Abstract
Background & Aims
B-vitamins and homocysteine may contribute to the development of gestational diabetes mellitus (GDM), but existing studies are inconsistent. We examined the cross-sectional associations of plasma folate, vitamins B6, B12, and homocysteine concentrations with GDM and glycaemia in a sample of multi-ethnic Asian pregnant women.
Methods
Plasma concentrations of folate, vitamins B6, B12, homocysteine and glucose were measured at 26-weeks’ gestation in 913 pregnant women. GDM was diagnosed using the 1999 World Health Organization criteria. Associations were examined with linear or logistic regression, adjusted for confounders and stratified by ethnicity.
Results
Higher plasma folate was associated with higher 2-hour glucose and higher odds of GDM [0.15 (0.02, 0.23) per 1-SD increment in folate, OR 1.29 (1.00, 1.60)], mainly among Indian mothers. Higher plasma vitamin B12 and homocysteine were associated with lower fasting and 2-hour glucose, and lower odds of GDM [-0.04 (-0.07, -0.01) per 1-SD increment in B12 and -0.09 (-0.18, -0.003) respectively, OR: 0.81 (0.68, 0.97); -0.05 (-0.08, -0.02) per 1-SD increment in homocysteine and -0.12 (-0.21, -0.02) respectively, OR: 0.76 (0.62, 0.92)]. The highest odds of GDM were observed among women with combined vitamin B12 insufficiency and high folate concentration [OR: 1.97 (1.05, 3.68)]. An association between higher vitamin B6 and higher 2-hour glucose shifted towards null adjusting for other B-vitamins.
Conclusions
Higher maternal folate coupled with vitamin B12 insufficiency was associated with higher GDM risk. This finding has potential implications for antenatal supplement recommendations but will require confirmation in future studies.
Keywords: Folate, Vitamin B6, Vitamin B12, Homocysteine, Pregnancy, Gestational Diabetes
Introduction
Gestational diabetes mellitus (GDM) is diagnosed when a woman has high blood sugar levels for the first time during pregnancy [1]. Globally, the prevalence of GDM is approximately 17%, but a number of Asian countries have a much higher prevalence of more than 20% [2]. The increased risk of GDM among certain Asian ethnic groups [3, 4], advanced maternal age, and the increasing prevalence of obesity in this region [5] are all contributing factors to the high prevalence of GDM in Asia. If poorly managed, this metabolic disorder increases risk of pre-eclampsia in mothers, and preterm birth, hypoglycemia and macrosomia in infants [2]. With GDM on the rise, there is urgent need for more cost-effective strategies to reduce the burden of maternal and offspring consequences.
Much research to date has been conducted on maternal macronutrient intake especially carbohydrates and fats in relation to the risk of GDM [6], but less is known about the role of micronutrients, including B-vitamins, in influencing GDM. It has been proposed that B-vitamins, in particular folate, vitamins B6 and B12, may be involved in the pathogenesis of glucose intolerance due to their ability to regulate synthesis of homocysteine [7], which at elevated concentrations has been linked with insulin resistance [8, 9]. Although there is no definitive evidence, it has been speculated that elevated homocysteine concentrations impairs endothelial function in skeletal muscles, adipose tissue and liver, thus reducing insulin delivery to these insulin-sensitive tissues [10, 11].
Results from studies relating maternal folate, vitamins B6 and B12 and homocysteine to blood glucose concentrations or GDM risk have been inconclusive. In a cohort study in China, daily intake of folate supplements during early pregnancy was associated with a higher risk of GDM [12]. Being vitamin B12 deficient when pregnant was associated with a higher risk of GDM and type 2 diabetes mellitus (T2DM) at five years postpartum in a subpopulation of South Asian Indians [13]. Lower vitamin B12 concentrations were observed in a group of Italian women with GDM compared to healthy subjects [14]; however, in several other European populations, folate and vitamin B12 concentrations did not differ substantially for women with GDM compared to those without [15–17]. Similarly, in a UK study, no significant association between plasma vitamin B12 concentration and fasting plasma glucose was observed [18]. An intervention study in the Netherlands demonstrated a reduction in postprandial glucose concentrations in pregnant women after vitamin B6 supplementation [19]. Higher homocysteine concentrations have been associated with GDM women in some [14, 15, 17] but not all [16, 20, 21] cross-sectional studies in Western populations.
Most studies to date have been conducted in Western settings [14–21], but recent evidence suggests that associations between B-vitamins, homocysteine and GDM may differ in Asians [12, 13]. Therefore, our study aimed to examine the associations of plasma folate, vitamins B6, B12, and homocysteine with GDM risk and plasma glucose concentrations during pregnancy, using data from a mother-offspring cohort study which included mothers of Chinese, Malay, and Indian ethnicity. We hypothesized that higher plasma concentrations of folate and vitamin B6, lower plasma concentration of vitamin B12, and a corresponding higher plasma concentration of homocysteine would be associated with higher plasma glucose concentrations and higher risk of GDM during pregnancy.
Materials and Methods
Study sample
The Growing Up in Singapore Towards healthy Outcomes (GUSTO) study is a prospective investigation of mother-offspring pairs in Singapore, which involves detailed assessments of important factors relating to health and wellbeing of pregnant women (18-46 years) and their offspring [22]. Recruitment took place during June 2009 and September 2010 at the National University Hospital (NUH) and KK Women’s and Children’s Hospital (KKH), where women less than 14 weeks pregnant were approached to participate. Participants have to be citizens or permanent residents of Singapore, and of Chinese, Malay or Indian descent with homogenous parental ethnic background. Only women who intended to deliver in the two hospitals, to live in Singapore for the following five years, and consented to donate birth tissues at delivery were eligible to participate. Women who were undergoing chemotherapy, receiving psychotropic drugs or diagnosed with type1 diabetes were not eligible. A total of 1247 pregnant women participated at baseline, and socio-demographic information and medical history were obtained. A detailed description of study methodology have been published [22]. The GUSTO study has received ethics approval from the Institutional Review Board of NUH and KKH. All participants provided written informed consent before being entered into the study.
