perennial cover (0.12 %) and both native and non-native perennials had higher cover at sites with high earthworm density and in fenced than open plots (Fig. 6; Table 2).
Overall, native woody cover was significantly higher than non-native woody cover (15 and 10 % averaged across all years and treatments, respectively). We found a significant interaction between plant species origin and earthworm density (Table 2): native woody cover was significantly higher at low earthworm density sites than at high earthworm sites, whereas non-native woody cover was not significantly correlated with earthworm density. We also found a significant interaction between origin and fencing: native woody cover was significantly higher in fenced than open plots (Fig. 6), whereas non-native woody cover was significantly higher in open than in fenced plots.
Separate analyses for May 2016 (N = 10 sites, two sites excluded from analysis due to fence damage) showed similar cover at low and high earthworm density sites and between open and fenced plots (P > 0.05), but a significant effect of site invasion (F1,14=5.33, P = 0.04). Total cover was significantly higher at the five sites dominated by non-native vegetation (42 ± 17.1 %; mean ± 1SE; N = 5 sites; values reflect mean of median cover per plot) than at the five sites dominated by native vegetation (23 ± 3.9 %). We found no significant effect of fencing or earthworm density on vegetation height (P > 0.5).
Species richness and diversity indexes
Over the 5-year study period we recorded a total of 192 species (36 graminoids, 17 (bi)annuals, 8 ferns, 78 perennial forbs and 53 woody species). The majority of species (92 %) were native. Species richness was not affected by fencing, earthworm density or site invasion, but significantly decreased over the study period at both the 1 m2 quadrat scale and 900 m2 plot scale. Species richness over 2009–2012 averaged 7.3 ± 0.17 species per 1 m2 quadrat and significantly decreased from 7.82 species on average in 2009 to 7.00 species in 2012 (year effect ± SE: −0.29 ± 0.09; χ2=9.1; df = 1, P < 0.01), and significantly decreased from 30.00 ± 3.34 species per plot in 2009 to 27.45 ± 2.94 species in 2012 (year effect ± SE: −0.85 ± 0.25; χ2=10.47; df = 1, P < 0.001).
Diversity (Shannon index) per open or fenced plot slightly but significantly increased over the study period from 1.70 ± 0.17 in 2009 to 1.80 ± 0.16 in 2012 (year effect ± SE: −0.035 ± 0.01; χ2=5.07; df = 1, P = 0.02). Similarly evenness (Pielou’s index) significantly increased from 0.50 ± 0.04 in 2009 to 0.54 ± 0.04 in 2012 (year effect ± SE: 0.015 ± 0.003; χ2=6.6; df = 1, P = 0.01). Neither diversity nor evenness were correlated with fencing, earthworm density or site invasion.
Species richness in May 2016 averaged 24.5 ± 2.92 species per plot, Shannon Diversity Index averaged 3.0 ± 0.15 and Pielou’s evenness averaged 0.95 ± 0.01. Fencing and earthworm density had no effect on either measure.
Community composition
PerMANOVA results indicated that species composition differed according to earthworm density (F1,954=39.83, R2=0.04; P < 0.01) and fencing (F1,954=3.39, R2=0.003; P = 0.01), with a significant interaction between earthworms and fencing (F1,954=2.85, R2=0.002; P = 0.01). Study factors, although significant, explained a small percentage of the variation (indicated by R2 values). We found no significant year effect, and no interactions between study year and fencing or earthworms on plant species composition. The interaction between earthworm density and fencing did not change over time and therefore we do not attribute it to our fencing treatment.
NMDS ordination showed no difference in understory plant community composition between open and fenced plots (R2=0.001, P=0.5), either at the beginning (2009) or end (2012) of the study period (R2=0.0001, P=0.9; Fig. 7). Earthworm density, on the other hand, had a significant goodness of fit (R2=0.18, P < 0.001) with low- and high-density earthworm sites separating along the first axis. Sites grouped according to dominant plant species. For example, sites dominated by M. vimineum (sites 3 and 12) and by B. thunbergii (sites 1 and 6) formed clear separate clusters. On the other hand, A. petiolata sites (sites 5 and 7) clustered with sites dominated by native vegetation.
Figure 7.
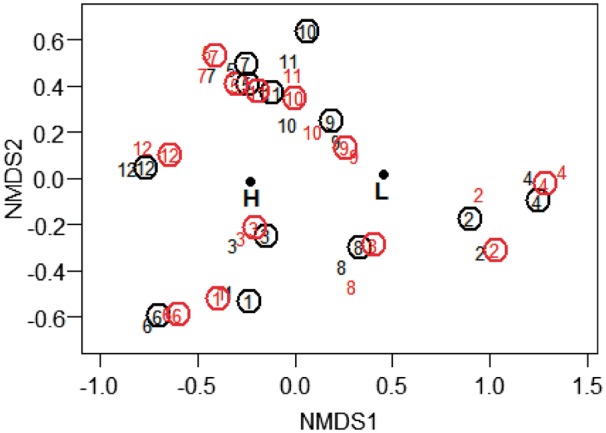
NMDS of the understory vegetation layer in May and July 2009 and 2012 in open and fenced plots at 12 sites at West Point NY (N = 10 permanent monitoring quadrats per plot). Numbers indicate study sites. Black and red numbers indicate sampling year (2009 and 2012, respectively) and circled numbers represent fenced plots. Earthworm density, a categorical factor indicating low (L) or high (H) earthworm density is represented by its compositional centroid. Points are jittered to allow visualization.
