Abstract
Objective
Late-onset Pompe disease (LOPD) is a lysosomal storage disease resulted from deficiency of the enzyme acid α-glucosidase. Patients usually develop a limb-girdle pattern of myopathy and respiratory impairment, and enzyme replacement therapy (ERT) is the only specific treatment available. Recently, LOPD has been associated with low bone mineral density (BMD), but the effect of ERT on BMD is inconclusive. In this report we described our early observations on the change of BMD after ERT in Chinese LOPD patients.
Results
We studied four Chinese LOPD patients with different severities of myopathy. All were underweight, and three had osteoporosis at baseline. We found significant weight gain in three patients after ERT and all four patients showed improvement in BMD. The biggest improvement, 84.4% increase in BMD, was seen in a lady with the most prominent weight recovery. Our results suggest that ERT improves BMD in Chinese LOPD and weight gain could be a major contributor to this effect.
Keywords: Glycogen storage disease type II, Lysosomal storage diseases, Osteoporosis, Enzyme replacement therapy, Bone density, Body weight
Introduction
Pompe disease is an autosomal recessive disorder caused by the deficiency of a lysosomal enzyme, acid α-glucosidase, resulting in glycogen accumulations and consequent autophagic buildup [1–3]. The late-onset form (LOPD) is characterized by a limb-girdle pattern of myopathy and respiratory impairment [4]. At present, enzyme replacement therapy (ERT) with alglucosidase alfa is the only specific treatment available. It modestly improves mobility and stabilizes respiratory function in patients with LOPD [5].
Recently, low bone mineral density (BMD) and osteoporosis has been reported in LOPD [6, 7]. These, and together with the observations on many other non-muscle disease manifestations such as small-fiber neuropathy, torturous vessels, minor cardiac abnormalities etc., have redefined LOPD as a multi-system disease [8, 9]. At the moment, it is unclear whether these non-myopathy parameters would be modified by ERT. We examined six patients with dual X-ray absorptiometry (DXA) upon initiation of ERT and found that three had osteoporosis and one had osteopenia. Herein, we present our observations on the first four patients who completed the follow-up DXA study.
Main text
Methods
Four Chinese LOPD patients who underwent DXA (Prodigy Advance, GE Healthcare) at baseline before ERT and a follow-up study after ERT were included. Patients 1 and 2 were brothers, patients 3 and patient 4 were sisters. Patient 1 was a juvenile-onset patient with an aggressive disease course; he was wheelchair-bound and required full-day non-invasive ventilation (NIV) support upon initiation of ERT. Because of his previous spinal surgery for scoliosis, patient 1’s DXA was performed on the forearm and hip instead of the usual lumbar spine and hip. Patient 2 was fully ambulatory and did not require NIV. Both patient 3 and patient 4 presented with type II respiratory failure in their early 30s. They were ambulatory and on nocturnal NIV. We prescribed calcium, vitamin D and l-alanine supplements to all of our LOPD patients. Patient 3 and patient 4 also received alendronate for osteoporosis after the baseline DXA study. Alglucosidase alfa infusion was given at the standard regime of 20 mg/kg every 2 weeks.
We serially monitored the patients’ mobility and pulmonary function with the 6-min walk test (6MWT) and spirometry, respectively, according to our internal management protocol for all LOPD patients on ERT, in order to justify the continuation of ERT in these patients through public funding. We also used a modified Walton scale according to Slonim et al. (0: all activities normal, to 7: wheelchair bound) to assess the muscle weakness in relation to their daily functions [10]. A follow-up DXA was arranged for patients 1, 2 and 3 after 5 years of ERT. The DXA was scheduled earlier for patient 4 because of her significant weight gain since the ERT.
Results
The four patients were in different stages of severity across the disease spectrum (Table 1). They were all slim and underweight. BMD measurements at baseline revealed that patients 1, 3 and 4 had osteoporosis, while patient 2 was in the normal range. All four patients had significant respiratory impairment before ERT as measured by spirometry, though patient 2 did not require NIV.
Table 1.
