Abstract
Exposure to stress is highly correlated with the emergence of mood-related illnesses. Because major depressive disorder often emerges in adolescence, we assessed the effects of social defeat stress on responses to depressive-like behaviors in juvenile mice. To do this, postnatal day (PD) 35 male c57BL/6 mice were exposed to 10 days of social defeat stress (PD35–44), while control mice were handled daily. Twenty-four hours after the last episode of defeat (PD45), separate groups of mice were tested in the social interaction, forced swimming, sucrose preference, and elevated plus-maze behavioral assays (n = 7–12 per group). Also, we examined body weight gain across days of social defeat and levels of blood serum corticosterone 40 min after the last episode of defeat stress. Our data indicates that defeated mice exhibited a depressive-like phenotype as inferred from increased social avoidance, increased immobility in the forced swim test, and reduced sucrose preference (a measure of anhedonia), when compared to non-defeated controls. Defeated mice also displayed an anxiogenic-like phenotype when tested on the elevated plus-maze. Lastly, stressed mice displayed lower body weight gain, along with increased blood serum corticosterone levels, when compared to non-stressed controls. Overall, we show that in adolescent male c57BL/6 mice, social defeat stress induces a depression- and anxiety-like phenotype 24 h after the last episode of stress. These data suggest that the social defeat paradigm may be used to examine the etiology of stress-induced mood-related disorders during adolescence.
Keywords: Anhedonia, animal model, anxiety, bullying, depression, forced swim test, juvenile, mood disorders
Introduction
Exposure to stress has been linked to the etiology of mood-related disorders (Krishnan & Nestler, 2008). In particular, stressful events have been causally linked to anxiety-related and major depression syndromes (Tsoory et al., 2007). Throughout the literature, preclinical, clinical, and postmortem investigations have primarily examined how both stress and antidepressant pharmacotherapy influence neurobiological homeostatic mechanisms that in turn may underlie the neurobiology of major depressive disorder (Krishnan et al., 2007; Kupfer et al., 2012). To date, most of these studies have examined the underpinnings of mood-related disorders using adult populations. This is surprising given that epidemiological reports suggest that the first incidence/episode of depression is most often reported prior to adulthood (Lewinsohn et al., 1993; Paus et al., 2008).
Adolescence, the transitional stage between childhood and adulthood, is characterized by distinct neurobiological changes that underlie the emergence of sex differences (Eiland & Romeo, 2013; Spear, 2000), as well as other age-specific behavioral phenotypes, including increased social activity, playfulness and risk-seeking behavior (Doremus-Fitzwater et al., 2010; Laviola et al., 2002; Richards et al., 2012). As such, juveniles are more likely to experience stressful life events (Charney & Manji, 2004), and are simultaneously more sensitive to the deleterious effects of stress (Stone & Quartermain, 1997; McCormick et al., 2010). This makes adolescence a unique developmental stage to examine how specific stressors, such as social stress, influence and/or precipitate the development of mood-related disorders. At the preclinical level, the social defeat paradigm is considered one of the most robust models of stress-induced mood-related illnesses (Berton et al., 2006). When compared to other animal models of depression, such as chronic unpredictable mild stress (Willner et al., 1987) or the forced swim test (Porsolt et al., 1977), the social defeat paradigm possesses higher face, predictive, and ethological validity that results in enduring behavioral and neurobiological changes that mimic several symptoms of the human condition (Berton et al., 2006; Krishnan & Nestler, 2008). For example, in adult rodents, social defeat stress results in increased activation of the hypothalamic-pituitary-adrenal (HPA) axis, as inferred by elevated levels of blood serum corticosterone (Buwalda et al., 1999), in addition to decreased preference for sucrose (a measure of anhedonia; Willner et al., 1987), increased sensitivity to helplessness measures (Warren et al., 2013), and increased social avoidance – behaviors collectively described as a depressive-like phenotype (Krishnan & Nestler, 2008).