Individual species
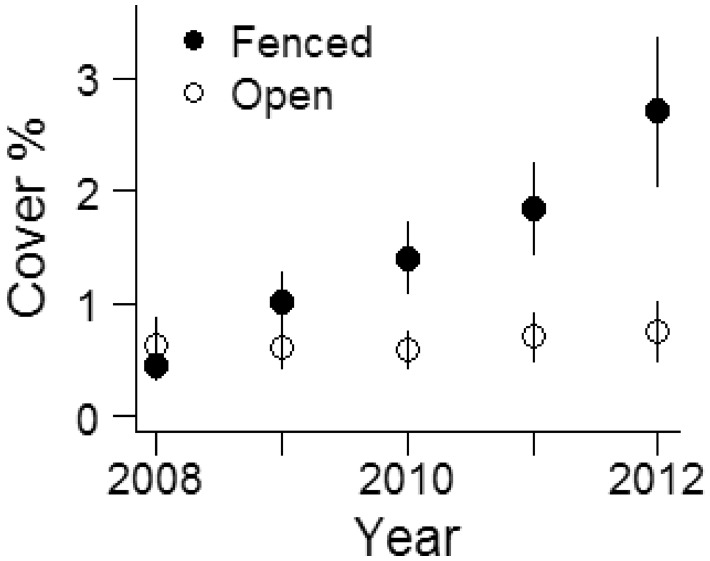
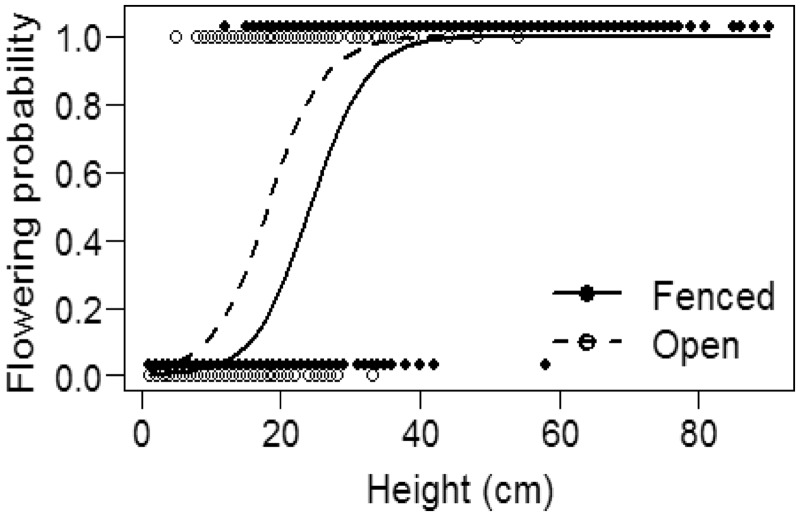
Between 2008 and 2012, E. divaricata mean cover in permanent vegetation quadrats significantly increased in all fenced plots, and remained relatively constant in all open plots (significant year × fencing interaction; interaction effect ± SE: −0.016 ± 0.05; χ2=10.1, df = 1, P = 0.001; Fig. 8). Frequency remained relatively constant in all plots during the same period (data not shown). We measured 942 individual E. divaricata stems, distributed across six sites (only sites where the species was present were included in the study). Plants in the fenced plots were significantly taller (Est ± 1SE: 20.27 ± 3.5, t=−5.7, χ2=17.6, df = 1, P < 0.001) and more likely to flower (Est ± 1SE: 1.5 ± 0.4, z = 3.7, P < 0.001). Interestingly, fertile plants in open plots were significantly shorter than fertile plants in fenced plots (Fig. 9).
Figure 8.
Eurybia divaricata cover (%) in open and fenced plots (N = 10 one-m2 quadrats per plot) in July 2008–2012 at six sites at West Point, NY. Only sites where E. divaricata was present were included in the study. Data are means ± 1SE.
Figure 9.
Flowering probability of Eurybia divaricata as a function of stem height in open and fenced plots at six sites at West Point, NY in June 2012. Lines depict model predictions.
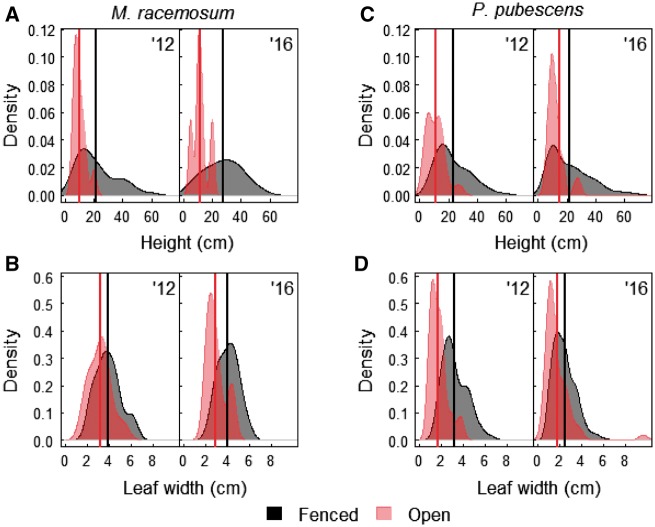
Maianthemum racemosum was uncommon at both sites in both years. In total, we found and measured 50 M. racemosum in 2012 and 24 in 2016, of which 12 (24 %) and 5 (21 %) were in open plots in 2012 and 2016, respectively. Stem height (Est ± 1SE: −0.74 ± 0.14, t=−5.2, χ2=7.1, df = 1, P = 0.01) and leaf width (Est ± 1SE: −0.27 ± 0.06, t=4.33, χ2=6.4, df = 1, P = 0.01) were significantly higher in fenced (mean ± 1SE; height: 23.69 ± 1.8 cm; leaf width: 3.96 ± 0.14 cm) than open plots (height: 10.4 ± 1.1 cm; leaf width: 3.11 ± 0.23 cm; Fig. 10). We found no flowering M. racemosum in open plots at either site, but 32 % and 16 % of plants flowered in fenced plots at sites 5 and 8, respectively. Flowering plants were significantly taller (Est ± 1SE: 0.75 ± 0.15, t=5.1, χ2=21.9, df = 1, P < 0.001) than non-flowering plants at both sites.
Figure 10.
Probability distribution of stem height (cm) and leaf width (cm) of Maianthemum racemosum (A and B) and Polygonatum pubescens (C and D) in open and fenced plots at two sites at West Point, NY in June 2012 and 2016. Fences were erected in 2008. Black and red solid lines represent means at fenced and open plots, respectively.