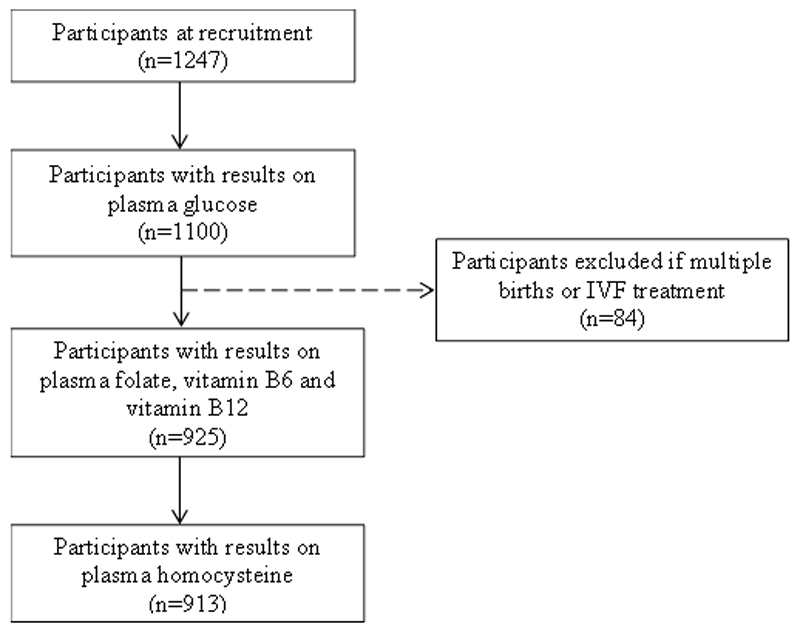
Participants returned to the hospital for a follow-up visit at 26-28 weeks of gestation, during which diet and lifestyle information and blood samples were obtained. The current analyses included women who provided sufficient blood for analyses of plasma folate, vitamins B6 and B12, homocysteine and glucose. Women who had multiple births or received in vitro fertilization treatment were excluded (n=84). Hence, the final analyses included a total of 913 participants (Figure 1). The 334 women excluded from the analyses tended to be younger (mean ± SD: 29.3 ± 5.3 vs 30.6 ± 5.2 years), and a higher proportion of them attained secondary education or lower (37.7% vs 30.7%) and received less than $2000 monthly income (22.4% vs 14.9%) compared to those included.
Figure 1.
Participant flow diagram for analysis of associations between plasma B-vitamins, homocysteine and gestational diabetes mellitus in the Growing Up in Singapore Towards healthy Outcomes study.
Assessment of plasma B vitamins (folate, vitamins B6 and B12) and homocysteine
Blood samples were retrieved from participants during the follow-up clinic visit at 26-28 weeks gestation, after an overnight (8-10 hours) fast using standard venipuncture techniques. All blood samples were kept in EDTA tubes, processed and stored at -80°C within 4 hours of collection, and thawed just prior to analysis. Plasma concentrations of folate and vitamin B12 were quantified using the competitive electrochemiluminescence immunoassay (ADVIA Centaur Immunoassay System, SIEMENS) at the NUH Referral laboratory. Between-assay CVs were 6.0-10.5% for 4.1-22.3 nmol/L plasma folate samples; and 4.3-8.8% for 160-490 pmol/L plasma vitamin B12 samples. Plasma vitamin B6 concentration was measured using reversed-phase HPLC with post–column derivatization and fluorometric detection, with between-assay CV of <5% at the MRC Human Nutrition Research, Elsie Widdowson Laboratory, UK. Plasma homocysteine was determined using HPLC (1100 series, Agilent Technologies) and mass-spectrometry (API 3000, AB Sciex) as described by Midttun et al. [23] at the Bevital AS laboratory. The between-assay CV was <2%.
Assessment of GDM
GDM was diagnosed at the same clinic visit, based on plasma glucose concentrations measured at a fasting state and two hours after a 75g oral glucose tolerance test (OGTT) was administered. Plasma glucose concentrations were analyzed using the colorimetry method (Advia 2400 Chemistry system, Siemens Medical Solutions Diagnostics; and Beckman LX20 Pro analyzer, Beckman Coulter). Participants were classified as having GDM, based on the 1999 World Health Organization standard criteria [24], if they met one of the following: (1) ≥7.0 mmol/L of fasting plasma glucose concentrations, (2) ≥7.8 mmol/L of plasma glucose concentrations 2-hour post-OGTT (1-hour blood samples were not collected).
Assessment of covariates
Potential confounding variables were identified from previous studies [13, 18]. Socio-demographic variables included: maternal age, ethnicity (Chinese, Malay, Indian), education (none/primary/secondary, post-secondary, university), and monthly household income ($0-1999, 2000-5999, ≥6000). Health behavior indicators included: cigarette smoking (non-smoker, former smoker, current smoker), alcohol intake, and physical activity – defined as minutes of metabolic equivalents of task (MET. mins) per week [25]. Pre-pregnancy BMI and a number of maternal conditions: parity, previous history of GDM and family history of T2DM, were also included.
Statistical analyses
Concentrations of plasma B vitamins and homocysteine were summarized according to maternal characteristics. Differences in concentrations between groups were compared using non-parametric analyses, namely the Wilcoxon rank-sum and Kruskal-Wallis tests, as distributions for plasma B-vitamins and homocysteine were skewed. For significant results, post-hoc analyses were carried out with Sidak adjustment to identify groups which differed.
The values for plasma folate, vitamins B6 and B12, and homocysteine were log-transformed, and then converted into SD scores to facilitate comparison across exposures. The effect sizes for plasma glucose concentrations and GDM represent changes in outcomes per 1-SD increment in log-transformed concentrations of plasma B-vitamins and homocysteine.