Although few investigations have examined the effects of early-life social defeat stress on mood-eliciting behavioral tasks in adulthood (Buwalda et al., 2013; Ver Hoeve et al., 2013; Watt et al., 2009), the social defeat model has not been thoroughly examined as a potential model of stress-induced depression within the juvenile period of development. Thus, the current investigation was designed to assess behavioral responsivity to a range of emotion-eliciting stimuli shortly (24 h) after chronic social defeat stress in adolescent (postnatal day [PD] 35) male c57BL/6 mice.
Materials and methods
Animals
Five week-old male c57BL/6 mice, and adult (retired) male CD1 breeders, were used in this study. The c57BL/6 mice were obtained from the Department of Psychology Mouse-Breeding Colony at California State University San Bernardino (CSUSB), while the CD1 retired breeders were purchased from Charles River Laboratories. Mice were housed in standard polypropylene cages containing wood shavings (c57BL/6, four per cage; CD1, one per cage) and placed on a 12-h light/dark cycle (lights on at 7:00 A.M.) with unrestricted access to food and water. Experiments were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and with approval of the Institutional Animal Care and Use Committee at CSUSB.
Social defeat stress and experimental design
Because social defeat stress follows the resident/intruder paradigm (Kudryavtseva et al., 1991; Miczek, 1979), where conflict stress involves the threat from a more dominant resident counterpart, we selected to use the inbred CD1 strain of mice as aggressors for this investigation (Parmigiani et al., 1999). Briefly, CD1 male mice with consistent attack latencies (≤30 s on three consecutive screening tests) were housed in cages fitted with perforated Plexiglas separators, which allow sensory contact without physical contact, and used to stress/defeat the experimental c57BL/6 mice. Specifically, adolescent (PD35) c57BL/6 mice were exposed to a 10 min long defeat episode, and then housed for the remainder of the day in the compartment next to the aggressor. In the event that the CD1 mouse was exhibiting overly aggressive attacks (continuous biting even after the experimental mouse displayed submissive posturing), the defeat bout was immediately terminated (Golden et al., 2011; Iñiguez et al., 2014). This procedure was repeated for 10 consecutive days (PD35–44) with different CD1 aggressors each day. The age at the start of social defeat stress (PD35) was selected because it roughly approximates mid-adolescence (Eiland & Romeo, 2013; Tirelli et al., 2003), a developmental stage in which the onset of major depressive disorder is most often reported in humans (Burke et al., 1990). Non-defeated (control) adolescent mice were handled daily and housed in similar cages, one on each side of a perforated Plexiglas partition. Immediately after the last defeat episode (i.e. PD44), both stressed (defeated) and non-stressed (control) mice were single housed. Twenty-four hours later (PD45), separate groups of c57BL/6 mice were randomly assigned to the different behavioral tasks described below. Separate groups of adolescent c57BL/6 mice were used for each experiment in order to avoid possible carryover effects (Table 1). All behaviors were recorded via an automated video tracking system (Noldus®), except for the forced swim test, which was scored by observers blind to stress conditions. Lastly, for the corticosterone immunoassay experiment, a separate group of mice was killed 40 min after the 10th defeat episode (PD44) in order to examine the activation of the HPA axis as a function of social defeat stress (Krishnan et al., 2007; Warren et al., 2013).
Table 1.
Experimental groups.
| Group | Subjects | Age | Interval | Procedure | |
|---|---|---|---|---|---|
| 1 | Control Defeated |
n =10 n =10 |
PD 35–44 | 24 h | Social interaction |
| 2 | Control Defeated |
n =8 n =10 |
PD 35–44 | 24 h | Forced swim test |
| 3 | Control Defeated |
n =10 n =12 |
PD 35–44 | 24 h | Sucrose preference |
| 4 | Control Defeated |
n =9 n =7 |
PD 35–44 | 24 h | Elevated plus-maze |
| 5 | Control Defeated |
n =10 n =9 |
PD 35–44 | 40 min | Corticosterone assay |
PD, postnatal day.