We measured 109 P. pubescens stems in 2012 and 274 in 2016. Similar to M. racemosum we recorded fewer stems in open (45 and 51 in 2012 and 2016, respectively) compared with fenced plots (64 and 223 in 2012 and 2016, respectively) despite the larger sampling area in open plots. We found a significant positive effect of fencing on stem height (22.32 ± 0.82 cm in fenced vs 13 ± 1.62 cm in open; Est ± 1SE: −0.56 ± 0.12, t=−4.67, χ2=6.9, df = 1, P = 0.01). In contrast, leaf width did not differ between open and fenced plots (2.6 ± 0.08 cm in fenced vs 1.7 ± 0.12 cm in open; P > 0.05; Fig. 10). At site 5, plants had a significantly higher probability of flowering in fenced than open plots (Est ± 1SE: −1.19 ± 0.43, z = 2.73, P = 0.006). At site 8, we recorded only one flowering individual in the open plot in 2012 and no flowering individuals in 2016, preventing formal analysis. Nevertheless, 41 % and 43 % of plants in the fenced plot flowered in 2012 and 2016, respectively. Flowering plants were significantly taller than non-flowering plants (Est ± 1SE: 0.99 ± 0.05, t = 20.69, χ2=287.9, df = 1, P < 0.001).
Discussion
We used two commonly employed methods, deer culling and fencing, to assess whether significant deer population reduction or even deer browse cessation has potential to allow for forest understory recovery. Our results indicate that based on how we define recovery, we can either accept or reject our first hypothesis. If we define recovery as increased abundance, however subtle, of native understory forest plant species, then we can accept our first hypothesis that reduced deer browse pressure can result in recovery of understory communities. At both Fermilab and West Point, cover of native species increased and cover of non-native species decreased in response to reduced deer abundance. At Fermilab recovery was dramatic with cover and height increasing rapidly, but this response was driven by explosive growth of early successional species already present in the community. At West Point change was more nuanced as total cover (native and non-native combined) was similar inside and outside fenced plots over the study period. At both locations, species present before deer reduction remained present afterwards, and few new species appeared.
However, if we define recovery more specifically, for example as increased cover and diversity of a broad array of plant species typical of the forest understory and including deer-favoured and late successional species, then we must reject our first hypothesis. Culling or fencing alone, despite greatly reducing deer browse pressure, was unable to bring back preferred deer browse species to a point that their presence was detectable at the community monitoring level—not even over a decadal time period. Limited recovery of species highly favoured by deer occurred (Hypothesis 2) but was only detectable when monitoring individual species. And this recovery occurred only for species that remained present within the community. This is, in part, explained by the present-day infrequent occurrence, particularly of reproductive individuals, of these deer-favoured species over much of their range (Ludwig and Conklin 1992; Wang and Mopper 2008; Knight et al. 2009a). Our results corroborate others in describing a lack of a vegetative response to fencing or substantial deer reductions, particularly for those species that appear threatened by excessive deer browse (Royo et al. 2010; Goetsch et al. 2011; Pendergast et al. 2016). Without further intervention, even after complete exclusion of deer, forests may remain in a depauperate stable state (Augustine et al. 1998; Nuttle et al. 2014) that developed over many decades in response to high deer populations.
Our first hypothesis was articulated in anticipation of a demographic response of those species that have suffered high browse intensity over extended time periods. Neither at West Point, nor at Fermilab, did we have access to detailed community composition data from before deer population increases, thus we are not fully able to “ground truth” the response, or better lack thereof, of the understory vegetation community. But in both regions floras and early forest community descriptions imply substantially different plant community compositions (Pepoon 1927; Raup 1938; Swink and Wilhelm 1979), and to a major extent the deer browse sensitive species are those that are now missing in the local forests or occur at greatly suppressed abundances (Ludwig and Conklin 1992; Werier and Barbour 2012).
An obvious question then is whether it was in fact the increase in deer populations, rather than abundance of non-native plants and non-native earthworms, that is responsible for the now widespread depauperate plant communities at our sites? Such negative effects of high deer populations on local plant and animal diversity, abundance, and cover have been described for many other locations and regions using long-term datasets (Côté et al. 2004; Wiegmann and Waller 2006; Rogers et al. 2009; Goetsch et al. 2011; Martin et al. 2011; Frerker et al. 2014; Chips et al. 2015) and our sites have likely undergone similar “sorting” processes. While we lack detailed historic records from our study locations, we documented that protecting individuals of preferred browse species such as T. recurvatum, E. divaricata, M. racemosum and P. pubescens from deer browse by fencing, or lowering deer browse pressure by culling, had beneficial effects on growth and reproductive output (Figs. 4 and 8–10), two important factors that may ultimately contribute to positive population growth rates (Wang and Mopper 2008; Knight et al. 2009a; Kalisz et al. 2014). This benefit accrued despite the remaining potential threats of biotic influences (invasive plants and introduced earthworms) or other regional factors such as climate change, changes in nutrient deposition or previous land use history that were not affected by deer culling or fencing. Thus, we find support for our third hypothesis. Together, this provides clear and compelling evidence that deer are the responsible driver (MacDougall and Turkington 2005) for demise of native species, and that the rise of at least the three non-native invasive plant species we investigated is but a symptom of other underlying stressors (see also Blossey et al, this issue). Thus, these three non-native species can be considered passengers rather than drivers of change in plant community composition (MacDougall and Turkington 2005; Dávalos et al. 2015b).
Our results further demonstrate that the effect of deer browse on vegetation is influenced by earthworm density, and deer browse and earthworms interact to influence growth of different plant life forms (Figs. 5 and 6). We are unable to assess how plant invasion and deer browse intensity have interacted historically to facilitate changes in native or introduced plant abundance. But earthworm abundance itself is a function of deer presence, deer exclusion results in declines of earthworms over time (Dávalos et al. 2015c), and earthworms also decline as forests age (Simmons et al. 2015) suggesting a complicated web of interactions that we are only slowly beginning to understand (Dobson and Blossey 2015; Craven et al. 2016). At West Point, both native and non-native vegetation composition was strongly affected by earthworm density at the beginning of our investigations (Fig. 5), which in turn may have been influenced by site-specific factors such as soil pH, moisture or invasion history (Dávalos et al. 2015c; Paudel et al. 2016) and both native and introduced species responded more strongly to deer exclusion in areas with high earthworm density.
Our work confirms the work by others that non-native vegetation cover, abundance, growth and population growth rates decline when deer browse pressure is reduced or even eliminated (Kalisz et al. 2014; Dávalos et al. 2015b) although see Averill et al. (this issue). We suggest that non-native plants, at least the three species in our study (A. petiolata, B. thunbergii, M. vimineum) are indicators of earthworm invasion and high deer abundance, and that these two factors pose greater threats, singly and in combination, to native vegetation than do non-native plants.