Associations of plasma B vitamins, homocysteine with fasting and 2-hour plasma glucose concentrations were examined using linear regressions, whereas the associations with GDM were examined with logistic regressions. The models were first adjusted for maternal age, ethnicity, education, income, smoking, alcohol intake, physical activity, pre-pregnancy BMI, parity, family history of diabetes and previous GDM (Model 1); and then included mutual adjustment of folate, vitamins B6 and B12 (Model 2). The latter was done to elucidate the independent association of each B-vitamins with plasma glucose concentrations and GDM and as B-vitamins intakes may be clustered due to similar dietary sources, and because they share the same metabolic pathway [7] thus may influence synthesis of each other.
In addition, the odds of GDM from combinations of folate and vitamin B12 status were estimated as two other studies have shown that high folate coupled with vitamin B12 deficiency was associated with GDM and T2DM risks [13, 26]. Participants were categorized into those with insufficient (<221nmol/L) or adequate vitamin B12. Within each group, they were split according to tertiles of folate. Women with lowest folate concentrations (tertile-1) were used as the reference within each group of vitamin B12 status. We used vitamin B12 insufficiency and tertiles of folate concentrations instead of deficiency status, as the combination of folate (<6.8nmol/L) and vitamin B12 (<148pmol/L) deficiencies [27] resulted in a small number of participants (n=29) with low power to detect statistically significant differences between groups.
Stratified analyses according to ethnicity were performed to account for ethnic differences in insulin resistance [3]. Multiplicative interaction terms were included to assess potential effect modifications of ethnicity on the associations of plasma B-vitamins and homocysteine with plasma glucose concentrations and GDM. Chinese ethnicity was the reference.
Approximately 93% (n=850) of the study sample had complete data for confounding variables. Missing values were imputed with the sample’s average (mode for categorical variables or median for continuous variables). Pre-pregnancy BMI was imputed using BMI obtained at recruitment (<14 weeks gestation) which showed a high correlation with BMI before pregnancy (r=0.965). To explore potential bias introduced by imputation of missing data, we repeated the analyses excluding participants with missing data. Similar study estimates and significance levels were observed; hence results were only reported for main analyses.
The above statistical analyses were performed using Stata version14 (StataCorp LP, College Station, TX, USA). P-values <0.05 were taken as significant.
Results
Participant characteristics
Concentrations of plasma folate, vitamins B6, B12, and homocysteine according to maternal characteristics are presented in Table 1. Women with lower folate concentrations tended to be younger, be Malay, have a lower educational level, have a lower income, and to be former smokers. Those with lower vitamin B12 concentrations tended to be Indian, be overweight or obese, be multiparous, and to have a family history of diabetes. Malay women were more likely to have lower vitamin B6 but higher homocysteine concentrations. Based on clinically used cut-offs [27], vitamins B6 (<20nmol/L) and B12 deficiencies (<148pmol/L) were observed in 16% (n=150) of the study sample, but less than 1% (n=24) of the cohort were folate deficient (<6.8nmol/L). A higher proportion of Malay women were vitamin B6 deficient (21.0% vs 13.5% Chinese and 17.2% Indians), and a higher proportion of Indian women were vitamin B12 deficient (27.8% vs 13.7% Chinese and 14.3% Malay). All plasma B-vitamins were negatively correlated with plasma homocysteine (folate: r= -0.21, vitamin B6: r= -0.21, vitamin B12: r= -0.17; all P-values<0.001).
Table 1.
Concentrations of plasma folate, vitamin B6, vitamin B12 and homocysteine according to maternal characteristics (n=913)
| n (%) | Folate, nmol/L | P | B6, nmol/L | P | B12, pmol/L | P | Hcy, µmol/L | P | |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| <35 years | 705 (77.2) | 33.8 (21.8, 43.7) | 0.001 | 57.9 (25.1, 109.7) | 0.106 | 208 (165, 258) | 0.987 | 4.8 (4.2, 5.6) | 0.940 |
| ≥35 years | 208 (22.8) | 37.1 (30.1, 47.7) | 71.2 (29.0, 121.4) | 211 (168, 253) | 4.9 (4.2, 5.6) | ||||
| Ethnicity | |||||||||
| Chinese | 498 (54.5) | 35.8 (27.8, 47.1)a | 0.001 | 63.0 (29.2, 115.6)a | 0.026 | 219 (176, 259)a | 0.001 | 4.8 (4.2, 5.4)a | 0.001 |