Social interaction test
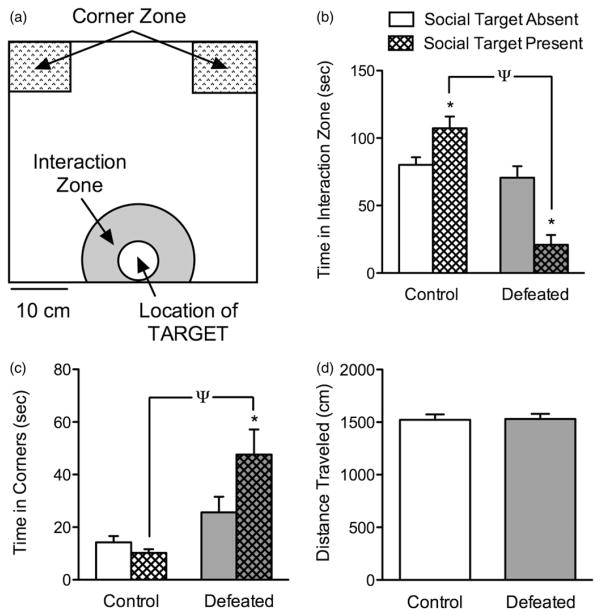
The social interaction test is used to assess social avoidance behavior (Berton et al., 2006). This is a two-step test (Krishnan et al., 2008), conducted under red light conditions. In the first 2.5 min session, a c57BL/6 mouse is allowed to freely explore an open field arena (40 cm length × 40 cm width × 40 cm height; Figure 1a shows the schematic). Along one side of the arena is a circular (7 cm diameter) wire cage (Stoelting Co., Wood Dale, IL) that remains empty during the first trial (target absent). The experimental c57BL/6 mouse is then removed from the open field arena and a novel CD1 male mouse is placed into the wire cage. In the second 2.5 min trial (target present), the experimental c57BL/6 mouse is reintroduced into this arena now containing a social target (unfamiliar CD1 mouse) within the wire cage. In this investigation, time (s) spent in the interaction zone (8 cm wide corridor surrounding the wire cage) and the corner zones (10 × 10 cm) were the dependent variables (Iñiguez et al., 2014). Additionally, we recorded the distance traveled (cm) during the first 2.5 min of the social interaction test to examine whether basal locomotor activity could be influenced by social defeat stress.
Figure 1.
Social defeat stress induces avoidance behaviors in adolescent c57BL/6 male mice. (a) Schematic of the social interaction/avoidance arena illustrating the geographic location and size of the interaction zone and corner zones with respect to the enclosure (circular wire cage) in which a social target (CD1 mouse) is positioned. (b) Control (non-stressed; n =10) mice showed significantly higher levels of interaction time (p<0.05), whereas defeated mice (n =10) spent significantly less time interacting (p<0.05), in the presence of a social target. (c) This avoidance behavior as a result of social defeat stress was also evident when assessing time in the corner zones, in which defeated (stressed) mice spent significantly more time in the corner zones in the presence of the social target (p<0.05). (d) No differences in locomotor activity (distance traveled in cm) during the first 2.5 min of the social interaction test was observed between the groups (p>0.05). *p<0.05, within group comparison (presence versus absence of social target). Ψp<0.05, between group comparison (control versus defeated). Data are presented as mean + SEM.
Forced swim test
The forced swim test is a behavioral procedure in which rodents are forced to swim under inescapable conditions. Initially, rodents engage in escape-like behaviors but eventually adopt a posture in which they make only the movements necessary to maintain their head above water; however, antidepressant treatment can significantly increase their escape-directed behaviors (Iñiguez et al., 2010a), an effect that has been correlated with antidepressant efficacy in humans (Porsolt et al., 1977). Conversely, an animal that spends more time immobile is considered to be more sensitive to the effects of inescapable stress (Iñiguez et al., 2010b). This task was carried out according to published protocols (Iñiguez et al., 2014). Specifically, mice were forced to swim once in a 4 L Pyrex glass beaker containing 3 L of water (24 ± 1 °C) for 6 min. All cylinders were emptied and cleaned between mice. The time (s) to initially adopt a posture of immobility (latency to immobility), as well as the total time (s) spent immobile, during the last 5 min of the test, were the dependent variables.