There are strong legacy effects of long-term high deer abundance and non-native earthworm abundance (Dávalos et al. 2015c) that impact recovery of vegetation and community composition even after deer reduction. Under continuous intense browsing, forest understory vegetation can be nearly eliminated, leaving an empty forest floor (Goetsch et al. 2011). Once highly favoured and/or uncommon species are eliminated or greatly reduced, the trajectory of community recovery after deer reduction will shift, and the resultant community may have low similarity to the previous community. Deer legacy effects include altered tree recruitment with resulting ripple effects on invertebrate and bird communities (Nuttle et al. 2011), and altered herbaceous forest layer that does not have a height refuge to escape deer browse (Nuttle et al. 2014; Chips et al. 2015). Deer and introduced earthworm abundance interact (Dávalos et al. 2015c) and may affect soil microbial community ability to facilitate or suppress germination and growing conditions for other native species through plant-soil feedbacks (Kardol et al. 2007, 2014). These indirect yet important non-consumptive deer effects have been overlooked until recently and they include demographic effects (Heckel et al. 2010; Dávalos et al. 2014) as well as evolutionary effects on plant height, palatability, or height needed to initiate reproductive efforts (Martin et al. 2015; Prendeville et al. 2015; Holeski et al. 2016).
Our work indicates that recovery following deer reduction is dependent upon the species that are already present (as plants or in seedbanks), and species that seed in (mostly wind or animal dispersed). Rescue from long-lived existing seed banks, as is typical for many wetland plant species (van der Valk 1981), does not represent an important demographic response available for many forest understory species (Nuzzo et al. 2015). Forest seed banks are typically dominated by early successional species dependent on high light conditions and disturbances for germination and growth, with a noticeable absence of perennial monocots and long-lived herbaceous species with low reproductive effort and extended germination requirements (Matlack and Good 1990; Hopfensperger 2007; Bossuyt and Honnay 2008; Schmidt et al. 2009; Esmailzadeh et al. 2011; Levine et al. 2012; Beauchamp et al. 2013). Furthermore, deer browsing affects seedbank composition and reduces germinant species diversity and richness, and total number of germinants (DiTommaso et al. 2014). Even if propagules arrive (as seeds or transplants), deer abundance may influence germination and growth (Dávalos et al. 2015a), and plant–plant competitive effect may also hinder establishment. In addition, non-native earthworms influence seedbank composition, seedling emergence and seedling survival (Willems and Huijsmans 1994; Regnier et al. 2008; Eisenhauer et al. 2010; Clause et al. 2015; Dobson and Blossey 2015; Nuzzo et al. 2015). Maintaining presence and abundance of large reproductive individuals will be paramount for conservation of many deer browse preferred herbaceous species (Wang and Mopper 2008; Knight et al. 2009a; Dávalos et al. 2014; Kalisz et al. 2014).
We expected that using typical plant community monitoring in 1 m2 quadrats would allow us to capture (1) changes in plant community structure associated with reduced deer browse pressure, and (2) the recovery of species highly favoured by deer. Our data show that we can accept the first expectation (albeit the effect was very limited and we found it only at Fermilab for early successional species) but reject the second. At Fermilab, understory composition prior to the cull changed gradually, slightly differing year after year (Fig. 3), whereas after the cull vegetation composition did not differ among years, emphasizing the rapid dominance of a few early successional species, potentially suggesting a continued lagged or legacy response that was not remedied by a greatly reduced deer population. At West Point we did not capture a community response to fencing at any of the 12 sites (Fig. 7). At our sites monitoring at the community level was unable to capture the subtle positive effects of deer browse reduction on individual species, at least over the time frames used in our studies. Three of the four species that we monitored (M. racemosum, P. pubescens and T. recurvatum) require 2 years to emerge from seed, and 7–10 years to produce a first flower (Baskin and Baskin 1992; Case and Case 1997; Cullina 2000). To effectively capture a demographic success (initial colonization and a population increase) in these and similar slow-growing species, it may be necessary to monitor for a decade or longer using traditional permanent 1 m2 quadrats.
We are not the first to document the limitations of plant community monitoring: a recent meta-analysis reported on inconsistencies in outcomes using this approach (Habeck and Schultz 2015). Interestingly, community monitoring is able to capture the initial “erosion” of diversity when deer colonize previously deer free islands or refugia (Côté et al. 2004; Mudrak et al. 2009; Martin et al. 2011), but is less effective at capturing recovery after deer reduction. Furthermore, community monitoring can be a powerful tool but requires substantial expertise to identify all plants to species, and is time consuming and hence expensive. Our results show that it also may not be the best approach to capture the initial response of vegetation to deer reductions. Monitoring individual deer-favoured plant species is both easier and less time-consuming assuming they still exist in local forests albeit at greatly reduced abundances. The response of individuals of deer favoured plant species clearly demonstrates that abundant deer herbivory reduces performance of these species, potentially for decades.
The differences in the height needed to initiate flowering between plants in open and fenced plots further suggests that chronic deer herbivory may over time favour plants with lower stature in areas where they are exposed to high deer herbivory. How these eco–evolutionary interactions play out over the long term, and how they could affect plant demography remains to be investigated. It is possible that decades of intensive deer browse may result in individuals of the remaining plant species that through phenotypic plasticity are reasonably well able to cope with deer browse but are poor performers or contributors to population growth rates due to reduced reproductive activity and stature. This may negatively affect the long-term viability of populations exposed to chronic deer herbivory.
Legacy effects of prolonged high deer browse intensity often result in understories that show little response to deer reductions, especially by vulnerable species (Royo et al. 2010; Nuttle et al. 2014; Pendergast et al. 2016). Expectations for rapid wholesale beneficial changes or return of species that have suffered from excessive deer browse pressure for decades and that have retreated to small refugia or have been lost from entire forests are not met by our evidence or results by others (Carson et al. 2005; Comisky et al. 2005; Ripple and Beschta 2005; Krueger and Peterson 2006; Habeck and Schultz 2015). Many of the currently remaining small populations may face an extinction debt (Vellend et al. 2006; Rogers et al. 2009) and we may lose them unless appropriate conservation measures are implemented. This may include not only greatly reduced deer abundance to provide safe spaces for recruitment, but also active restoration if we want to retain these species as part of our biological communities and heritage.
