Abstract
Background and Purpose
Multimodal interventions possess the strongest evidence in the long-term management of patellofemoral pain, but despite receiving evidence-based treatments that are initially effective many patients report recurrent or persistent symptoms for years after the initial diagnosis. Untreated psychological factors could be a possible explanation for persistent symptoms and poor treatment outcome. The purpose of this case report was to describe and evaluate the effects of a multimodal rehabilitation program that included pain education, a graded program of lower limb strengthening, and running retraining on pain, kinesiophobia, and function in a runner with patellofemoral pain.
Case description
The subject was a 37-year-old female runner reporting a 12-month history of anterior knee pain with previous failed physiotherapeutic treatment. She discontinued running when symptoms gradually worsened, approximately six months after initial onset. She was advised to avoid painful activities. Clinical examination revealed pain during the performance of a weight-bearing functional task, fear of movement, and functional limitations. Treatment focused on pain education, self-management strategies, and progressive loading of the involved tissues through a graduated program of exercises and running retraining.
Outcomes
Clinically meaningful improvements were seen in pain, kinesiophobia, and function following a 21-week multimodal rehabilitation program.
Discussion
This case report illustrates several important aspects of clinical reasoning contributing to successful outcomes for a runner with patellofemoral pain. The multimodal rehabilitation program utilized was based upon the neurophysiology of pain (pain education) rather than the tissue pathology model. The findings from this case report may be used to benefit clinicians with similar subject presentations and drive future research into the use of these interventions based upon neurophysiology models of pain in the treatment of patellofemoral pain.
Level of Evidence
Level 4
Keywords: Kinesiophobia, pain education, patellofemoral pain, running
BACKGROUND AND PURPOSE
Patellofemoral pain (PFP) is a prevalent multifactorial knee condition, accounting for 16.5% of all consultations in sports medicine clinics.1 Its impact is profound, often reducing the ability of those with PFP to perform sporting, physical activity and work-related activities pain-free. Multimodal interventions, which commonly consist of combined quadriceps and gluteal strengthening, along with appropriate patient education, possess the strongest evidence in the long term management of PFP.2 Furthermore, running gait retraining has been shown to be effective in reducing pain and improving function in runners with PFP.3 Nevertheless, despite receiving evidence-based treatments that are initially effective, the long term prognosis for PFP is still poor, with many patients reporting recurrent or persistent symptoms for years after the initial diagnosis.4
Growing evidence suggests that psychological features such as pain-related fear, anxiety, depression, catastrophising, and self-efficacy play a role in the development and maintenance of persistent musculoskeletal pain.5 They have also been associated with pain and disability and identified as barriers to recovery and as factors that limit the potential for improvement with rehabilitation. As a persistent musculoskeletal condition that is no longer considered self-limiting, PFP may also be characterised by the coexistence of physical and non-physical features.6,7 Psychological features such as fear-avoidance beliefs, anxiety, kinesiophobia and catastrophising are known to be prevalent and predictive of outcome in patients with PFP. Untreated psychological factors could be a possible explanation for persistent symptoms and poor treatment outcome. Therefore, co-interventions to reduce catastrophising beliefs and kinesiophobia may enhance the results. A rationale for an assessment and treatment approach that moves the focus away from a traditional biomedical and structural model of pain, towards one directed at the neurophysiology of pain, has been recently suggested.8
The purpose of this case report was to describe and evaluate the effects of a multimodal rehabilitation program that included pain education, a graded program of lower limb strengthening exercises, and running retraining on pain, kinesiophobia, and function in a runner with PFP.
CASE DESCRIPTION
The subject in this study was a 37-year-old female reporting a 12-months history of right anterior knee pain, with an insidious onset. She had been a recreational runner for fifteen years and participated in road races including half marathons and marathons. Without symptoms, the subject ran approximately 40 km per week on paved roads. However, she discontinued running when symptoms gradually worsened, approximately six months after initial onset. The subject denied any trauma to the knee and any previous history of knee or lower limb problems. She reported no other health problems. The subject saw an orthopaedic surgeon five months prior, who diagnosed her with patellofemoral pain syndrome. Magnetic resonance imaging at that time revealed small patellar osteophytes and high signal intensity of the Hoffa fat pad. She was advised to avoid running, cycling, and painful activities. Swimming was suggested, which the subject had diligently performed ever since. Anti-inflammatory medication had been prescribed for this condition, but the subject found no relief with this intervention. Previous physiotherapy treatment involved two months of quadriceps strengthening exercises, patellar taping, physical modalities, and foot insoles, with very little benefit. The subject was very worried regarding the progressively worsening of symptoms and because the orthopaedic surgeon suggested an arthroscopic lateral retinacular release if the pain did not improve. Furthermore, the previous physiotherapist highlighted an abnormal patellar tracking as cause of her pain and the fact that this could lead to a premature wear of the cartilage if she did not avoid running and painful activities.