Sucrose preference
The sucrose preference test was assayed using published protocols (Iñiguez et al., 2009). This test consisted of a 2-bottle procedure in which mice were given the choice between consuming water or a 1% sucrose solution. This test has been widely used across the literature to examine the effects of stress-induced anhedonia (Willner et al., 1987), a reduced ability to experience pleasure. Adolescent mice were habituated to drink water from two bottles during the last 5 days of social defeat (PD40–44). On PD45, 24 h after the last social defeat episode, animals were single housed in a cage that had two drinking bottles. One of the bottles had water, while the other bottle had a 1% sucrose solution. Water and sucrose consumption was measured the following day (8:00 A.M.). The position of the sucrose bottle was counterbalanced (left versus right) across the different cages to control for potential side-preference bias. Preference for sucrose over water (sucrose/[sucrose + water]) was used as a measure for sensitivity to reward (Warren et al., 2011).
Elevated plus-maze
The elevated plus-maze is a classic test of anxiety-like behavior (Montgomery, 1955), that uses the natural reluctance of rodents to explore open spaces (Pellow et al., 1985). The maze (Stoelting Co., Wood Dale, IL) was made of gray plastic and consisted of two perpendicular intersecting runways (5 cm wide × 35 cm long). One runway had tall walls (closed arms; 15 cm in height), and the other one had no walls (open arms). The arms were connected together by a central area (5 × 5 cm), and the maze was elevated 40 cm from the floor. At the beginning of the test, under controlled light conditions (~90 lux), rodents were placed in the central area, facing one of the open arms, and the cumulative time (s) spent in the open- and closed-arms was recorded (Iñiguez et al., 2014). In addition, the total distance (cm) traveled was recorded throughout the 5 min test.
Corticosterone immunoassay
A separate set of adolescent c57BL/6 mice was used to examine how repeated episodes of social defeat would influence the activation of the HPA axis, as inferred by levels of trunk blood serum corticosterone (Backström & Winberg, 2013). Forty minutes after the 10th episode of social defeat (PD44), mice were decapitated, and trunk blood was collected into standard Heparin-coated collection tubes and placed on ice (Krishnan et al., 2007). Blood was centrifuged (1500g) for 15 min at 4 °C. Serum supernatant was collected, and stored at −20 °C, until corticosterone levels were assayed as previously described (Warren et al., 2013), per manufacturer’s instructions (Assay Designs, Ann Arbor, MI).
Statistical analysis
Assignment of adolescent mice to the different experimental conditions was random. Data was analyzed using ANOVA techniques, with stress (control versus defeat), presence of social target (absent versus present; repeated measure), and body weight (across days of defeat; repeated measure) as sources of variance. Two-tailed Student’s t-tests were used for analyses implicating two-group comparisons. Data are presented as the mean + SEM. Statistical significance was defined as p<0.05.
Results
Social interaction
The effects of 10 days of social defeat stress during adolescence on social behaviors, 24 h after the last defeat (PD45), are shown in Figure 1(b–d). A two-way ANOVA, with stress and presence of social target as independent variables, indicated that the time spent in the interaction zone (Figure 1b) was dependent on a stress main effect (F(1,36) = 39.9, p<0.0001), and a stress by presence of target interaction (F(1,36) = 25.76, p<0.001). Post hoc analyses indicated that control (non-stressed) adolescent mice (n =10) showed significantly higher levels of social interaction when the target was present (target absent versus present, p<0.05). In contrast, social interaction levels were significantly reduced in mice exposed to social defeat (n =10) in the presence of a social target (target absent versus present, p<0.05), or when compared to non-stressed controls (between group comparison, p<0.05).