Recognizing the limitations of approaches and metrics (such as plant community monitoring) to measure incremental long-term plant responses to changes in deer populations and deer browse pressure is an important part of management of forest understory communities. This includes the recognition that forest recovery needs to be appropriately defined and “simple” suggestions such as “increased diversity” or “increased native cover” as an outcome of deer management may be insufficient to safeguard or recover browse sensitive species that have dramatically declined over the past decades. Defining recovery through growth, ability to reproduce and positive population growth rates for individual species may be much more appropriate.
Our assessment of plant individuals shows that browse sensitive individuals (if still present in the areas of interest) can be utilized to assess incremental initial success, or lack thereof, of deer management (culling, fencing, hunting, sterilization or no management). Where these species are absent, planting of indicator species may be warranted (see Blossey et al. 2017).
Conclusions
Deer culling or fencing results in reduced non-native plant cover and increased native plant cover, but little change in plant community composition (although a few early successional native forbs can increase rapidly under reduced deer browse). We found that deer herbivory interacts with impacts of introduced earthworms in shaping plant communities, especially growth of different life forms. We also found that non-native plant species (at least the three in this study) had minimal effect on plant community composition. Rather, they are indicators of other stressors, especially presence of non-native earthworms and deer abundance.
Plant community monitoring fails to capture initial and subtle effects of reduced or even cessation of deer browse on browse sensitive species, especially when these species are rare or are not present in standardized monitoring quadrats, as occurred in our study. Measuring responses of individual plants (growth, flowering and reproductive success) provides a more sensitive and powerful assessment of forest understory responses to deer management.
Land managers need to appropriately define recovery goals of deer management and develop suitable metrics. Defining recovery through growth, ability to reproduce and positive population growth rates for individual species is more appropriate than simply stating ‘increased species diversity’ or ‘increased native cover’. Reducing deer populations may provide safe spaces for recovery of deer sensitive species, but active restoration may be required if we want to retain these species as part of our ecosystems.
Sources of Funding
Funding for the Fermilab research was provided by U.S. DOE Fermilab National Environmental Research Park (0perated by Fermi Research Alliance, LLC under Contract No. DE-AC02-07CH11359 with the United States Department of Energy) to V.N. Funding for the West Point research was provided by the Strategic Environmental Research and Development Program (SERDP) of the U.S. Department of Defense (Grant RC-1542 to BB).
Contributions by the Authors
V.N. conceived the Fermilab study design, and B.B. and V.N. conceived the West Point study design; V.N. collected plant data, and A.D. and V.N. collected earthworm data. A.D. conducted all analyses. B.B. led the writing and all authors participated in writing the manuscript.
Conflicts of Interest Statement
No conflicts of interest.
Acknowledgements
We thank Rod Walton, Coordinator, Fermilab National Environmental Research Park (NERP) for facilitating access and assisting with data collection, and US Army Garrison West Point Natural Resource Manager Christopher Pray and other West Point personnel for allowing and facilitating access to field sites. We also thank all field assistants and all members of the Ecology and Management of Invasive Plants Program at Cornell University for their contributions during this research.
Literature Cited
- Ammer C, Vor T, Knoke T, Wagner S.. 2010. Der wald-wild-konflikt: Analyse und lösungsansätze vor dem hintergrund rechtlicher, ökologischer und ökonomischer zusammenhänge. Universitätsverlag Göttingen, Göttingen, Germany. [Google Scholar]
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Australian Ecology 26:32–46. [Google Scholar]
- Augustine DJ, Frelich LE, Jordan PA.. 1998. Evidence for two alternate stable states in an ungulate grazing system. Ecological Applications 8:1260–1269. [Google Scholar]
- Baskin JM, Baskin CC.. 1992. Seed germination biology of the weedy biennial Alliaria petiolata. Natural Areas Journal 12:191–197. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S.. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. http://CRANR-projectorg/package=lme4.
- Beauchamp VB, Ghuznavi N, Koontz SM, Roberts RP.. 2013. Edges, exotics and deer: the seed bank of a suburban secondary successional temperate deciduous forest. Applied Vegetation Science 16:571–584. [Google Scholar]
- Beisner BE, Haydon DT, Cuddington K.. 2003. Alternative stable states in ecology. Frontiers in Ecology and the Environment 1:376–382. [Google Scholar]
- Blossey B, Dávalos A, Nuzzo V.. 2017. An indicator approach to capture impacts of white-tailed deer and other ungulates in the presence of multiple associated stressors. AoBPlants (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt B, Honnay O.. 2008. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. Journal of Vegetation Science 19:875–884. [Google Scholar]
- Carson WP, Banta JA, Royo AA, Kirschbaum C.. 2005. Plant communities growing on boulders in the Allegheny National Forest: evidence for boulders as refugia from deer and as a bioassay of overbrowsing. Natural Areas Journal 25:10–18. [Google Scholar]
- Case FW Jr, Case RB.. 1997. Trilliums. Portland, OR: Timber Press. Inc. [Google Scholar]
- Chips MJ, Yerger EH, Hervanek A, Nuttle T, Royo AA, Pruitt JN, McGlynn TP, Riggall CL, Carson WP.. 2015. The indirect impact of long-term overbrowsing on insects in the Allegheny National Forest region of Pennsylvania. Northeastern Naturalist 22:782–797. [Google Scholar]