The subject featured in this case report gave written informed consent to participate in the study and was informed that the data concerning the case report would be submitted for publication.
OUTCOME MEASURES
The outcome measures utilized in this case report included the Tampa Scale for Kinesiophobia-11 (TSK-11), the Knee Outcome Survey – Activities of Daily Living Scale questionnaire (KOS-ADLS), perceived knee pain during the performance of a forward step-down test (FSD) measured using the Numeric Pain Rating Scale (NPRS), and self-reported posterolateral hip pain (NPRS). The outcome measures were collected at initial examination, after eight weeks, and again at 21 weeks.
The TSK-11 is an 11-item questionnaire designed to assess fear of movement and reinjury while offering the advantage of brevity. All items are based on a 4-point Likert scale in which patient options range from strongly disagree to strongly agree. The TSK-11 scores range from 11 to 44, with higher scores indicating a higher degree of kinesiophobia. Despite the shortened format, the TSK-11 has demonstrated similar factor structure, internal consistency, test-retest reliability, and validity to the original TSK-17.9 The shortened version has been used extensively in orthopaedic populations, including patients with lower extremity disability.10 A minimally clinical important difference (MCID) in the level of kinesiophobia has been determined to be a 4-point difference in TSK-11 scores.9
The KOS-ADLS is a 14-item knee-specific questionnaire designed to evaluate symptoms and functional limitations experienced during activities of daily living in individuals with various knee disorders.11 Six items assess knee symptoms (pain, stiffness, swelling, instability, weakness, and limping) and eight items assess functional limitations (walking on a level surface, ascending and descending stairs, standing, kneeling, squatting, sitting, and rising from a chair). Each item is scored on a 6-point Likert scale (0–5 points). Total score on 70 is converted to a 0–100 point scale with 100 indicating the absence of symptoms and functional limitations. The KOS-ADLS has been validated in patients with PFP, with a reported MCID of 7 points.12 A systematic review pertaining to self-reported questionnaires used in individuals with PFP recommended the KOS-ADLS over other knee-specific scales based on its psychometric properties and clinical applicability for runners.13
The FSD is a clinical test designed to replicate stair descent, a functional task often limited by PFP.14 This test has been shown to be significantly correlated with pain in patients with PFP, and has demonstrated high intrasubject reliability which is a prerequisite for detecting true changes after treatment.15,16
The 11-point NPRS is a self-administered measure of the intensity of pain, ranging from 0 (no pain) to 10 (the worst imaginable pain). The NPRS has been validated in patients with PFP, with a reported MCID of 2 points.12 The NPRS was administered verbally.
EXAMINATION
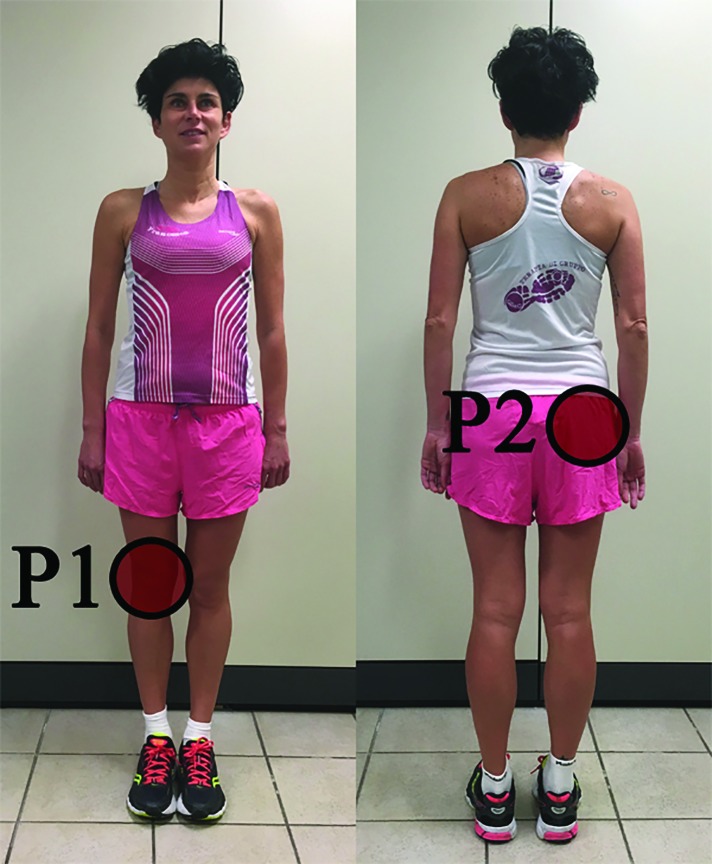
The subject described two areas of pain. The first area was located over the anterior region of the right knee (labeled P1, Figure 1). Her highest pain intensity on the NPRS in this location was rated as 7/10. The subject described the pain as intermittent and acute, provoked while sitting for longer than 30 min with her knee in a flexed position, descending stairs, squatting, and walking more than 30 min. Symptom eased with rest. The second area of pain was located in the posterolateral region of the right hip (labeled P2, Figure 1). The intensity of pain was rated as 5/10. The subject described the pain as intermittent and deep, provoked while sitting for longer than 30 min with her knee in a flexed position and standing for a longer than two hours.
