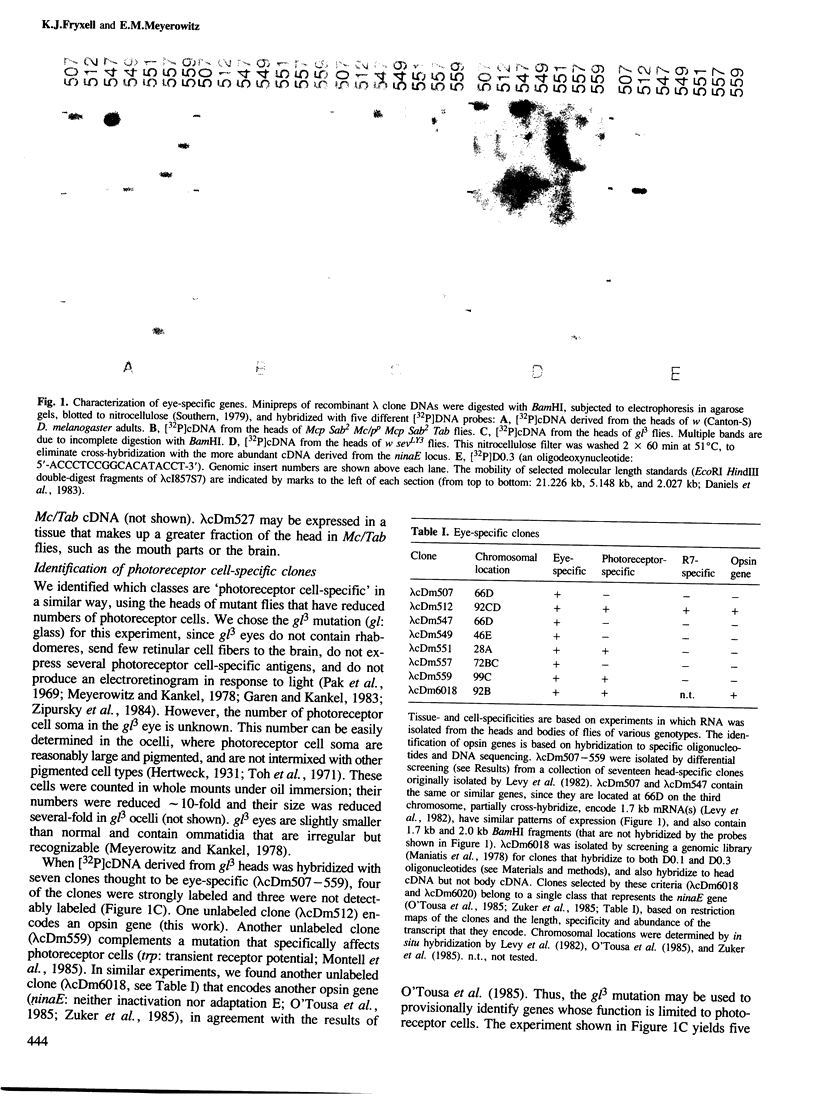
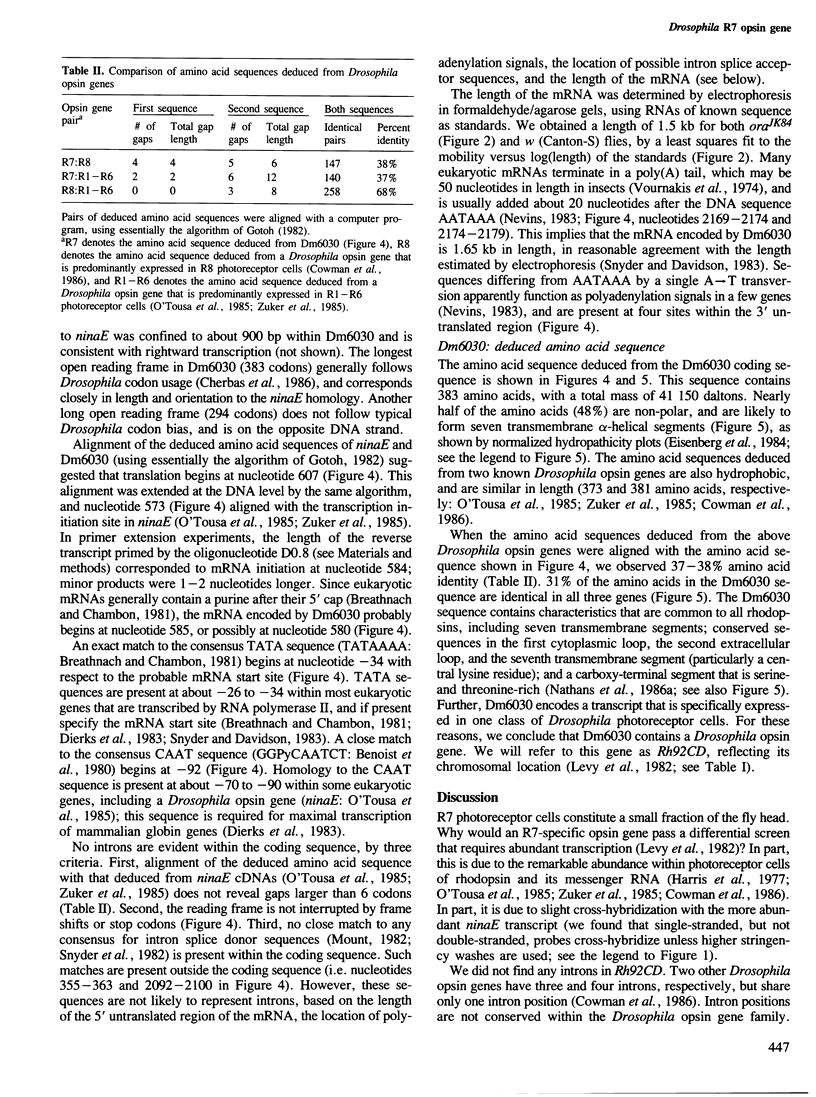
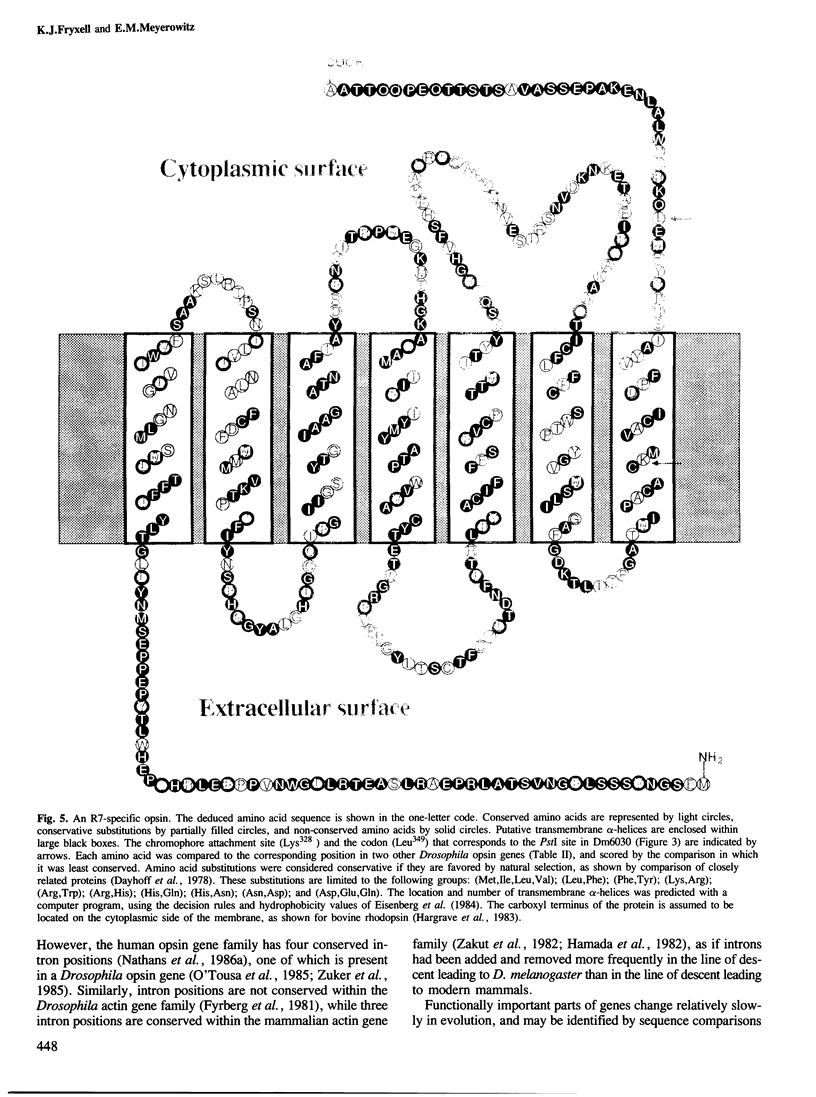
Abstract
We have used two techniques to isolate and characterize eye-specific genes from Drosophila melanogaster. First, we identified genes whose expression is limited to eyes, photoreceptor cells, or R7 photoreceptor cells by differential screening with [32P]cDNAs derived from the heads of mutant flies that have reduced amounts of these tissues and cells (Microcephalus, glass3, and sevenless, respectively). Secondly, we identified opsin genes by hybridization with synthetic [32P]oligonucleotides that encode domains that have been conserved between some opsin genes. We found seven clones that contain genes expressed only in the eye or optic lobes of Drosophila; three are expressed only in photoreceptor cells. One is expressed only in R7 photoreceptor cells and hybridizes to some of the previously mentioned oligonucleotides. The complete DNA sequence of the R7-specific opsin gene and its 5' and 3' flanking regions was determined. It is quite different from other known Drosophila opsin genes, in that it is not interrupted by introns and shares only 37-38% amino acid identity with the proteins encoded by these genes. The predicted protein structure contains many characteristics that are common to all rhodopsins, and the sequence differences help to identify four domains of the rhodopsin molecule that have been conserved in evolution.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Schulz R. A., Koehler M. M., Savakis C., Cherbas P. Structure of the Eip28/29 gene, an ecdysone-inducible gene from Drosophila. J Mol Biol. 1986 Jun 20;189(4):617–631. doi: 10.1016/0022-2836(86)90492-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Zuker C. S., Rubin G. M. An opsin gene expressed in only one photoreceptor cell type of the Drosophila eye. Cell. 1986 Mar 14;44(5):705–710. doi: 10.1016/0092-8674(86)90836-6. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Garen S. H., Kankel D. R. Golgi and genetic mosaic analyses of visual system mutants in Drosophila melanogaster. Dev Biol. 1983 Apr;96(2):445–466. doi: 10.1016/0012-1606(83)90182-3. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Gotoh O. An improved algorithm for matching biological sequences. J Mol Biol. 1982 Dec 15;162(3):705–708. doi: 10.1016/0022-2836(82)90398-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. Molecular structure and evolutionary origin of human cardiac muscle actin gene. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5901–5905. doi: 10.1073/pnas.79.19.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Harris W. A., Ready D. F., Lipson E. D., Hudspeth A. J., Stark W. S. Vitamin A deprivation and Drosophila photopigments. Nature. 1977 Apr 14;266(5603):648–650. doi: 10.1038/266648a0. [DOI] [PubMed] [Google Scholar]
- Harris W. A., Stark W. S., Walker J. A. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976 Apr;256(2):415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M., Kent S., Caruthers M., Dreyer W., Firca J., Giffin C., Horvath S., Hunkapiller T., Tempst P., Hood L. A microchemical facility for the analysis and synthesis of genes and proteins. Nature. 1984 Jul 12;310(5973):105–111. doi: 10.1038/310105a0. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Ganguly R., Ganguly N., Manning J. E. The selection, expression, and organization of a set of head-specific genes in Drosophila. Dev Biol. 1982 Dec;94(2):451–464. doi: 10.1016/0012-1606(82)90362-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Kankel D. R. A genetic analysis of visual system development in Drosophilia melanogaster. Dev Biol. 1978 Jan;62(1):112–142. doi: 10.1016/0012-1606(78)90096-9. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Martin C. H. Adjacent chromosomal regions can evolve at very different rates: evolution of the Drosophila 68C glue gene cluster. J Mol Evol. 1984;20(3-4):251–264. doi: 10.1007/BF02104731. [DOI] [PubMed] [Google Scholar]
- Montell C., Jones K., Hafen E., Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985 Nov 29;230(4729):1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neal M. W., Florini J. R. A rapid method for desalting small volumes of solution. Anal Biochem. 1973 Sep;55(1):328–330. doi: 10.1016/0003-2697(73)90325-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Pak W. L., Grossfield J., White N. V. Nonphototactic mutants in a study of vision of Drosophila. Nature. 1969 Apr 26;222(5191):351–354. doi: 10.1038/222351a0. [DOI] [PubMed] [Google Scholar]