The time spent in the corner zones (Figure 1c), another measure of social avoidance (Krishnan et al., 2008), also indicated that the total time spent in the corners was dependent on a stress main effect (F(1,36) = 17.77, p<0.001), as well as a stress by presence of target interaction (F(1,36) = 5.05, p<0.03). Specifically, socially defeated c57BL/6 mice spent significantly more time in the corner zones in the presence of the social target (target absent versus present, p<0.05), or when compared to controls (target present, p<0.05). Importantly, no differences in total distance (cm) traveled were observed between the groups during the target absent (2.5 min) condition of the social interaction test (Figure 1d; p>0.05).
Forced swim test
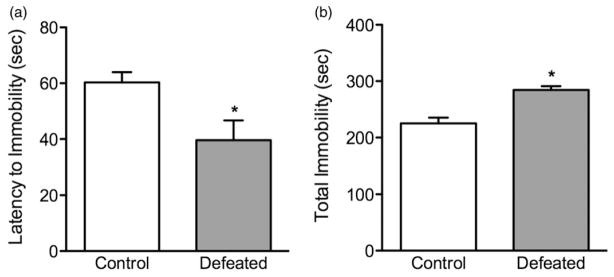
Adolescent social defeat stress increases sensitivity to behavioral despair in the forced swim test (Figure 2). Twenty-four hours after the last exposure to social defeat stress (PD45), adolescent mice were exposed to a 6-min episode of inescapable swimming stress. Here, the defeated mice (n =10) displayed shorter latencies (s) to adopt a posture of immobility (t(16) = 2.76, p<0.01; Figure 2a), and spent significantly more time (s) in the immobile position (t(16) = 2.32, p<0.02; Figure 2b), when compared to non-stressed control mice (n =8).
Figure 2.
Effects of adolescent social defeat stress on forced swimming behavior, 24 h after the last defeat episode (postnatal day 45). Defeated (stressed; n =10) adolescent mice exhibited a depressive-like behavior as inferred by (a) lower time to adopt an initial posture of immobility and conversely (b) spending significantly more time in the immobile position, when compared to control (non-stressed; n =8) mice. *Significantly different from controls (p<0.05). Data are presented as mean time (s) + SEM.
Sucrose preference
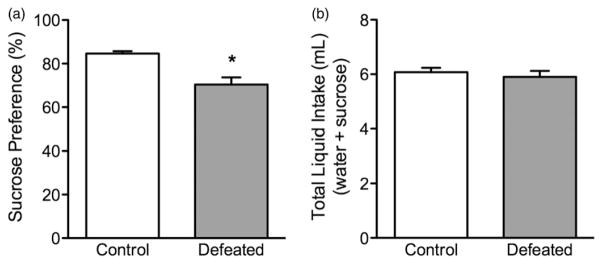
Adolescent social defeat stress decreases preference for sucrose in adolescent c57BL/6 mice (Figure 3). Twenty-four hours after the last episode of social defeat stress, the defeated mice (n =12) showed a significant decrease in preference for a 1% sucrose solution (Figure 3a), when compared to non-stressed controls (n =10); t(20) = 3.73, p<0.01. No differences in total liquid (mL) intake (sucrose + water) were observed between the groups (p>0.05; Figure 3b).
Figure 3.
Effects of social defeat stress on sucrose preference in adolescent male c57BL/6 mice. (a) Twenty-four hours after social defeat (postnatal day 45), stressed (defeated; n =12) adolescent mice displayed a decreased preference for a 1% sucrose solution, when compared to control (non-stressed; n =10) mice. (b) No differences in total liquid intake (sucrose + water) were detected between the groups. *Significantly different from controls (p<0.05). Data are presented as percentage or total mL consumed (mean + SEM).