Figure 1.
Pain diagram. P1 and P2 indicate two distinct areas of perceived pain by the subject, P1 = primary pain area, P2 = secondary pain area.
Active and passive examination of the lumbar spine was performed to rule out spine contributions to her symptoms. Active examination tests for the lumbar spine consisted of active range of motion, followed by active range of motion with overpressure in flexion, extension, and sidebending and rotation. Passive testing was performed using both central and lateral posterior-to-anterior spring tests from the fifth lumbar to the tenth thoracic spinal segments. There was no reproduction of her buttock or knee symptoms with active or passive testing to the lumbar spine. The subject's knee symptoms were not reproduced by the femoral slump test. Observation of the subject's knee revealed no swelling, bruising, or obvious bony deformity. Observation did not show also any differences in lower limb muscle trophism or significant postural asymmetries.
Palpation assessment of the knee showed tenderness over the medial aspect of the patella. No pain was noted on the patellar tendon or along knee medial or lateral joint lines. Soft tissue palpation revealed tender nodules in taut tissue band in the gluteus medius muscle, indicative of the likelihood of trigger points (TrPs) in the affected musculature. Deep palpation of gluteus medius muscle reproduced 7/10 pain in the P2 region.
The subject's range of motion for hip, knee and ankle, measurements were pain free and symmetrical bilaterally except for hip internal rotation with right internal rotation measured at 25 degrees and left internal rotation measured at 40 degrees.
Performance of the FSD reproduced 7/10 pain over the anterior region of the knee. This functional task also showed excessive dynamic knee valgus. At this stage it was felt the examination had reached the maximum possible level of testing without exacerbating the subject symptoms, therefore the examination ended. The subject's score on the KOS-ADLS was 49/100 and 30/44 on the TSK-11. Her goal was to reduce pain and return to running.
CLINICAL IMPRESSION
Subject report of anterior knee pain, aggravated by activities that load the patellofemoral joint (PFJ) during weight bearing, tenderness on patellar facet palpation, and pain on prolonged sitting were consistent with diagnosis of PFP.17 TrPs in the gluteus medius muscle were suspected as a potential source of pain in the posterolateral hip region. Subjects with PFP have a significant prevalence of TrPs in the gluteus medius that may be an important variable to consider in the evaluation and treatment of this condition.18 The subject showed elevated signs of fear and anxiety of movement and physical activity.
INTERVENTION
The subject was seen for a total of 20 visits over the course of 20 weeks.
First session
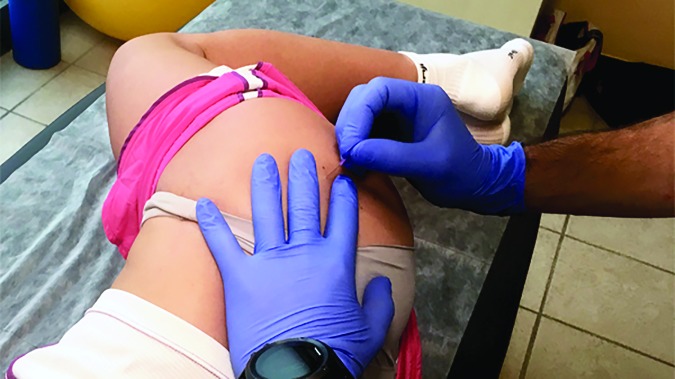
Immediate pain relief should be a priority in order to gain subject trust, facilitate active engagement and optimize long term outcomes.2 Therefore, posterolateral hip pain (P2) was treated in the first session. According to the literature, the ability to definitively ascertain the exact location of a TrP is questionable, and examiner experience plays a positive role in determining the presence of a TrP.19,20,21 Identification of a tender nodule in a taut band of muscle along with reproduction of the subject's subjective report of pain is the most clinically accurate way to recognize the presence of a TrP.22 Risks and potential complications were advised and written consent was obtained outlining common and serious adverse events associated with dry needling (DN) interventions. Common complications include bruising, vasovagal response, bleeding, and muscle soreness. More serious (but rare) complications include infection, broken needle, and pneumothorax.23 There were no reported contraindications to the use of DN. DN techniques are proposed to quickly reduce pain, remove peripheral sources of persistent nociceptive input, and improve range of motion in a host of pathological conditions, such as chronic lateral hip pain.24 DN was performed to the gluteus medius muscle at the area determined by deep palpation as a possible locations of the TrPs (Figure 2). The DN technique utilized ten fast-in/out movements in a cone pattern to attempt to target as many sensitive loci as possible within the tender nodule in the taut band of muscle.