| Malay | 250 (27.4) | 29.3 (16.0, 40.3)a,b | 52.8 (23.1, 102.3)a | 212 (168, 266) b | 5.0 (4.3, 5.9)a | ||||
| Indian | 165 (18.1) | 35.7 (24.0, 44.7)b | 64.5 (25.4, 109.5) | 186 (142, 226)a,b | 4.9 (4.2, 5.6) | ||||
| Education | |||||||||
| None/Primary/ Secondary | 279 (30.6) | 32.5 (19.3, 40.4)a,b | 0.001 | 62.3 (24.5, 114.5) | 0.701 | 204 (166, 258) | 0.256 | 4.9 (4.3, 5.7) | 0.120 |
| Post-secondary | 318 (34.8) | 33.7 (24.1, 44.0)a,c | 63.3 (28.4, 114.7) | 208 (162, 252) | 4.9 (4.2, 5.6) | ||||
| University | 316 (34.6) | 37.7 (28.1, 48.9)b,c | 56.5 (27.0, 107.1) | 218 (170, 261) | 4.7 (4.2, 5.4) | ||||
| Monthly household income (SGD) | |||||||||
| <1999 | 127 (13.9) | 31.8 (18.3, 44.2)a | 0.001 | 50.2 (23.3, 113.0) | 0.198 | 209 (174, 249) | 0.376 | 4.9 (4.2, 5.8) | 0.339 |
| 2000-5999 | 543 (59.5) | 33.8 (23.5, 42.5)b | 67.9 (27.8, 113.6) | 206 (162, 258) | 4.8 (4.2, 5.6) | ||||
| ≥6000 | 243 (26.6) | 37.9 (30.0, 51.1)a,b | 52.6 (26.1, 108.2) | 215 (175, 263) | 4.8 (4.2, 5.4) | ||||
| Pre-pregnancy BMI (kg/m2) | |||||||||
| Under (<18.5) | 110 (12.1) | 34.6 (24.4, 44.1) | 0.204 | 55.1 (24.9, 109.4) | 0.625 | 230 (176, 285)a,b | 0.001 | 4.9 (4.1, 5.6) | 0.541 |
| Normal (18.5-22.9) | 457 (50.1) | 35.3 (25.0, 45.9) | 60.8 (26.1, 114.9) | 220 (175, 273)c,d | 4.8 (4.2, 5.5) | ||||
| Over (23.0-27.5) | 215 (23.5) | 33.7 (24.1, 44.3) | 67.1 (27.8, 109.3) | 200 (158, 245)a,c | 4.8 (4.3, 5.6) | ||||
| Obese (>27.5) | 131 (14.3) | 32.2 (20.8, 42.6) | 58.5 (21.5, 116.4) | 187 (139, 226)b,d | 5.0 (4.2, 5.7) | ||||
| Smoking during pregnancy | |||||||||
| Non-smoker | 788 (86.3) | 35.0 (25.4, 45.0)a | 0.001 | 62.7 (27.0, 113.4) | 0.253 | 214 (168, 258) | 0.061 | 4.8 (4.2, 5.5) | 0.364 |
| Former smoker | 101 (11.1) | 26.2 (14.0, 38.5)a | 52.8 (23.3, 104.7) | 192 (163, 235) | 4.8 (4.0, 5.7) | ||||
| Current smoker | 24 (2.6) | 36.3 (21.6, 44.8) | 89.3 (35.0, 105.1) | 194 (173, 286) | 5.2 (4.4, 6.0) | ||||
| Exercise (MET.mins/week) | |||||||||
| Low (<600) | 291 (31.9) | 34.3 (25.0, 43.8) | 0.060 | 61.8 (28.7, 108.2) | 0.814 | 218 (175, 256) | 0.148 | 4.8 (4.2, 5.5) | 0.793 |
| Moderate (600-1500) | 278 (30.4) | 35.3 (24.7, 46.9) | 66.9 (27.0, 113.1) | 215 (168, 264) | 4.8 (4.3, 5.5) | ||||
| High (>1500) | 344 (37.7) | 33.5 (22.9, 43.0) | 56.9 (24.6, 116.0) | 202 (162, 252) | 4.9 (4.2, 5.7) | ||||
| Alcohol consumption | |||||||||
| No | 894 (97.9) | 34.5 (24.5, 44.6) | 0.210 | 61.8 (26.3, 113.0) | 0.115 | 209 (165, 256) | 0.104 | 4.8 (4.2, 5.6) | 0.445 |
| Yes | 19 (2.1) | 32.6 (18.8, 38.5) | 33.9 (18.8, 93.2) | 250 (187, 281) | 5.1 (3.7, 6.2) | ||||
| Parity | |||||||||
| Nulliparous | 388 (42.5) | 34.7 (24.5, 44.9) | 0.420 | 63.6 (27.8, 113.0) | 0.396 | 220 (177, 264) | 0.005 | 4.8 (4.2, 5.5) | 0.659 |
| Multiparous | 525 (57.5) | 34.1 (24.1, 44.1) | 57.2 (24.7, 111.5) | 203 (162, 252) | 4.8 (4.2, 5.6) | ||||
| Family history of diabetes | |||||||||
| No | 642 (70.3) | 34.8 (24.4, 44.9) | 0.618 | 62.9 (26.1, 113.6) | 0.482 | 215 (169, 266) | 0.001 | 4.8 (4.2, 5.6) | 0.939 |
| Yes | 271(29.7) | 33.6 (24.4, 43.5) | 55.0 (25.0, 106.5) | 197 (159, 245) | 4.9 (4.3, 5.5) | ||||
| Previous GDM | |||||||||
| No | 878 (96.2) | 34.5 (24.1, 44.5) | 0.742 | 60.8 (25.9, 113.0) | 0.300 | 209 (166, 258) | 0.533 | 4.8 (4.2, 5.6) | 0.434 |
| Yes | 35 (3.8) | 34.5 (24.8, 41.4) | 40.7 (21.8, 85.0) | 205 (158, 252) | 4.7 (4.2, 5.3) | ||||
Values are medians (IQRs) or n (%). P-values are for Wilcoxon rank-sum or Kruskal-Wallis tests of differences between groups (a, b, c, d groups with the same superscript symbols have significantly different concentrations). Hcy, homocysteine.
Imputed missing values: n=12 education, n=12 monthly household income, n=57 pre-pregnancy BMI, n=3 smoking status, n=1 alcohol consumption, n=1 parity, n=23 family history of diabetes, n=23 previous GDM
Associations with plasma glucose concentrations and GDM risk
18% of participants were diagnosed with GDM. The associations of maternal plasma folate, vitamins B6 and B12 and homocysteine with plasma glucose concentrations and GDM are presented in Table 2. Higher folate concentrations (1-SD or 1.9nmol/L increment) were associated with higher 2-hour glucose concentrations and higher odds of GDM in all adjusted models (Model 2: 2-hour glucose β: 0.15, 95% CI: 0.04, 0.25; GDM OR: 1.29, 95% CI: 1.01, 1.60). Higher folate concentrations were marginally associated with lower fasting glucose concentrations, but this was not statistically significant in adjusted models.
Table 2.