- Clause J, Forey E, Lortie CJ, Lambert AM, Barot S.. 2015. Non-native earthworms promote plant invasion by ingesting seeds and modifying soil properties. Acta Oecologica 64:10–20. [Google Scholar]
- Comisky L, Royo AA, Carson WP.. 2005. Deer browsing creates rock refugia gardens on large boulders in the Allegheny National Forest, Pennsylvania. American Midland Naturalist 154:201–206. [Google Scholar]
- Côté SD, Rooney TP, Tremblay J-P, Dussault C, Waller DM.. 2004. Ecological impacts of deer overabundance. Annual Review of Ecology and Systematics 35:113–147. [Google Scholar]
- Craven D, Thakur MP, Cameron EK, Frelich LE, Beauséjour R, Blair RB, Blossey B, Burtis J, Choi A, Dávalos A, Fahey TJ, Fisichelli N, Gibson K, Handa IT, Hopfenspberger K, Loss SR, Nuzzo V, Maerz JC, Sackett T, Scharenbroch B, Smith SM, Vellend M, Umek LG, Eisenhauer N.. 2016. The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Global Change Biology doi: 10.1111/gcb.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullina W. 2000. The New England Wild Flower Society guide to growing and propagating wildflowers of the United States and Canada. New York: Houghton Mifflin Company. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B.. 2014. Demographic responses of rare forest plants to multiple stressors: the role of deer, invasive species and nutrients. Journal of Ecology 102:1222–1233. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B.. 2015a. Interactive effects of deer, earthworms and non-native plants on rare forest plant recruitment. Biodiversity and Conservation 187:173–181. [Google Scholar]
- Dávalos A, Nuzzo V, Blossey B.. 2015b. Single and interactive effects of deer and earthworms on non-native plants. Forest Ecology and Management 351:28–35. [Google Scholar]
- Dávalos A, Simpson E, Nuzzo V, Blossey B.. 2015c. Non-consumptive effects of native deer on introduced earthworm abundance. Ecosystems 18:1029–1042. [Google Scholar]
- DeNicola AJ. 2008. Sharpshooting suburban white-tailed deer reduces deer–vehicle collisions. Human–Wildlife Conflicts 2:28–33. [Google Scholar]
- DiTommaso A, Morris SH, Parker JD, Cone CL, Agrawal AA.. 2014. Deer browsing delays succession by altering aboveground vegetation and belowground seed banks. PLoS One 9:e91155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A, Blossey B.. 2015. Earthworm invasion, white-tailed deer and seedling establishment in deciduous forests of north-eastern North America. Journal of Ecology 103:153–164. [Google Scholar]
- Eisenhauer N, Butenschoen O, Radsick S, Scheu S.. 2010. Earthworms as seedling predators: Importance of seeds and seedlings for earthworm nutrition. Soil Biology & Biochemistry 42:1245–1252. [Google Scholar]
- Esmailzadeh O, Hosseini SM, Tabari M.. 2011. Relationship between soil seed bank and above-ground vegetation of a mixed-deciduous temperate forest in Northern Iran. Journal of Agricultural Science and Technology 13:411–424. [Google Scholar]
- Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soule ME, Virtanen R, Wardle DA.. 2011. Trophic downgrading of planet earth. Science 333:301–306. [DOI] [PubMed] [Google Scholar]
- Fletcher JD, Shipley LA, McShea WJ, Shumway DL, 2001. Wildlife herbivory and rare plants: the effects of white-tailed deer, rodents, and insects on growth and survival of Turk's cap lily. Biological Conservation 1: 229–238. [Google Scholar]
- Forsyth DM, Wilmshurst JM, Allen RB, Coomes DA.. 2010. Impacts of introduced deer and extinct moa on New Zealand ecosystems. New Zealand Journal of Ecology 34:48–65. [Google Scholar]
- Foster CN, Barton PS, Lindenmayer DB.. 2014. Effects of large native herbivores on other animals. Journal of Applied Ecology 51:929–938. [Google Scholar]
- Frelich LE, Lorimer CG.. 1985. Current and predicted long-term effects of deer browsing in hemlock forests in Michigan, USA. Biological Conservation 34:99–120. [Google Scholar]
- Frerker K, Sabo A, Waller D.. 2014. Long-term regional shifts in plant community composition are largely explained by local deer impact experiments. PLoS One 9:e115843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetsch C, Wigg J, Royo AA, Ristau T, Carson WP.. 2011. Chronic over browsing and biodiversity collapse in a forest understory in Pennsylvania: results from a 60 year-old deer exclusion plot. Journal of the Torrey Botanical Society 138:220–224. [Google Scholar]
- Griggs JA, Rock JH, Webster CR, Jenkins MA.. 2006. Vegetative legacy of a protected deer herd in Cades Cove, Great Smoky Mountains National Park. Natural Areas Journal 26:126–136. [Google Scholar]
- Habeck CW, Schultz AK.. 2015. Community-level impacts of white-tailed deer on understorey plants in North American forests: a meta-analysis. AoB Plants 7:plv119.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck JR. 1959. A vegetational study of the central Wisconsin winter deer range. Jour Wildlife Management 23:273–278. [Google Scholar]
- Halls LK. (Ed.), 1984. White-tailed deer: ecology and management. Harrisburg: Stackpole Books. [Google Scholar]
- Heckel CD, Bourg NA, McShea WJ, Kalisz S.. 2010. Nonconsumptive effects of a generalist ungulate herbivore drive decline of unpalatable forest herbs. Ecology 91:319–326. [DOI] [PubMed] [Google Scholar]
- Holeski LM, McKenzie SC, Kruger EL, Couture JJ, Rubert-Nason K, Lindroth RL.. 2016. Phytochemical traits underlie genotypic variation in susceptibility of quaking aspen (Populus tremuloides) to browsing by a keystone forest ungulate. Journal of Ecology 104:850–863. [Google Scholar]
- Hopfensperger KN. 2007. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 116:1438–1448. [Google Scholar]
- Horsley SB, Stout SL, DeCalesta DS.. 2003. White-tailed deer impact on the vegetation dynamics of a northern hardwood forest. Ecological Applications 13:98–118. [Google Scholar]