Figure 2.
Dry needling of the gluteus medius muscle.
Appropriate patient education is essential to effective PFP management.2 Therefore, she was given the ‘Managing My Patellofemoral Pain’ education leaflet (created by Barton CJ and Rathleff MS) to facilitate knowledge retention and reduce emphasis on biomechanics.25 To facilitate adherence to home exercise and optimize recovery, she was given information on the importance of an active role over passive intervention and positive dose–response relationship between exercise and recovery.26
Intervention: Weeks 1-8
The subject was instructed to perform an exercise program divided into two phases. She was asked to perform prescribed exercises three times/week. Weekly meetings were scheduled to ensure proper execution of exercises and gradual progression of loads. Exercises were dosed and progressed according to pain levels and number of repetitions reached. The subject used the pain-monitoring model to grade the level and dosage of exercise and physical activity.27 According to the pain-monitoring model, the pain was allowed to reach level 5 on the visual analog scale, where 0 is no pain and 10 is the worst pain imaginable, during and after the exercise training, but should have subsided by the following morning. She was educated that it was not injurious to feel some pain during exercise during the exercise training. Increased fear of painful movement may be negative for recovery if it causes the subject to not load enough during the exercise program. Allowing for some amount of pain may be necessary to ensure that the load is sufficient to create meaningful adaptive changes in the PFJ. Descriptions of biomedical models of pain (e.g. abnormal patellar tracking, pronated foot or limb malalignment) and anatomic findings (patellar osteophytes and high signal intensity of Hoffa fat pad) were actively discouraged and challenged with pain described as a consequence of ‘de-conditioned’ tissue. A painful loaded exercise program focused on functional gains instead of symptom modification may have a key rule in fear avoidance education reconceptualizing pain, de-emphasizing anatomic findings and lower limb structural anomalies, and encouraging the patients to take an active role in their recovery.28,29
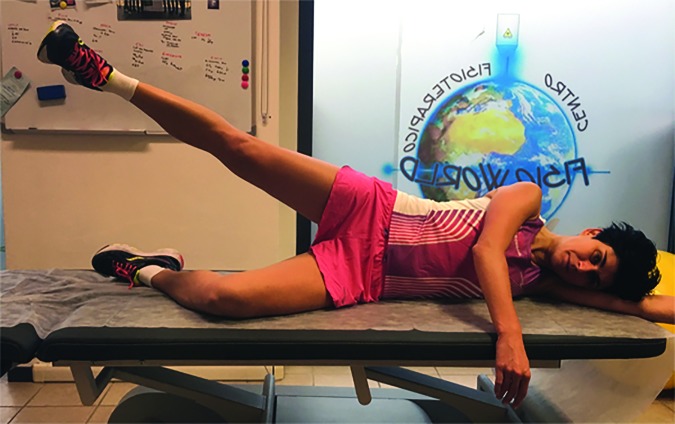
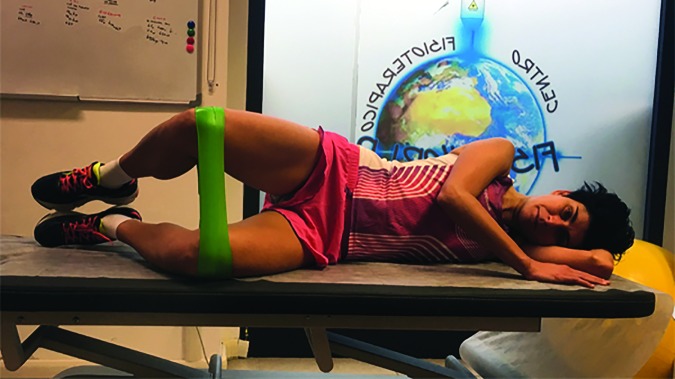
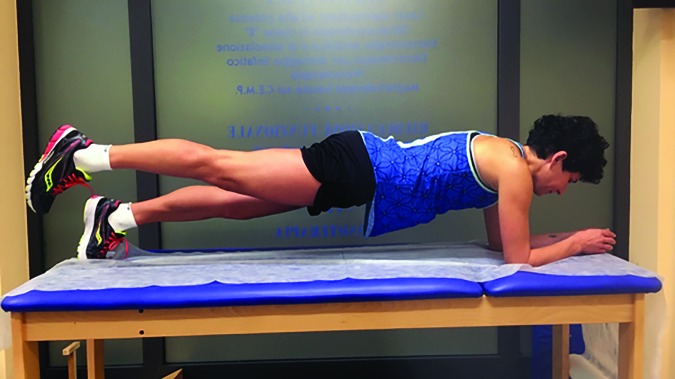
In Phase one floor exercises were performed to recruit gluteal, quadriceps, and core muscles (Figures 3-8).30,31 Repetitions were externally paced by a metronome. Externally paced resistance training may be used to introduce a skill based element that may improve motor control.32
Figure 3.