Associations of maternal plasma folate, vitamins B6 and B12, and homocysteine with fasting and 2-hour plasma glucose, and gestational diabetes mellitus (n=913)
| Fasting Plasma Glucose | 2-hour Plasma Glucose | Gestational Diabetes | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | OR (95% CI) | P | |
| Folate | ||||||
| Unadjusted | -0.03 (-0.06, 0.001) | 0.056 | 0.26 (0.16, 0.35) | 0.001 | 1.40 (1.17, 1.68) | 0.001 |
| Model 1 | -0.02 (-0.06, 0.01) | 0.124 | 0.17 (0.08, 0.27) | 0.001 | 1.30 (1.07, 1.59) | 0.009 |
| Model 2a | -0.02 (-0.05, 0.02) | 0.358 | 0.15 (0.04, 0.25) | 0.014 | 1.29 (1.01, 1.60) | 0.039 |
| Vitamin B12 | ||||||
| Unadjusted | -0.06 (-0.09, -0.03) | 0.007 | -0.09 (-0.19, 0.001) | 0.050 | 0.83 (0.70, 0.98) | 0.028 |
| Model 1 | -0.04 (-0.07, -0.01) | 0.008 | -0.06 (-0.15, 0.03) | 0.224 | 0.86 (0.72, 1.03) | 0.094 |
| Model 2b | -0.04 (-0.07, -0.01) | 0.016 | -0.09 (-0.18, -0.003) | 0.044 | 0.81 (0.68, 0.97) | 0.024 |
| Vitamin B6 | ||||||
| Unadjusted | -0.02 (-0.05, 0.01) | 0.284 | 0.17 (0.08, 0.26) | 0.001 | 1.20 (1.01, 1.43) | 0.039 |
| Model 1 | -0.02 (-0.05, 0.01) | 0.159 | 0.13 (0.04, 0.22) | 0.003 | 1.20 (0.996, 1.44) | 0.055 |
| Model 2c | -0.005 (-0.04, 0.03) | 0.747 | 0.10 (-0.0005, 0.19) | 0.051 | 1.14 (0.94, 1.39) | 0.175 |
| Homocysteine | ||||||
| Unadjusted | -0.03 (-0.06, 0.005) | 0.106 | -0.17 (-0.27, -0.08) | 0.001 | 0.75 (0.63, 0.90) | 0.002 |
| Model 1 | -0.03 (-0.06, -0.0002) | 0.048 | -0.15 (-0.24, -0.06) | 0.001 | 0.75 (0.62, 0.91) | 0.003 |
| Model 2d | -0.05 (-0.08, -0.02) | 0.002 | -0.12 (-0.21, -0.02) | 0.012 | 0.76 (0.62, 0.92) | 0.006 |
Effect estimates are per 1-SD increment in log-transformed concentrations of plasma folate, vitamins B6 and B12, and homocysteine.
Model 1 adjusted for maternal age, ethnicity, education, income, smoking, alcohol intake, physical activity, pre-pregnancy BMI, parity, family history of diabetes, and previous occurrence of GDM.
Model 2 adjusted as for Model 1 and aplasma B6 and B12; bplasma folate and B6; cplasma folate and B12; dplasma folate, B6, and B12
Higher vitamin B12 concentrations (1-SD or 1.4pmol/L increment) were associated with lower fasting and 2-hour glucose concentrations (borderline significance) and lower odds of GDM in the crude model. Associations with fasting glucose remained significant in all adjusted models (Model 2: β: -0.04, 95% CI: -0.07, -0.01). Interestingly, the attenuated associations between vitamin B12, and 2-hour glucose and GDM in Model 1 became significant when further adjusted for other B-vitamins (Model 2: 2-hour glucose β: -0.09, 95% CI: -0.18, -0.003; GDM OR: 0.81, 95% CI: 0.68, 0.97). We found pre-pregnancy BMI to be the main confounding variable attenuating the associations in Model 1.
Significant associations were observed for higher vitamin B6 concentrations (1-SD or 2.4nmol/L increment) with higher 2-hour glucose concentrations and higher odds of GDM in Model 1, although this was attenuated when adjusted for other B-vitamins.
We observed associations between higher homocysteine concentrations (1-SD or 1.2μmol/L increment) and lower 2-hour glucose concentrations and lower odds of GDM in all models (Model 2: 2-hour glucose β: -0.12, 95% CI: -0.21, -0.02; GDM OR: 0.76, 95% CI: 0.62, 0.92). Likewise, higher homocysteine concentrations were associated with lower fasting glucose concentrations after adjustment for confounders and B-vitamins (β: -0.05, 95% CI: -0.08, -0.02).
In examining combinations of folate and vitamin B12 status within the cohort, we observed highest odds of GDM for women with combined vitamin B12 insufficiency and highest concentrations of folate, compared to having vitamin B12 insufficiency but lowest concentrations of folate (Table 3). In contrast, no significant associations between increasing folate concentrations and GDM were found among women with normal vitamin B12 status.
Table 3.
Associations between combinations of maternal vitamin B12 insufficiency status and plasma folate concentrations, and gestational diabetes mellitus (n=913)
| B12-insufficient (<221pmol/L) | B12-normal (≥221pmol/L) | |||||
|---|---|---|---|---|---|---|
| n | OR (95% CI) | P | n | OR (95% CI) | P | |
| Folate, nmol/L (Median, IQR) | ||||||
| T1 (18.2, 11.6-24.4) | 193 | (reference) | 110 | (reference) | ||
| T2 (34.5, 32.3-37.1) | 164 | 1.94 (1.04, 3.62) | 0.036 | 141 | 0.82 (0.33, 2.04) | 0.669 |
| T3 (49.7, 44.5-58.5) | 156 | 1.97 (1.05, 3.68) | 0.034 | 149 | 1.42 (0.61, 3.30) | 0.413 |
Effect estimates are presented as OR (95% CI) for GDM.
All models adjusted for maternal age, ethnicity, education, income, smoking, alcohol intake, physical activity, pre-pregnancy BMI, parity, family history of diabetes, previous occurrence of GDM, and plasma B6.
When the analyses were stratified by ethnicity, the associations of folate and 2-hour glucose and odds of GDM were mainly significant among Indian women, but no substantial associations were observed in Chinese and Malay women (Table 4). Likewise, higher homocysteine concentrations were significantly associated with lower 2-hour glucose concentrations, and marginally associated with lower odds of GDM in Indian women but not in Chinese and Malay women. However, the tests for interaction between ethnicity and folate or homocysteine in relation to 2-hour glucose (ethnicity x folate: P=0.106; ethnicity x homocysteine: P=0.191) and GDM (ethnicity x folate: P=0.425; ethnicity x homocysteine: P=0.909) were not statistically significant, possibly due to a smaller sample size (insufficient power) for Indian women.