- Howland B, Stojanovic D, Gordon IJ, Manning AD, Fletcher D, Lindenmayer DB.. 2014. Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. Plos ONE 9:e105966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima H, Ueno M.. 2016. Spatial heterogeneity in the carrying capacity of sika deer in Japan. Journal of Mammalogy 97:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisz S, Spigler R, Horvitz C.. 2014. In a long-term experimental demography study, excluding ungulates reversed invader's explosive population growth rate and restored natives. Proceedings of the National Academy of Sciences of the United States of America 111:4501–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardol P, Cornips NJ, van Kempen MML, Bakx-Schotman JMT, van der Putten WH.. 2007. Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecological Monographs 77:147–162. [Google Scholar]
- Kardol P, Dickie IA, St John MG, Husheer SW, Bonner KI, Bellingham PJ, Wardle DA.. 2014. Soil-mediated effects of invasive ungulates on native tree seedlings. Journal of Ecology 102:622–631. [Google Scholar]
- Knight TM, Caswell H, Kalisz S.. 2009a. Population growth rate of a common understory herb decreases non-linearly across a gradient of deer herbivory. Forest Ecology and Management 257:1095–1103. [Google Scholar]
- Knight TM, Dunn JL, Smith LA, Davis J, Kalisz S.. 2009b. Deer facilitate invasive plant success in a Pennsylvania forest understory. Natural Areas Journal 29:110–116. [Google Scholar]
- Krueger LM, Peterson CJ.. 2006. Effects of white-tailed deer on Tsuga canadensis regeneration: Evidence of microsites as refugia from browsing. American Midland Naturalist 156:353–362. [Google Scholar]
- Leonardsson J, Lof M, Gotmark F.. 2015. Exclosures can favour natural regeneration of oak after conservation-oriented thinning in mixed forests in Sweden: a 10-year study. Forest Ecology and Management 354:1–9. [Google Scholar]
- Leopold A, Sowls LK, Spencer DL.. 1947. A survey of over-populated deer ranges in the United States. Journal of Wildlife Management 11:162–177. [Google Scholar]
- Levine CR, Winchcombe RJ, Canham CD, Christenson LM, Ronsheim ML.. 2012. Deer impacts on seed banks and saplings in eastern New York. Northeastern Naturalist 19:49–66. [Google Scholar]
- Ludwig DR, Conklin B.. 1992. Status of white-tailed deer (Odocoileus virginianus) within the Forest Preserve District of DuPage County, IL. Glen Ellyn: Forest Preserve District of DuPage County, p. 105. [Google Scholar]
- MacDougall AS, Turkington R.. 2005. Are invasive species the drivers or passengers of change in degraded ecosystems. Ecology 86:42–55. [Google Scholar]
- Martin LJ, Agrawal AA, Kraft CE.. 2015. Historically browsed jewelweed populations exhibit greater tolerance to deer herbivory than historically protected populations. Journal of Ecology 103:243–249. [Google Scholar]
- Martin TG, Arcese P, Scheerder N.. 2011. Browsing down our natural heritage: Deer impacts on vegetation structure and songbird populations across an island archipelago. Biological Conservation 144:459–469. [Google Scholar]
- Matlack GR, Good RE.. 1990. Spatial heterogeneity in the soil seed bank of a mature coastal plain forest. Bulletin of the Torrey Botanical CLub 117:134–152. [Google Scholar]
- McGraw JB, Furedi MA.. 2005. Deer browsing and population viability of a forest understory plant. Science 307:920–922. [DOI] [PubMed] [Google Scholar]
- McShea WJ, Rappole JH.. 2000. Managing the abundance and diversity of breeding bird populations through manipulation of deer populations. Conservation Biology 14:1161–1170. [Google Scholar]
- Millar RB, Anderson MJ.. 2004. Remedies for pseudoreplication. Fisheries Research 70:397–407. [Google Scholar]
- Mudrak EL, Johnson SE, Waller DA.. 2009. Forty-seven year changes in vegetation at the Apostle Islands: effects of deer on the forest understory. Natural Areas Journal 29:167–176. [Google Scholar]
- Nuttle T, Ristau TE, Royo AA.. 2014. Long-term biological legacies of herbivore density in a landscape-scale experiment: forest understoreys reflect past deer density treatments for at least 20 years. Journal of Ecology 102:221–228. [Google Scholar]
- Nuttle T, Yerger EH, Stoleson SH, Ristau TE.. 2011. Legacy of top-down herbivore pressure ricochets back up multiple trophic levels in forest canopies over 30 years. Ecosphere 2:art 4. [Google Scholar]
- Nuzzo V, Dávalos A, Blossey B.. 2015. Invasive earthworms shape forest seed bank composition. Diversity and Distributions 21:560–570. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H.. 2012. vegan: Community Ecology Package. http://CRAN.R-project.org/package=vegan.
- Paudel S, Wilson GW, MacDonald B, Longcore T, Loss SR.. 2016. Predicting spatial extent of invasive earthworms on an oceanic island. Diversity and Distributions 22:1013–1023. [Google Scholar]
- Pendergast TH, Hanlon SM, Long ZM, Royo AA, Carson WP.. 2016. The legacy of deer overabundance: long-term delays in herbaceous understory recovery. Canadian Journal of Forest Research 46:362–369. [Google Scholar]
- Pepoon HS. 1927. An annotated flora of the Chicago Region. Chicago: Lakeside Press, R.R. Donnelly and Sons. [Google Scholar]
- Perea R, Girardello M, San Miguel A.. 2014. Big game or big loss? High deer densities are threatening woody plant diversity and vegetation dynamics. Biodiversity and Conservation 23:1303–1318. [Google Scholar]
- Porter WF, Underwood HB.. 1999. Of elephants and blind men: deer management in the U.S. National Parks. Ecological Applications 9:3–9. [Google Scholar]
- Prendeville HR, Steven JC, Galloway LF.. 2015. Spatiotemporal variation in deer browse and tolerance in a woodland herb. Ecology 96:471–478. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://wwwR-projectorg/. [Google Scholar]
- Raup HM. 1938. Botanical studies in the Black Rock Forest. The Black Rock Forest Bulletin 7:1–166. [Google Scholar]
- Regnier E, Harrison SK, Liu J, Schmoll JT, Edwards CA, Arancon N, Holloman C.. 2008. Impact of an exotic earthworm on seed dispersal of an indigenous US weed. Journal of Applied Ecology 45:1621–1629. [Google Scholar]
- Ripple WJ, Beschta RL.. 2005. Refugia from browsing as reference sites for restoration planning. Western North American Naturalist 65:269–273. [Google Scholar]