Side-lying hip abduction.
Figure 8.
Side plank.
Figure 4.
Side-lying clam with elastic band.
Figure 5.
Supine two-leg bridge.
Figure 6.
Supine one-leg bridge.
Figure 7.
Plank.
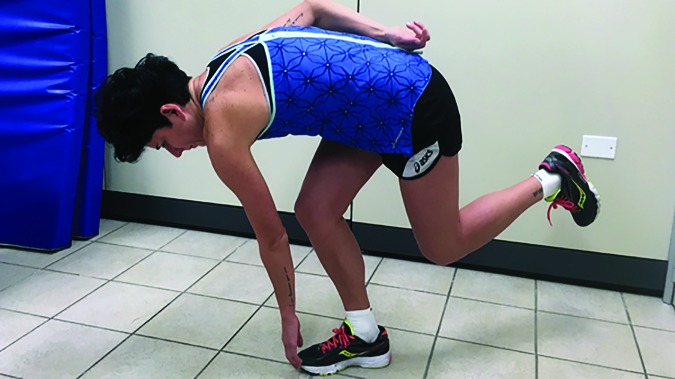
The main focus of Phase two of the treatment program was to progressively increase the exercises functional demand and load on PFJ by progressing hip and quadriceps exercises to weight bearing (Figures 9,10). Abdominal planks were progressed through higher levels of difficulty (Figures 11,12). An external focus of attention (attention directed towards the outcome or effects of the movement) was also added by using simple strategies, such us a cup full of water (the subject was asked to perform exercises without spilling water) (Figure 13), or a SenMoCOR™ (Sensory Motor Control-Oriented Rehabilitation) System (the subject performed exercises with immediate visual feedback using laser and laser target) (Figure 14). An externally focused exercise training may enhance skill acquisition more efficiently, promote utilization of unconscious or automatic processes, increase compliance, and increase the potential to transfer of improved motor skills to sport.33,34
Figure 9.

Squat with elastic band.
Figure 10.
One-leg squat.
Figure 11.
One-leg plank.
Figure 12.
Side plank with external focus.
Figure 13.

Step down with resistance from an elastic band and external focus.
Figure 14.

Squat with Sensory Motor Control-Oriented Rehabilitation.
Furthermore, the subject was advised to gradually start cycling with similar advice given on not to fear or avoid some amount of pain. In the authors’ clinical experience, cycling represents an effective exercise strategy for improving strength of the kinetic chain and load capacity of PFJ. The subject reported complete pain relief in P2 and hip internal rotation range of motion was symmetrical bilaterally after the first session. Therefore, other DN sessions or treatments had not been performed for this symptom.
Interventions: Weeks 9-20
The subject was evaluated on an instrumented treadmill (MyRun, Technogym, FC, Italy) at a preferred running pace of 6 minutes/km. Foot strike pattern was determined visually by looking at the slow motion video recording. Variables of interest were cadence (steps per minute), ground contact time (the time the foot is in contact with the ground during each step), and vertical oscillation (the amount of “bounce” in running motion), collected using Garmin Fenix 3 watch and Garmin HRM-Tri strap (Garmin Ltd, Southampton, UK). The subject had a forefoot strike pattern. The subject's running cadence was 168 steps per minute, ground contact time was 272 ms, and vertical oscillation was 9 cm. She reported 5/10 pain over the anterior region of the knee. The primary intervention was running gait retraining with step rate manipulation. To reduce PFJ contact forces and stress while running, she was asked to increase her step rate to 185 steps per minute. There is strong evidence indicating reduced PFJ stress/load with increasing the step rate by 10%.3,35,36 Step rate was maintained using an audible metronome set to 185 beats per minute. The subject was also provided with simple verbal cues that included “run quietly” and “reduce noise”. The subject was able to successfully achieve this cadence. After the step rate manipulation, the cadence was 185, ground contact time was 242 ms, and vertical oscillation was 7.7 cm. Pain improved to 3/10.
The subject received a personalized running program provided over 12 weeks (three sessions per week with at least one rest day between running days). She was advised to maintain her new step rate during running practice with help of the metronome and to avoid downhill running. She was instructed to utilize constant auditory cueing from the metronome until she was able to easily and consistently match the cadence goal. The run time was gradually increased from five minutes to 30 minutes over the 12 sessions. No specific recommendation was provided regarding lower limb alignment during running. Weekly meetings were scheduled to ensure gradual progression of running training. She was not to exceed a 5/10 pain intensity during training, according to the pain-monitoring model. The subject was advised to continue the home exercise program and cycling as cross-training.