Table 4.
Associations of maternal plasma folate, vitamins B6 and B12, and homocysteine with fasting and 2-hour plasma glucose, and gestational diabetes mellitus according to ethnicity (n=913)
| Fasting Plasma Glucose | 2-hour Plasma Glucose | Gestational Diabetes | |||||
|---|---|---|---|---|---|---|---|
| n | β (95% CI) | P | β (95% CI) | P | OR (95% CI) | P | |
| Folatea | |||||||
| Chinese | 498 | -0.03 (-0.08, 0.01) | 0.131 | 0.08 (-0.06, 0.23) | 0.263 | 1.11 (0.84, 1.47) | 0.444 |
| Malay | 250 | 0.01 (-0.07, 0.09) | 0.776 | 0.09 (-0.11, 0.29) | 0.370 | 1.17 (0.65, 2.12) | 0.590 |
| Indian | 165 | 0.001 (-0.08, 0.08) | 0.969 | 0.35 (0.10, 0.60) | 0.007 | 2.28 (1.24, 4.18) | 0.008 |
| Vitamin B12b | |||||||
| Chinese | 498 | -0.03 (-0.07, 0.01) | 0.161 | -0.07 (-0.20, 0.05) | 0.227 | 0.86 (0.68, 1.08) | 0.208 |
| Malay | 250 | -0.05 (-0.12, 0.02) | 0.174 | -0.06 (-0.25, 0.13) | 0.527 | 0.71 (0.43, 1.17) | 0.183 |
| Indian | 165 | -0.03 (-0.11, 0.04) | 0.353 | -0.15 (-0.38, 0.08) | 0.206 | 0.78 (0.49, 1.25) | 0.307 |
| Vitamin B6c | |||||||
| Chinese | 498 | 0.003 (-0.04, 0.04) | 0.897 | 0.09 (-0.04, 0.22) | 0.162 | 1.18 (0.91, 1.53) | 0.216 |
| Malay | 250 | -0.05 (-0.13, 0.02) | 0.168 | 0.03 (-0.17, 0.23) | 0.765 | 1.24 (0.74, 2.08) | 0.420 |
| Indian | 165 | 0.005 (-0.07, 0.08) | 0.903 | 0.14 (-0.10, 0.38) | 0.254 | 0.96 (0.59, 1.57) | 0.887 |
| Homocysteined | |||||||
| Chinese | 498 | -0.03 (-0.08, 0.01) | 0.136 | -0.12 (-0.25, 0.02) | 0.099 | 0.78 (0.59, 1.02) | 0.075 |
| Malay | 250 | -0.06 (-0.13, 0.002) | 0.058 | -0.04 (-0.21, 0.13) | 0.648 | 0.79 (0.50, 1.25) | 0.312 |
| Indian | 165 | -0.03 (-0.10, 0.03) | 0.305 | -0.23 (-0.44, -0.01) | 0.037 | 0.62 (0.39, 1.01) | 0.053 |
Effects estimates are per1-SD increment in log-transformed concentrations of plasma folate, vitamins B6 and B12, and homocysteine.
All models adjusted for maternal age, education, income, smoking, alcohol intake, physical activity, pre-pregnancy BMI, parity, family history of diabetes, previous occurrence of GDM, and aplasma B6 and B12; bplasma folate and B6; cplasma folate and B12;dplasma folate, B6, and B12.
Discussion
In line with our hypothesis, we observed higher folate concentrations to associate with higher 2-hour glucose concentrations and higher odds of GDM; and higher vitamin B12 concentrations to associate with lower fasting and 2-hour glucose concentrations and lower odds of GDM. Conversely, higher homocysteine concentrations were associated with lower fasting and 2-hour glucose concentrations and lower odds of GDM. The associations of folate or homocysteine with GDM and glycemia were largely driven by associations in women of Indian ethnicity. Higher vitamin B6 concentrations were associated with higher 2-hour glucose concentrations but this was not independent of other B-vitamins. Most strikingly, we observed that combined vitamin B12 insufficiency and high folate concentrations was associated with higher odds of GDM compared to women with normal vitamin B12 status and high folate concentrations.
Concordant with the cohort study in China which showed daily folate supplement intake in the first trimester to be associated with higher odds of GDM later in gestation [12], we showed that higher folate concentrations were associated with higher 2-hour glucose concentrations and higher odds of GDM. This association, albeit significant, were slightly confounded by other B-vitamins. Concomitantly, we observed an association between higher vitamin B12 concentrations and lower fasting and 2-hour glucose concentrations, and lower odds of GDM, similar to an Italian study [14], where women without GDM have higher vitamin B12 concentrations then women with GDM. This association was attenuated when adjusted for pre-pregnancy BMI, indicating that BMI explains part of the association of vitamin B12 with plasma glucose concentrations and GDM, which have been demonstrated in previous studies [13, 18]. The attenuated association, however, became significant when further adjusted for other B-vitamins and homocysteine. One possible explanation is that BMI may simultaneously affect concentrations of other B-vitamins and homocysteine which have varying impacts on blood glucose and GDM, and may have masked the true effect of vitamin B12 on glucose tolerance.
Our results further demonstrated that the combination of vitamin B12 insufficiency and high folate concentrations was associated with higher GDM risk, suggesting that an imbalance in the two B-vitamins may be responsible for glucose intolerance. These findings add evidence to a study in Mysore, India [16], where a combination of higher maternal folate concentrations and vitamin B12 deficiency was observed to be associated with higher prevalence of GDM. Our results are reminiscent of the Pune Maternal Nutrition study findings [26], where lowest maternal vitamin B12 coupled with highest folate concentrations were associated with greater insulin resistance in their offspring at six years of age [26]. In our stratified analyses, we found that the significant associations between higher folate and higher 2-hour glucose concentrations, and higher odds of GDM were mainly among Indians, who are also the ethnic group with the highest prevalence of high folate and vitamin B12 insufficiency combination. We speculate that this may be due to a lower consumption of animal foods in Indian women as reflected by a higher proportion of them adopting a vegetarian diet (7.9% vs 1.4% Chinese and 2% Malay). The stronger association among Indian subjects may also be in part attributable to their genetic susceptibility to glucose intolerance [28] rather than differences in dietary practice alone. This finding, however, requires replication with a larger sample size for Indian subjects.