Baseline characteristics of patients at study entry before enzyme replacement therapy
| Genotype | Age | Gender | Walton score | Assisted ventilation | |
|---|---|---|---|---|---|
| Patient 1 | c.1082C>T (p.Pro361Leu) c.1309C>T (p.Arg437Cys) |
25 | M | 7 | Full day NIV |
| Patient 2 | c.1082C>T (p.Pro361Leu) c.1309C>T (p.Arg437Cys) |
21 | M | 2 | Nil |
| Patient 3 | c.2238G>C (p.Trp746Cys) c.1935C>A (p.Asp645Glu) |
39 | F | 2 | Nocturnal NIV |
| Patient 4 | c.2238G>C (p.Trp746Cys) c.1935C>A (p.Asp645Glu) |
37 | F | 2.5 | Nocturnal NIV |
Patients 1 and 2 were brothers, patients 3 and 4 were sisters
In the follow-up reassessment after ERT (Figs. 1, 2; Table 2), patient 2 showed significant and sustained improvement in mobility and pulmonary function. Pulmonary function in patients 1 and 4 had improved slightly, and the effect was maintained over the study period. Patient 4 also showed better mobility and physical endurance; her Walton score decreased from 2.5 to 2. Patient 3 had initial mild improvement in mobility and pulmonary function, but both parameters dropped to baseline levels later. None of the four patients showed any change in requirement or degree of NIV support, and except patient 4, their respective Walton scores also remained the same.
Fig. 1.
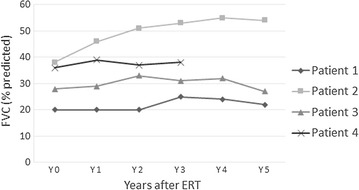
Serial changes in FVC after ERT
Fig. 2.
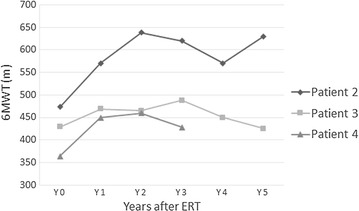
Serial changes in 6MWT (m) after ERT
Table 2.
Changes in mobility, pulmonary function, body mass index and bone mineral density after enzyme replacement therapy at the time of follow-up DXA
| Duration of ERT (months) | 6MWT (m) | Walton score | FVC (L) (% predicted) | BMI (kg/m2) | BMD (g/cm2) | ∆BMD (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | ||||
| Patient 1 | 62 | NA | NA | 7 | 7 | 0.94 (20%) | 1.02 (22%) | 14.2 | 17.0 | 33% radiusa | 0.765 | 0.825 | +7.8 |
| Z-score | −1.9 | −1.3 | |||||||||||
| Hip total | 0.675 | 0.749 | +11 | ||||||||||
| Z-score | −2.5 | −1.9 | |||||||||||
| Patient 2 | 61 | 473 | 630 | 2 | 2 | 1.63 (38%) | 2.32 (54%) | 16.2 | 19.6 | Spine L1–L4 | 1.209 | 1.328 | +9.8 |
| Z-score | 0.9 | 2.0 | |||||||||||
| Hip total | 0.928 | 0.939 | +1.2 | ||||||||||
| Z-score | −0.5 | −0.4 | |||||||||||
| Patient 3 | 61 | 429 | 425 | 2 | 2 | 0.74 (28%) | 0.70 (27%) | 14.3 | 13.6 | Spine L1–L4 | 0.959 | 1.027 | +7.1 |
| Z-score | −1.3 | −0.7 | |||||||||||
| Hip total | 0.576 | 0.642 | +11.5 | ||||||||||
| Z-score | −3.1 | −2.4 | |||||||||||
| Patient 4 | 33 | 364 | 428 | 2.5 | 2 | 0.97 (36%) | 0.99 (38%) | 16.2 | 20.6 | Spine L1–L4 | 0.940 | 1.341 | +42.7 |
| Z-score | −1.5 | 1.9 | |||||||||||
| Hip total | 0.41 | 0.773 | +84.4 | ||||||||||
| Z-score | −4.3 | −1.5 | |||||||||||
Patients 1 and 2 were brothers, patients 3 and 4 were sisters
FVC forced vital capacity, BMI body mass index, NA not applicable
aPatient 1 had spinal surgery for scoliosis, BMD was measured from forearm instead of lumbar vertebra
Significant weight gains of 22, 21 and 27% was observed in patients 1, 2 and 4, respectively. Patient 3 did not gain weight after ERT and remained severely underweight. BMD had increased in all four patients after ERT. There was an increase of approximately 10% in patients 1, 2 and 3 and an astonishing 84.4% at the hip in patient 4. The improved Z-scores showed that patients 1, 3 and 4 had moved from osteoporosis to osteopenia after ERT.