OUTCOMES
Outcomes are shown in Table 1. Over time, KOS-ADLS score improved from a 49/100 to 90/100 and TSK-11 from 30/44 to 16/44. Subject met the MCID of 7 points on the KOS-ADLS and 4 points on the TSK-11. Additionally, perceived knee pain during the performance of FSD decreased from 7/10 to 2/10, which was successful in obtaining the required 2-point change to be a clinically meaningful improvement. The subject reported complete pain relief in the posterolateral region of the hip. Despite clinically relevant improvements with treatment, kinematics during the performance of FSD was unchanged, questioning the role of biomechanics as a contributor to pain in this subject. As previously described, factors thought to relate to PFP often remain after patients’ symptoms have resolved making their clinical importance difficult to determine.37 Subjectively, the subject reported that she was able to run without limitation. The subject also noted that her running pace had improved from 6:00 minutes/km to 5:45 minutes/km, but it is unclear whether this was related to the increased step rate, improved pain, reduced kinesiophobia, or factors related to training. An e-mail received nine months following discharge noted that the subject completed a marathon symptom free.
Table 1.
Outcome measures
| Initial exam | Week 8 | Week 21 | |
|---|---|---|---|
| TSK-11 | 30 | 22 | 16 |
| KOS-ADLS | 49 | 76 | 90 |
| Pain during FSD (NPRS) | 7 | 3 | 2 |
| Pain in P2 (NPRS) | 5 | 0 | 0 |
TSK-11 = Tampa Scale for Kinesiophobia-11, KOS-ADLS = Knee Outcome Survey – Activities of Daily Living Scale, FSD = Forward Step Down, NPRS = Numeric Pain Rating Scale.
DISCUSSION
This case report provides several important aspects related to clinical reasoning contributing to the successful outcomes for a runner with PFP. There is no consensus on the pathogenesis of PFP, with numerous biomechanical factors attributed to the greater PFJ stress and the development of pain and disability.6 As a result, various conservative interventions based upon tissue pathology models of pain have been proposed, including hip and quadriceps strengthening, patellar taping, braces, foot orthoses, stretching, and soft tissue manipulation.2 PFP is not a self-limiting condition, with recurrent or persistent symptoms often remaining for years after the initial diagnosis.4 Furthermore, the presence of structural abnormalities of the PFJ is not associated with pain and disability in many patients.38 A possible reason for the continued pain is that PFP is a multifactorial condition and the treatments may not address all of the contributing factors in each patient.
As in other musculoskeletal conditions in which chronic pain occurs, psychological and social factors may integrate these factors in order to explain and understand persistent or recurrent symptoms. Patients with long-lasting PFP show signs of kinesiophobia, pain catastrophising, fear-avoidance belief, anxiety, and excessively negative thoughts towards their pain and function.5,6,7 These factors were strong predictors of pain intensity, function, and disability in PFP patients.6,7 Therefore, co-interventions to reduce catastrophizing thinking and kinesiophobia may improve the long-term outcomes. A loaded exercise program that allowed for some pain during exercise and running retraining, with pain described as ‘de-conditioned’ tissue, along with appropriate patient pain education and self-management strategies, may desensitize the central nervous system and address fear avoidance, kinesiophobia, and catastrophising beliefs.8,29 A pain-monitoring model may guide patients on how to cope with and respond to pain during and after exercises.27 This model, within a framework that suggests ‘hurt does not equal harm’, may encourage patients to take an active role in their recovery, improve adherence to the exercise program, identify an appropriate load during exercises and running, and shift the focus to functional gains with rehabilitation instead of pain. Progressive loading of the PFJ may reduce local hyperalgesia and may alter central pain processing in individuals with PFP.39,40 Therefore, progressive hip and quadriceps strengthening may improve a patient's envelope of function by enhancing load tolerance of the PFJ.
Whether patellar taping should be first-line treatment for PFP is unclear.30 Patellar taping was not used because a previous healthcare practitioner had already used it without benefit to the subject. In addition, patellar taping could reinforce the subject's beliefs on a pathoanatomical source of pain, such as an abnormal patellar tracking, and consequently result in more kinesiophobia.
The subject tolerated the DN intervention to the hip region very well with no side effects reported following treatment. She reported a hypoalgesic effect following the DN intervention and only complained of minimal muscle soreness that lasted approximately two hours following treatment at the area of needle penetration. The use of DN demonstrated positive outcomes for reducing pain associated with lower quarter TrPs in the short-term.41 Immediate pain relief should be a priority to gain patient trust, facilitate active engagement, and optimize outcomes.2
There are a number of limitations associated with this case report. A number of pathologies may cause posterolateral hip pain worsened with prolonged standing and sitting and should be considered in the clinician's differential diagnosis. This case report uses only a single subject, as is typical of a case report. This is an inherent limitation offering only results that relate to this single subject that cannot be generalized to larger populations. The same physiotherapists who performed the initial evaluation also performed the treatments and completed the final evaluation. In future studies, using a different and potentially blinded evaluator could minimize potential bias.