- Rogers DA, Rooney TP, Hawbaker TJ, Radeloff VC, Waller DM.. 2009. Paying the extinction debt in southern Wisconsin forest understories. Conservation Biology 23:1497–1506. [DOI] [PubMed] [Google Scholar]
- Royo AA, Stout SL, deCalesta DS, Pierson TG.. 2010. Restoring forest herb communities through landscape-level deer herd reductions: Is recovery limited by legacy effects? Biological Conservation 143:2425–2434. [Google Scholar]
- Schabel HG. 2001. Deer and Dauerwald in Germany: any progress? Wildlife Society Bulletin 29:888–898. [Google Scholar]
- Schank JC, Koehnle TJ.. 2009. Pseudoreplication is a pseudoproblem. Journal of Comparative Psychology 123:421–433. [DOI] [PubMed] [Google Scholar]
- Schmidt I, Leuschner C, Moelder A, Schmidt W.. 2009. Structure and composition of the seed bank in monospecific and tree species-rich temperate broad-leaved forests. Forest Ecology and Management 257:695–702. [Google Scholar]
- Schweitzer D, Garris JR, McBride AE, Smith JAM.. 2014. The current status of forest Macrolepidoptera in northern New Jersey: evidence for the decline of understory specialists. Journal of Insect Conservation 18:561–571. [Google Scholar]
- Severinghouse CW, Brown CP.. 1956. History of the white-tailed deer in New York. New York Fish and Game Journal 3:130–167. [Google Scholar]
- Simmons W, Davalos A, Blossey B.. 2015. Forest successional history and earthworm legacy affect earthworm survival and performance. Pedobiologia 58:153–164. [Google Scholar]
- Soliveres S, van der Plas F, Manning P, Prati D, Gossner MM, Renner SC, Alt F, Arndt H, Baumgartner V, Binkenstein J, Birkhofer K, Blaser S, Blüthgen N, Boch S, Böhm S, Börschig C, Buscot F, Diekötter T, Heinze J, Hölzel N, Jung K, Klaus VH, Kleinebecker T, Klemmer S, Krauss J, Lange M, Müller J, Oelmann Y, Overmann J, Pašalić E, Rillig MC, Schaefer HM, Schloter M, Schmitt B, Schöning I, Schrumpf M, Sikorski J, Socher SA, Solly EF, Sonnemann I, Sorkau E, Steckel J, Steffan-Dewenter I, Stempfhuber B, Tschapka M, Türke M, Venter PC, Weiner CN, Weisser WW, Werner M, Westphal C, Wilcke W, Wolters V, Wubet T, Wurst S, Fischer M, Allan E, 2016. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536:456–459. [DOI] [PubMed] [Google Scholar]
- Suding KN. 2011. Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annual Review of Ecology, Evolution, and Systematics 42:465–487. [Google Scholar]
- Suding KN, Gross KL, Houseman GR.. 2004. Alternative states and positive feedbacks in restoration ecology. Trends in Ecology & Evolution 19:46–53. [DOI] [PubMed] [Google Scholar]
- Swink F, Wilhelm G.. 1979. Plants of the Chicago region. Lisle: The Morton Arboretum. [Google Scholar]
- Tanentzap AJ, Bazely DR, Koh S, Timciska M, Haggith EG, Carleton TJ, Coomes DA.. 2011. Seeing the forest for the deer: Do reductions in deer-disturbance lead to forest recovery? Biological Conservation 144:376–382. [Google Scholar]
- Tanentzap AJ, Kirby KJ, Goldberg E.. 2012. Slow responses of ecosystems to reductions in deer (Cervidae) populations and strategies for achieving recovery. Forest Ecology and Management 264:159–166. [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA.. 2008. Global change and species interactions in terrestrial ecosystems. Ecology Letters 11:1351–1363. [DOI] [PubMed] [Google Scholar]
- USDA NRCS. 2017. The PLANTS Database (http://plants.usda.gov). National Plant Data Team, Greensboro, North Carolina, USA (21 May 2017).
- van der Plas F, Manning P, Soliveres S, Allan E, Scherer-Lorenzen M, Verheyen K, Wirth C, Zavala MA, Ampoorter E, Baeten L, Barbaro L, Bauhus J, Benavides R, Benneter A, Bonal D, Bouriaud O, Bruelheide H, Bussotti F, Carnol M, Castagneyroli B, Charbonnier Y, Coomes DA, Coppi A, Bestias CC, Dawud SM, De Wandeler H, Domisch T, Finer L, Gessler A, Granier A, Grossiord C, Guyot V, Hattenschwiler S, Jactel H, Jaroszewicz B, Joly FX, Jucker T, Koricheva J, Milligan H, Mueller S, Muys B, Nguyen D, Pollastrini M, Ratcliffe S, Raulund-Rasmussen K, Selvi F, Stenlid J, Valladares F, Vesterdal L, Zielinski D, Fischer M.. 2016. Biotic homogenization can decrease landscape-scale forest multifunctionality. Proceedings of the National Academy of Sciences of the United States of America 113:3557–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk AG. 1981. Succession in wetlands: a Gleasonian approach. Ecology 62:688–696. [Google Scholar]
- Vellend M, Verheyen K, Jacquemeyn H, Kolb A, van Calster H, Peterken G, Hermy M.. 2006. Extinction debt of forest plants persists for more than a century following habitat fragmentation. Ecology 87:542–548. [DOI] [PubMed] [Google Scholar]
- Wang HG, Mopper S.. 2008. Separate and interacting effects of deer florivory and salinity stress on iris herbivores. Oikos 117:564–570. [Google Scholar]
- Wardle DA, Bardgett RD.. 2004. Human-induced changes in large herbivorous mammal density: the consequences for decomposers. Frontiers in Ecology and the Environment 2:145–153. [Google Scholar]
- Werier D, Barbour JG.. 2012. Plant rarities at West Point: a 200 year overview including details from intensive surveys in 2011. Natural Resources Branch of the West Point Military Academy, West Point, New York, USA, p. 784.
- Wiegmann S, Waller DM.. 2006. Fifty years of change in northern upland forest understories: identity and traits of “winner” and “loser” plant species. Biological Conservation 129:109–123. [Google Scholar]
- Willems JH, Huijsmans KGA.. 1994. Vertical seed dispersal by earthworms: a quantitative approach. Ecography 17:124–130. [Google Scholar]
- Williams CE, Mosbacher EV, Moriarity WJ.. 2000. Use of turtlehead (Chelone glabra L.) and other herbaceous plants to assess intensity of white-tailed deer browsing on Allegheny Plateau riparian forests, USA. Biological Conservation 92:207–215. [Google Scholar]
- Wright DM, Tanentzap AJ, Flores O, Husheer SW, Duncan RP, Wiser SK, Coomes DA.. 2012. Impacts of culling and exclusion of browsers on vegetation recovery across New Zealand forests. Biological Conservation 153:64–71. [Google Scholar]