The exact mechanism linking the combined effects of low vitamin B12 and high folate on glucose intolerance and insulin resistance is still unclear. One possible explanation is that when vitamin B12 is insufficient, the conversion of 5-methyltetrahydrofolate to tetrahydrofolate is inhibited [7]. This disrupts the production of purines and thymidine for DNA/RNA synthesis, and impaired DNA synthesis particularly of mitochondrial DNA was observed to be associated with development of insulin resistance [29].
An association between higher vitamin B6 and higher 2-hour glucose concentrations was observed but this is not independent of the influence of other B-vitamins. This matches results from one intervention study where vitamin B6 supplementation reduced postprandial glucose concentrations in pregnant women [19]. However, this study lacked a control group for comparison, and without knowledge on participants’ folate and vitamin B12 status, it is difficult to attribute the observed beneficial effects to vitamin B6 supplementation alone.
We found higher homocysteine concentrations to be associated with lower fasting and 2-hour glucose concentrations and lower odds of GDM. This is in contrast to the proposed mechanism of hyperhomocysteinemia promoting insulin resistance [8, 9]. Studies relating homocysteine to GDM and plasma glucose have reported associations in different directions, which may be due to the large variation in homocysteine concentrations. Homocysteine concentrations in our cohort were much lower compared to commonly cited cut-offs of >16mmol/L for elevated plasma homocysteine [27], and also lower than those reported in studies relating hyperhomocysteinemia to a higher risk of GDM (≥6mmol/L) [14, 15, 17, 30], thus may not be of sufficient concentrations to achieve the adverse metabolic effects.
This is the first description of the independent associations of plasma folate, vitamins B6 and B12, and homocysteine on GDM risk and plasma glucose concentrations, with analyses controlled for possible confounding of exposures on each other. Additionally, we observed that the associations of folate with fasting and postprandial glucose were in opposing directions, indicating that folate may affect the development of GDM via different pathways (e.g. improve hepatic insulin sensitivity but worsen muscle insulin resistance). Using plasma glucose in addition to diagnosis of GDM also allowed us to study associations of B-vitamins with glucose intolerance as a continuum rather than being limited to clinical cut-offs. Most importantly, this is the first study that included three Asian ethnic groups and we showed that the associations between B-vitamins, homocysteine and GDM outcomes were stronger among Indian women compared to the other two ethnic groups.
Several limitations should be noted. First, plasma B-vitamins, homocysteine and glucose were measured at the same time, thus the causal direction cannot be established. Second, nutrient biomarkers may not be a direct reflection of dietary status as they are influenced by genetic and metabolic factors of individuals [31]. However, in the absence of detailed information on B-vitamins supplementation (e.g. dose and frequency), plasma concentrations prove to be advantageous over using self-reported nutrient estimates. Third, the use of the 1999 WHO criteria to diagnose GDM could have underestimated the prevalence of GDM due to the higher cut-offs employed for both fasting and 2-hour plasma glucose, but the lack of data on 1-hour plasma glucose concentrations limited the use of the 2013 WHO criteria. Lastly, missing observations were imputed with mode or median values but this did not substantially change our study results.
Conclusions
We observed that higher folate concentrations during pregnancy were associated with higher postprandial glucose concentrations and higher odds of GDM, while higher vitamin B12 concentrations were associated with lower fasting and postprandial glucose concentrations and lower odds of GDM. Importantly, high folate concentrations coupled with vitamin B12 insufficiency were associated with higher odds of GDM. Given the widespread vitamin B12 insufficiency in our sample (48%), this strongly suggests a need to consider shifting our attention to address this nutritional issue within the population particularly in Indian mothers and others at risk of vitamin B12 insufficiency. Furthermore, we found higher homocysteine concentrations to associate with lower fasting and postprandial glucose concentrations and lower odds of GDM. These findings have much wider implications than for GDM alone, and could potentially contribute to reducing pregnancy complications and adverse birth outcomes associated with having GDM. Overall, the associations between B-vitamins and GDM outcomes found in our study, when confirmed by further research, highlight the need to carefully evaluate and manage folate and vitamin B12 status in pregnant women.
Acknowledgements
We will like to acknowledge the contribution of the GUSTO study group: Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter D. Gluckman, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee. We also thank Stephen Young, Medical Research Council Human Nutrition Research, for assistance with the vitamin B-6 assays.
Funding sources
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), and Nestec. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre. The funding sources have no involvement in study design; in collection, analysis and interpretation of data; in writing of this manuscript; and in decision to submit the article for publication.
Abbreviations
- GDM
Gestational Diabetes Mellitus
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- Hcy
Homocysteine
- KKH
KK Women’s and Children’s Hospital
- MET.mins
Minutes of Metabolic Equivalent of Task
- NUH
National University Hospital
- T2DM
Type 2 Diabetes Mellitus
Footnotes
Statement of authorship
JSL, WWP and MFFC designed the study. JSL analyzed and interpreted data, and wrote the manuscript. WWP contributed to data analysis. MFFC reviewed and edited the manuscript. JSL and MFFC had primary responsibility for final content. LPS, FKPY, KT, KMG and YSC designed and led the GUSTO study. All authors were involved in study conception and data interpretation, critically reviewed the manuscript for intellectual content, read and approved the final manuscript.
Conflict of interest statement
YSL, KMG and YSC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. These authors are part of an academic consortium that has received research funding from Abbot Nutrition, Nestec, and Danone. All other authors declare that there is conflict of interest associated with this manuscript.