As there is a lack of evidence describing the rehabilitation of psychological factors in subjects with PFP, as well as relationships with pain and physical function, additional systematic research is needed to determine the exact contribution of a painful loaded exercise program to the overall treatment approach provided to this population. The findings from this case report may be used to benefit clinicians with similar subject presentations and drive future research into the use of these interventions based upon neurophysiology models of pain and pain education in the treatment of PFP.
CONCLUSION
This case report describes the history, assessment and treatment of a runner with PFP who demonstrated clinically meaningful improvements in pain, kinesiophobia, and function following a 21-week multimodal rehabilitation program. This program was based upon the neurophysiology of pain rather than the tissue pathology model. Treatment focused on education and loading the tissues over many weeks through a graded program of loaded exercises and running retraining.
REFERENCES
- 1.Taunton JE Ryan MB Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton CJ Lack S Hemmings S, et al. The ‘Best Practice Guide to Conservative Management of Patellofemoral Pain’: incorporating level 1 evidence with expert clinical reasoning. Br J Sports Med. 2015;49(14):923-34. [DOI] [PubMed] [Google Scholar]
- 3.Esculier JF Bouyer LJ Roy JS. The Effects of a Multimodal Rehabilitation Program on Symptoms and Ground-Reaction Forces in Runners With Patellofemoral Pain Syndrome. J Sport Rehabil. 2016;25(1):23-30. [DOI] [PubMed] [Google Scholar]
- 4.Lankhorst NE van Middelkoop M Crossley KM, et al. Factors that predict a poor outcome 5-8 years after the diagnosis of patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2016;50(14):881-6. [DOI] [PubMed] [Google Scholar]
- 5.Maclachlan LR Collins NJ Matthews ML, et al. The psychological features of patellofemoral pain: a systematic review. Br J Sports Med. 2017;51(9):732-742. [DOI] [PubMed] [Google Scholar]
- 6.Domenech J Sanchis-Alfonso V López L, et al. Influence of kinesiophobia and catastrophizing on pain and disability in anterior knee pain patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1562-8. [DOI] [PubMed] [Google Scholar]
- 7.Piva SR Fitzgerald GK Wisniewski S, et al. Predictors of pain and function outcome after rehabilitation in patients with patellofemoral pain syndrome. J Rehabil Med. 2009;41(8):604-12. [DOI] [PubMed] [Google Scholar]
- 8.Smith BE Hendrick P Logan P. Patellofemoral pain: Challenging current practice - A case report. Man Ther. 2016;22:216-9. [DOI] [PubMed] [Google Scholar]
- 9.Woby SR Roach NK Urmston M, et al. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1-2):137-44. [DOI] [PubMed] [Google Scholar]
- 10.Kvist J Ek A Sporrstedt K, et al. Fear of re-injury: a hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2005;13(5):393-7. [DOI] [PubMed] [Google Scholar]
- 11.Irrgang JJ Snyder-Mackler L Wainner RS, et al. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-45. [DOI] [PubMed] [Google Scholar]
- 12.Piva SR Gil AB Moore CG, et al. Responsiveness of the activities of daily living scale of the knee outcome survey and numeric pain rating scale in patients with patellofemoral pain. J Rehabil Med. 2009;41(3):129-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esculier JF Roy JS Bouyer LJ. Psychometric evidence of self-reported questionnaires for patellofemoral pain syndrome: a systematic review. Disabil Rehabil. 2013;35(26):2181-90. [DOI] [PubMed] [Google Scholar]
- 14.Crossley KM Cowan SM McConnell J, et al. Physical therapy improves knee flexion during stair ambulation in patellofemoral pain. Med Sci Sports Exerc. 2005;37(2):176-83. [DOI] [PubMed] [Google Scholar]
- 15.Loudon JK Wiesner D Goist-Foley HL, et al. Intrarater Reliability of Functional Performance Tests for Subjects With Patellofemoral Pain Syndrome. J Athl Train. 2002;37(3):256-261. [PMC free article] [PubMed] [Google Scholar]
- 16.Nunes GS Stapait EL Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: Systematic review with meta-analysis. Phys Ther Sport. 2013;14(1):54-9. [DOI] [PubMed] [Google Scholar]
- 17.Crossley KM Stefanik JJ Selfe J, et al. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50(14):839-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roach S Sorenson E Headley B, et al. Prevalence of myofascial trigger points in the hip in patellofemoral pain. Arch Phys Med Rehabil. 2013;94(3):522-6. [DOI] [PubMed] [Google Scholar]
- 19.Tough EA White AR Richards S, et al. Variability of criteria used to diagnose myofascial trigger point pain syndrome - evidence from a review of the literature. Clin J Pain. 2007;23(3):278-86. [DOI] [PubMed] [Google Scholar]