Discussion
Low BMD causing a predisposition to fracture has been associated with LOPD [11, 12], but publications specifically addressing this are lacking. The only systematic study that involved 46 Pompe patients (both late- and infantile-onset forms) reported osteoporosis in 26%, and the majority of the affected were older, wheelchair-bound patients with long disease duration, leading to the speculation that weak loading force had led to osteoporosis [13]. Similar observations have been reported for other chronic myopathies, and the speculation was supported by an experimental study applying computed tomography to analyze the bone architecture [14, 15]. However, osteoporosis appears to be over-represented in LOPD compared to other severe hereditary myopathies such as Duchenne muscular dystrophy, in which most patients are wheelchair-bound as teenagers, while the majority of the LOPD patients are still ambulatory. Muscle strength per se is not a sufficient explanation for osteoporosis in LOPD. Chinese LOPD patients are characterized by an aggressive disease course with earlier emergence of symptoms, rapid deterioration and early respiratory failure, and most of them are slim and underweight [16, 17]. Since malnutrition and low body weight are major risk factors for low peak bone mass and BMD, this Chinese LOPD phenotype could be particularly vulnerable to osteoporosis. Our DXA results from a small sample of six patients with this typical phenotype do suggest that osteoporosis is more prevalent in Chinese LOPD. We believe that any BMD changes caused by ERT could be more readily observable in these high-risk patients.
Our four patients represented a spectrum of different disease severities, but they were all underweight at baseline before ERT. Three had osteoporosis, and one had normal BMD. All four patients showed improvement in BMD in the follow-up study. Patient 4 had the most prominent weigh gain, and her physical endurance improved, but there was no significant enhancement in her hip girdle muscle strength, and her mobility was only modestly better. Her huge gain in BMD was more likely a result of her recovery of body weight. Alendronate could not improve BMD by that much, and any positive effect from improvement in muscle strength should be modest. Patients 1 and 2 were both underweight and had similar weight gain after ERT. Patient 1 remained severely disabled, and patient 2 had good improvement in physical performance. The improvement in BMD was more marked in patient 1 than in patient 2, suggesting that the treatment effect could be more obvious in those with a lower baseline BMD and that strength might be less important than body weight. These observations agreed with the previous reports that described inconsistent ERT responses in LOPD patients with diverse characteristics [6, 18]. Nevertheless, patient 3 had similar disease severity to patient 4, but she did not gain weight, and her physical performance deteriorated slightly in the follow-up, yet she still had a 10% increase in BMD. Enzyme therapy could have a direct positive effect on bone turnover independent of weight and muscle strength.
Although it is acknowledged that Chinese LOPD patients commonly manifest an aggressive clinical course and low BMI is a poor prognostic marker in LOPD, the reason for such a high prevalence of malnutrition in Chinese LOPD is still poorly understood. Postulations include, among many, a chronic malabsorption state from gastrointestinal smooth muscle involvement in LOPD and a persistent catabolic state from respiratory failure [19, 20]. However, neither would be a strong contender with an impact on nutritional status to the degree we observe in LOPD.
Limitations
We observed a positive effect on BMD after ERT in a small group of Chinese LOPD patients, and suggested a few postulations to explain this association. However, our findings were mainly hypothesis generating, we were unable establish any causal relationship between ERT and BMD. Future studies should focus on catabolism, changes in body compositions, changes in bone turnover markers, and the balance of hormones and cytokines, both at baseline and with ERT, and take the secondary contributing factors into account to obtain a clearer picture on LOPD and bone health.
Authors’ contributions
Manuscript preparation and review, data interpretation: BS, YPC, WTW. Manuscript review and data interpretation: EKCY, SPLC, WHL. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All relevant data are presented in the manuscript. There is no additional data related to this report.
Consent for publication
Written informed consents were obtained from all patients.
Ethics approval and consent to participate
Written informed consents were obtained from all patients. The study was approved by the ethics committee of Kowloon West Cluster, Hospital Authority: KW/EX-12-139(7715).
Funding
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 6MWT
6-min walk test
- BMD
bone mineral density
- BMI
body mass index
- DXA
dual X-ray absorptiometry
- ERT
enzyme replacement therapy
- FVC
forced vital capacity
- LOPD
late-onset Pompe disease
- NIV
non-invasive ventilation
Contributor Information
Bun Sheng, Email: shengbun@hotmail.com.
Yim Pui Chu, Email: cyp285@pmh.ha.org.hk.
Wa Tai Wong, Email: wwt745@ha.org.hk.
Eric Kin Cheong Yau, Email: yaukc1@ha.org.hk.
Sammy Pak Lam Chen, Email: chenpls@ha.org.hk.
Wing Hang Luk, Email: lukwh@ha.org.hk.