Elevated plus-maze
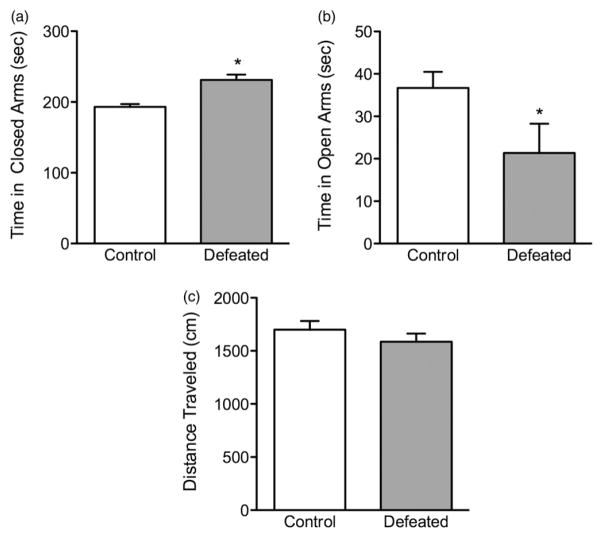
Figure 4 shows the effects of adolescent social defeat stress on the anxiogenic environment of the elevated plus-maze. When compared to non-stressed controls, (n =9), socially defeated adolescent mice (n =7) spent significantly more time in the closed arms (t(14) = 4.87, p<0.001; Figure 4a), while spending less time in the open arms (t(14) = 2.06, p<0.02; Figure 4b) of the maze. Importantly, no differences in total locomotor activity between defeated (stressed) and control mice were evident during the 5 min test (p>0.05; Figure 4c).
Figure 4.
Effects of 10 days of adolescent social defeat stress on anxiety-like behavior in the elevated plus-maze. Twenty-four hours after the last social defeat episode, the stressed (defeated; n =7) mice spent (a) significantly more time (s) in the closed arms, (b) while spending less time in the open arms of the maze, when compared to control (non-stressed; n =9) mice. (c) No differences in the total distance (cm) traveled within the 5 min test were observed between the groups (p>0.05). *Significantly different from controls (p<0.05). Data are presented as mean time (s) and distance traveled (cm) + SEM.
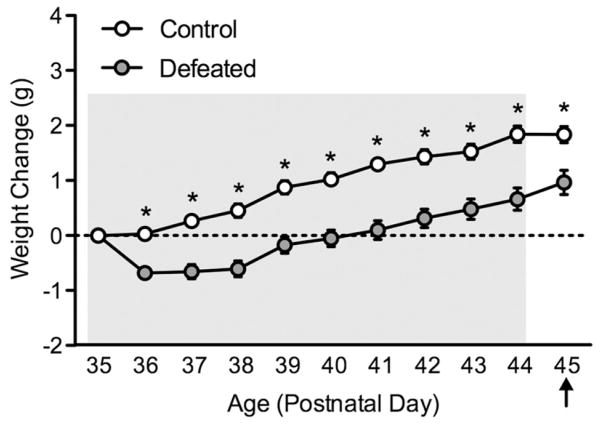
Body weight
Figure 5 shows the effects of 10 days of social defeat stress on body weight change in adolescent male c57BL/6 mice. Body weight was recorded throughout the 10 days of social defeat (PD35–44). Weight change was calculated by subtracting the body weight of the animal from the initial weight on PD35; thus a positive number would suggest weight increase, while a negative number would indicate body weight decrease (Warren et al., 2013). A mixed-design repeated measures ANOVA revealed that body weight changed as a function of stress (between group main effect: F(1,18) = 7.41, p<0.001), day of social defeat episode (repeated measure main effect: F(9,162) = 7.45, p<0.01), and a stress by day of defeat interaction (F(9,162) = 2.51, p<0.01). Post hoc analyses revealed that when compared to control mice (n =10), defeated mice (n =10) displayed lower weights as of the second day (i.e. PD36) of stress (p<0.05, respectively). Twenty-four hours after the last episode of stress (PD45), adolescent mice exposed to social defeat weighed significantly less than the control mice (t(18) = 2.14, p<0.05).