References
- [1].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–85. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- [3].Chong Y-S, Cai S, Lin H, Soh SE, Lee Y-S, Leow MK-S, et al. Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: a cohort study. BMC Pregnancy Childbirth. 2014;14(1):1–7. doi: 10.1186/1471-2393-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yajnik CS. The insulin resistance epidemic in India: fetal origins, later lifestyle, or both? Nutr Rev. 2001;59(1 Pt 1):1–9. doi: 10.1111/j.1753-4887.2001.tb01898.x. [DOI] [PubMed] [Google Scholar]
- [5].Tutino GE, Tam WH, Yang X, Chan JC, Lao TT, Ma RC. Diabetes and pregnancy: perspectives from Asia. Diabet Med. 2014;31(3):302–18. doi: 10.1111/dme.12396. [DOI] [PubMed] [Google Scholar]
- [6].Gunderson EP. Gestational diabetes and nutritional recommendations. Curr Diab Rep. 2004;4(5):377–86. doi: 10.1007/s11892-004-0041-5. [DOI] [PubMed] [Google Scholar]
- [7].Preedy VR. B Vitamins and Folate: Chemistry, Analysis, Function and Effects. 2nd ed. London: Royal Society of Chemistry; 2012. [Google Scholar]
- [8].Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JF, Jr, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state--impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41(11):1455–61. doi: 10.1515/CCLM.2003.223. [DOI] [PubMed] [Google Scholar]
- [9].Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care. 2001;24(8):1403–10. doi: 10.2337/diacare.24.8.1403. [DOI] [PubMed] [Google Scholar]
- [10].Serne EH, Stehouwer CD, ter Maaten JC, ter Wee PM, Rauwerda JA, Donker AJ, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99(7):896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- [11].Pinkney JH, Stehouwer CD, Coppack SW, Yudkin JS. Endothelial dysfunction: cause of the insulin resistance syndrome. Diabetes. 1997;46(Suppl 2):S9–13. doi: 10.2337/diab.46.2.s9. [DOI] [PubMed] [Google Scholar]
- [12].Zhu B, Ge X, Huang K, Mao L, Yan S, Xu Y, et al. Folic Acid Supplement Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: Evidence From a Prospective Cohort Study. Diabetes Care. 2016;39(3):e36–7. doi: 10.2337/dc15-2389. [DOI] [PubMed] [Google Scholar]
- [13].Krishnaveni GV, Hill JC, Veena SR, Bhat DS, Wills AK, Karat CL, et al. Low plasma vitamin B12 in pregnancy is associated with gestational 'diabesity' and later diabetes. Diabetologia. 2009;52(11):2350–8. doi: 10.1007/s00125-009-1499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seghieri G, Breschi MC, Anichini R, De Bellis A, Alviggi L, Maida I, et al. Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metabolism. 2003;52(6):720–3. doi: 10.1016/s0026-0495(03)00032-5. [DOI] [PubMed] [Google Scholar]
- [15].Guven MA, Kilinc M, Batukan C, Ekerbicer HC, Aksu T. Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch Gynecol Obstet. 2006;274(6):333–7. doi: 10.1007/s00404-006-0191-6. [DOI] [PubMed] [Google Scholar]
- [16].Idzior-Walus B, Cyganek K, Sztefko K, Seghieri G, Breschi MC, Walus-Miarka M, et al. Total plasma homocysteine correlates in women with gestational diabetes. Arch Gynecol Obstet. 2008;278(4):309–13. doi: 10.1007/s00404-008-0571-1. [DOI] [PubMed] [Google Scholar]
- [17].Tarim E, Bagis T, Kilicdag E, Erkanli S, Aslan E, Sezgin N, et al. Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2004;83(6):543–7. doi: 10.1111/j.0001-6349.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- [18].Knight BA, Shields BM, Brook A, Hill A, Bhat DS, Hattersley AT, et al. Lower Circulating B12 Is Associated with Higher Obesity and Insulin Resistance during Pregnancy in a Non-Diabetic White British Population. PLoS ONE [Electronic Resource] 2015;10(8):e0135268. doi: 10.1371/journal.pone.0135268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bennink HJ, Schreurs WH. Improvement of oral glucose tolerance in gestational diabetes by pyridoxine. Br Med J. 1975;3(5974):13–5. doi: 10.1136/bmj.3.5974.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lopez-Quesada E, Antonia Vilaseca M, Gomez E, Lailla JM. Are plasma total homocysteine and other amino acids associated with glucose intolerance in uncomplicated pregnancies and preeclampsia? Eur J Obstet Gynecol Reprod Biol. 2005;119(1):36–41. doi: 10.1016/j.ejogrb.2004.01.046. [DOI] [PubMed] [Google Scholar]
- [21].Mascarenhas M, Habeebullah S, Sridhar MG. Revisiting the role of first trimester homocysteine as an index of maternal and fetal outcome. Journal of Pregnancy. 2014;2014:123024. doi: 10.1155/2014/123024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Soh S-E, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- [23].Midttun Ø, Kvalheim G, Ueland PM. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem. 2013;405(6):2009–17. doi: 10.1007/s00216-012-6602-6. [DOI] [PubMed] [Google Scholar]
- [24].WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva: WHO; 1999. [Google Scholar]
- [25].Craig Cl, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- [26].Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51(1):29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Institute of Medicine (US) Standing Committee. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. 1998. [accessed 26 September.2016]. https://www.ncbi.nlm.nih.gov/books/NBK114310/ [PubMed] [Google Scholar]
- [28].Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43(10):984–9. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zheng LD, Linarelli LE, Liu L, Wall SS, Greenawald MH, Seidel RW, et al. Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clin Epigenetics. 2015;7(1):60. doi: 10.1186/s13148-015-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cho NH, Lim S, Jang HC, Park HK, Metzger BE. Elevated homocysteine as a risk factor for the development of diabetes in women with a previous history of gestational diabetes mellitus: a 4-year prospective study. Diabetes Care. 2005;28(11):2750–5. doi: 10.2337/diacare.28.11.2750. [DOI] [PubMed] [Google Scholar]
- [31].Willett W. Nutritional Epidemiology. 3rd ed. NY: Oxford University Press; 2012. [Google Scholar]