References
- 1.Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC-H, Bossen E, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006;86(12):1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- 2.Raben N, Ralston E, Chien Y-H, Baum R, Schreiner C, Hwu W-L, et al. Differences in the predominance of lysosomal and autophagic pathologies between infants and adults with Pompe disease: implications for therapy. Mol Genet Metab. 2010;101(4):324–331. doi: 10.1016/j.ymgme.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nascimbeni AC, Fanin M, Masiero E, Angelini C, Sandri M. The role of autophagy in the pathogenesis of glycogen storage disease type II (GSDII) Cell Death Differ. 2012;19(10):1698–1708. doi: 10.1038/cdd.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagemans ML, Winkel LP, Van Doorn PA, Hop WJ, Loonen MC, Reuser AJ, et al. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005;128(Pt 3):671–677. doi: 10.1093/brain/awh384. [DOI] [PubMed] [Google Scholar]
- 5.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362(15):1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 6.Papadimas GK, Terzis G, Methenitis S, Spengos K, Papadopoulos C, Vassilopoulou S, et al. Body composition analysis in late-onset Pompe disease. Mol Genet Metab. 2011;102(1):41–43. doi: 10.1016/j.ymgme.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Hobson-Webb LD, Proia AD, Thurberg BL, Banugaria S, Prater SN, Kishnani PS. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab. 2012;106(4):462–469. doi: 10.1016/j.ymgme.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Filosto M, Todeschini A, Cotelli MS, Vielmi V, Rinaldi F, Rota S, et al. Non-muscle involvement in late-onset glycogenosis II. Acta Myol. 2013;32(2):91–94. [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Desai AK, Kazi ZB, Corey K, Austin S, Hobson-Webb LD, et al. The emerging phenotype of late-onset Pompe disease: a systematic literature review. Mol Genet Metab. 2017;120(3):163–172. doi: 10.1016/j.ymgme.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Slonim AE, Bulone L, Goldberg T, Minikes J, Slonim E, Galanko J, et al. Modification of the natural history of adult-onset acid maltase deficiency by nutrition and exercise therapy. Muscle Nerve. 2007;35(1):70–77. doi: 10.1002/mus.20665. [DOI] [PubMed] [Google Scholar]
- 11.Bertoldo F, Zappini F, Brigo M, Moggio M, Lucchini V, Angelini C, et al. Prevalence of asymptomatic vertebral fractures in late-onset Pompe disease. J Clin Endocrinol Metab. 2015;100(2):401–406. doi: 10.1210/jc.2014-2763. [DOI] [PubMed] [Google Scholar]
- 12.Case LE, Hanna R, Frush DP, Krishnamurthy V, DeArmey S, Mackey J, et al. Fractures in children with Pompe disease: a potentiallong-term complication. Pediatr Radiol. 2007;37(5):437–445. doi: 10.1007/s00247-007-0428-y. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg LE, Zandbergen AA, van Capelle CI, de Vries JM, Hop WC, van den Hout JM, et al. Low bone mass in Pompe disease: muscular strength as a predictor of bone mineral density. Bone. 2010;47(3):643–649. doi: 10.1016/j.bone.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Philippe V, Pruna L, Abdel Fattah M, Pascal V, Kaminsky P. Decreased bone mineral density in adult patients with muscular dystrophy. Joint Bone Spine. 2011;78(6):651–652. doi: 10.1016/j.jbspin.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Khan A, Weinstein Z, Hanley DA, Casey R, McNeil C, Ramage B, et al. In vivo bone architecture in Pompe disease using high-resolution peripheral computed tomography. JIMD Rep. 2013;7:81–88. doi: 10.1007/8904_2012_146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CC, Chien YH, Lee NC, Chiang SC, Lin SP, Kuo YT, et al. Rapid progressive course of later-onset Pompe disease in Chinese patients. Mol Genet Metab. 2011;104(3):284–288. doi: 10.1016/j.ymgme.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Chu YP, Sheng B, Lau KK, Chan HF, Kam GY, Lee HH, et al. Clinical manifestation of late onset Pompe disease patients in Hong Kong. Neuromuscul Disord. 2016;26(12):873–879. doi: 10.1016/j.nmd.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Papadimas G, Terzis G, Papadopoulos C, Areovimata A, Spengos K, Kavouras S, et al. Bone density in patients with late onset Pompe disease. Int J Endocrinol Metab. 2012;10(4):599–603. doi: 10.5812/ijem.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardo J, Garcia-Sobrino T, Lopez-Ferreiro A. Gastrointestinal symptoms in late-onset Pompe disease: early response to enzyme replacement therapy. J Neurol Sci. 2015;353(1–2):181–182. doi: 10.1016/j.jns.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1253–1262. doi: 10.1152/japplphysiol.00790.2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are presented in the manuscript. There is no additional data related to this report.