Figure 5.
Effects of social defeat stress on body weight gain in adolescent male c57BL/6 mice. Social stress (postnatal day 35–44) resulted in significantly lower body weight gain across days of social defeat, starting on day 2 of stress exposure, when compared to non-stressed (control) mice (n =10 per group). Body weight remained significantly decreased in the defeated group 24 h after the last day of stress exposure (postnatal day 45). Arrow indicates day of behavioral testing. *Significantly different when compared to controls (p<0.05). Data are presented as weight change in grams (mean + SEM).
Corticosterone immunoassay
Figure 6 shows the effects of social defeat stress on blood serum corticosterone levels in adolescent c57BL/6 mice. Forty min after the last episode of social defeat (PD44), the stressed mice (defeated, n =9) exhibited significantly higher levels of corticosterone (t(17) = 2.59, p<0.001), when compared to non-stressed controls (n =10).
Figure 6.
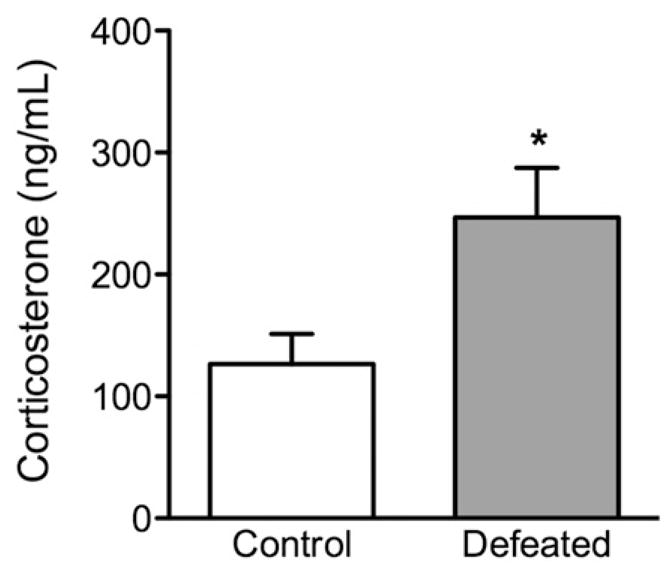
Effects of social defeat stress on adolescent neuroendocrine stress response. (a) Trunk blood of c57BL/6 mice was taken 40 min after the 10th episode of social defeat stress (i.e. postnatal day 44). Adolescent mice exposed to social defeat stress (defeated; n =9) displayed significantly increased serum corticosterone levels when compared to non-stressed mice (control; n =10). Data are presented as concentrations in ng/mL. *Significantly different than control mice (p<0.05). Data are presented as mean + SEM.
Discussion
The present investigation was designed to evaluate whether the social defeat stress model (Berton et al., 2006), a common paradigm used to examine the behavioral, physiological, and molecular underpinnings of mood-related disorders (Krishnan & Nestler, 2011; Trainor et al., 2011), would result in a depressive-like phenotype in adolescent c57BL/6 male mice. This approach was taken because stress exposure during the juvenile stage of development increases the vulnerability to develop mood-related disorders, such as depression- and anxiety-related syndromes (Spear, 2000; Wals & Verhulst, 2005). The social defeat paradigm was selected because social stressors are of particular relevance to juvenile populations, in the form of bullying and increased child-parent conflict (Gladstone et al., 2006). Our findings indicate that exposure to 10 days of defeat stress during adolescence (PD35–44) results in an overall depression- and anxiogenic-like phenotype as indicated by both behavioral and physiological responses that are commonly used to evaluate mood-related syndromes in murine models of depression (Krishnan & Nestler, 2011; Trainor et al., 2011).