- Pappin D. J., Findlay J. B. Sequence variability in the retinal-attachment domain of mammalian rhodopsins. Biochem J. 1984 Feb 1;217(3):605–613. doi: 10.1042/bj2170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A., Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974 Mar;71(3):708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom R. The time of action of three mutations affecting Drosophila eye morphogenesis. J Embryol Exp Morphol. 1979 Oct;53:225–235. [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976 Oct 15;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Davidson N. Two gene families clustered in a small region of the Drosophila genome. J Mol Biol. 1983 May 15;166(2):101–118. doi: 10.1016/s0022-2836(83)80001-1. [DOI] [PubMed] [Google Scholar]
- Snyder M., Hunkapiller M., Yuen D., Silvert D., Fristrom J., Davidson N. Cuticle protein genes of Drosophila: structure, organization and evolution of four clustered genes. Cell. 1982 Jul;29(3):1027–1040. doi: 10.1016/0092-8674(82)90466-4. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- To Y., Tominaga Y., Kuwabara M. The fine structure of the dorsal ocellus of the fleshfly. J Electron Microsc (Tokyo) 1971;20(1):53–66. [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Sevenless: a cell-specific homeotic mutation of the Drosophila eye. Science. 1986 Jan 24;231(4736):400–402. doi: 10.1126/science.231.4736.400. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Vournakis J. N., Gelinas R. E., Kafatos F. C. Short polyadenylic acid sequences in insect chorion messenger RNA. Cell. 1974 Nov;3(3):265–273. doi: 10.1016/0092-8674(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Zakut R., Shani M., Givol D., Neuman S., Yaffe D., Nudel U. Nucleotide sequence of the rat skeletal muscle actin gene. Nature. 1982 Aug 26;298(5877):857–859. doi: 10.1038/298857a0. [DOI] [PubMed] [Google Scholar]
- Zipursky S. L., Venkatesh T. R., Teplow D. B., Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984 Jan;36(1):15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- de Martynoff G., Pays E., Vassart G. Synthesis of a full length DNA complementary to thyroglobulin 33 S messenger RNA. Biochem Biophys Res Commun. 1980 Apr 14;93(3):645–653. doi: 10.1016/0006-291x(80)91127-4. [DOI] [PubMed] [Google Scholar]