- 20.Lucas N Macaskill P Irwig L, et al. Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain. 2009;25(1):80-9. [DOI] [PubMed] [Google Scholar]
- 21.Myburgh C Lauridsen HH Larsen AH, et al. Standardized manual palpation of myofascial trigger points in relation to neck/shoulder pain; the influence of clinical experience on inter-examiner reproducibility. Man Ther. 2011;16(2):136-140. [DOI] [PubMed] [Google Scholar]
- 22.Gerwin RD Shannon S Hong CZ, et al. Interrater reliability in myofascial trigger point examination. Pain. 1997;69(1-2):65-73. [DOI] [PubMed] [Google Scholar]
- 23.Brady S McEvoy J Dommerholt J, et al. Adverse events following trigger point dry needling: a prospective survey of chartered physiotherapists. J Man Manip Ther. 2014;22(3):134-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavkovich R. The use of dry needling for a subject with chronic lateral hip and thigh pain: a case report. Int J Sports Phys Ther. 2015;10(2):246-55. [PMC free article] [PubMed] [Google Scholar]
- 25.Barton CJ Rathleff MS. ‘Managing My Patellofemoral Pain’: the creation of an education leaflet for patients. BMJ Open Sport & Exercise Medicine. 2016;2:e000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathleff MS Roos EM Olesen JL, et al. Exercise during school hours when added to patient education improves outcome for 2 years in adolescent patellofemoral pain: a cluster randomised trial. Br J Sports Med. 2015;49(6):406-12. [DOI] [PubMed] [Google Scholar]
- 27.Thomeé R. A comprehensive treatment approach for patellofemoral pain syndrome in young women. Phys Ther. 1997;77(12):1690-703. [DOI] [PubMed] [Google Scholar]
- 28.Selhorst M Rice W Degenhart T, et al. Evaluation of a treatment algorithm for patients with patellofemoral pain syndrome: a pilot study. Int J Sports Phys Ther. 2015;10(2):178-88. [PMC free article] [PubMed] [Google Scholar]
- 29.Littlewood C Malliaras P Bateman M, et al. The central nervous system - An additional consideration in ‘rotator cuff tendinopathy’ and a potential basis for understanding response to loaded therapeutic exercise. Man Ther. 2013;18(6):468-72. [DOI] [PubMed] [Google Scholar]
- 30.Crossley KM van Middelkoop M Callaghan MJ, et al. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 2: recommended physical interventions (exercise, taping, bracing, foot orthoses and combined interventions). Br J Sports Med. 2016;50(14):844-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earl JE Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39(1):154-63. [DOI] [PubMed] [Google Scholar]
- 32.Rio E Kidgell D Moseley GL, et al. Tendon neuroplastic training: changing the way we think about tendon rehabilitation: a narrative review. Br J Sports Med. 2016;50(4):209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjaminse A Gokeler A Dowling AV, et al. Optimization of the anterior cruciate ligament injury prevention paradigm: novel feedback techniques to enhance motor learning and reduce injury risk. J Orthop Sports Phys Ther. 2015;45(3):170-82. [DOI] [PubMed] [Google Scholar]
- 34.Grooms D Appelbaum G Onate J. Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. J Orthop Sports Phys Ther. 2015;45(5):381-93. [DOI] [PubMed] [Google Scholar]
- 35.Lenhart RL Thelen DG Wille CM, et al. Increasing running step rate reduces patellofemoral joint forces. Med Sci Sports Exerc. 2014;46(3):557-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton CJ Bonanno DR Carr J, et al. Running retraining to treat lower limb injuries: a mixed-methods study of current evidence synthesised with expert opinion. Br J Sports Med. 2016;50(9):513-26. [DOI] [PubMed] [Google Scholar]
- 37.Dye SF. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;(436):100-10. [DOI] [PubMed] [Google Scholar]
- 38.van der Heijden RA, de Kanter JL, Bierma-Zeinstra SM, et al. Structural Abnormalities on Magnetic Resonance Imaging in Patients With Patellofemoral Pain: A Cross-sectional Case-Control Study. Am J Sports Med. 2016;44(9):2339-46. [DOI] [PubMed] [Google Scholar]
- 39.Pazzinatto MF de Oliveira Silva D Barton C, et al. Female Adults with Patellofemoral Pain Are Characterized by Widespread Hyperalgesia, Which Is Not Affected Immediately by Patellofemoral Joint Loading. Pain Med. 2016;17(10):1953-1961. [DOI] [PubMed] [Google Scholar]
- 40.Østerås B Østerås H Torstensen TA, et al. Dose-response effects of medical exercise therapy in patients with patellofemoral pain syndrome: a randomised controlled clinical trial. Physiotherapy. 2013;99(2):126-31. [DOI] [PubMed] [Google Scholar]
- 41.Morihisa R Eskew J McNamara A, et al. Dry Needling in subject with muscular trigger points in the lower quarter: a systematic review. Int J Sports Phys Ther. 2016;11(1):1-14. [PMC free article] [PubMed] [Google Scholar]