When tested on the social interaction test, defeated adolescent mice displayed an avoidant behavioral response, by spending significantly less time in the interaction zone, while spending more time in the corner zones, when compared to non-defeated controls. This stress-induced avoidance behavior mirrors that of adult c57BL/6 male mice exposed to social defeat stress (Iñiguez et al., 2010b), wherein this response is long-lasting (Krishnan et al., 2007) and reversed by chronic, but not acute, administration of traditional antidepressants (Berton et al., 2006). Furthermore, when assessing sensitivity to a subsequent helplessness-related stressor, namely the forced swim test, we found that defeated adolescent mice displayed increased sensitivity to despair measures, as inferred from decreased time to adopt a posture of immobility and a total increased time spent immobile, when compared to controls (Figure 2). This depression-like behavioral phenotype resembles that of early-adult (~PD56) c57BL/6 male mice exposed to a similar defeat stress regimen (Huang et al., 2013), yet here, we extend these findings to mid-adolescence (PD45), the developmental stage when the first incidence of depression is most commonly reported (Burke et al., 1990). Because social avoidance and increased helplessness behaviors are considered symptoms of numerous psychiatric illnesses in addition to major depression (APA, 2000; Berton et al., 2006), we assessed whether adolescent social defeat would influence sucrose preference in a two-bottle choice test as a complementary measure of depression-like behavior. Specifically, we selected this additional measure because anhedonia (Papp et al., 1991), the reduced ability to experience pleasure, is one of the core symptoms of clinical depression (APA, 2000), and rodents exposed to unpredictable environmental stressors typically exhibit a reduced preference for sweet solutions (Willner et al., 1987). Here, we found that defeated adolescent mice displayed a reduced preference for the sucrose solution, without changes in total fluid intake, when compared to controls (Figure 3). Collectively, the data from the social interaction, forced swim, and sucrose preference tests suggest that exposure to social defeat stress during adolescence results in the expression of core and common endophenotypes of major depressive disorder, which include avoidance, helplessness, and anhedonia (Krishnan & Nestler, 2008).
The co-occurrence of psychiatric illnesses, such as major depression and generalized anxiety disorder is very common in children and adolescents (Axelson & Birmaher, 2001). Consequently, an “anxious-depression” classification has been included in the new fifth edition of the diagnostic and statistical manual of mental disorders (DSM-5; Das-Munshi et al., 2008). For this reason, when assessing stress-induced mood-related phenotypes at the preclinical level, anxiety-related tests, in addition to behavioral despair measures, are commonly implemented (Beuke et al., 2003). Thus, we examined how adolescent social defeat stress would influence responses to the anxiogenic environment of the elevated plus-maze (Montgomery, 1955). We found that defeated mice spent significantly less time in the open arms, while spending more time in the closed arms of the maze, when compared to controls – a classic anxiogenic-like response (Pellow et al., 1985). This increased sensitivity to anxiety-like behavior in adolescent mice mimics that of adult rodents reported after social defeat (Berton et al., 2006), highlighting that social defeat stress influences sensitivity to anxiety-inducing environments in adolescent mice in a similar manner as adults (Warren et al., 2013).
In addition to mood-related behaviors, physiological changes – such as fluctuations in body weight and levels of blood corticosterone – are commonly used to evaluate depression-related phenotypes. For example, weight disorders are a common characteristic of pediatric major depressive disorder (APA, 2000), and therefore, we monitored weight gain across days of defeat stress. Not surprisingly, we found that defeated mice displayed decreased body weight gain as of the second day of defeat, and remained significantly lower 24 h after the last episode of stress (PD45). This stress-induced weight reduction could potentially suggest a weight disorder that mimics another endophenotype of clinical depression (APA, 2000). It is important to note that although the defeated mice weighed significantly lower than the non-stressed group, we found no differences in basal locomotor activity as a function of stress exposure across the different behavioral tests, indicating that the depressive-like phenotype (i.e. social avoidance, helplessness, and decreased preference for sucrose) was not attributed to stress-induced decreases in body weight. In addition to fluctuations in body weight, another common physiological mechanism by which the brain responds to stress is the activation of the HPA axis (McCormick et al., 2010). Accordingly, we examined levels of blood serum corticosterone 40 min after the last episode of social defeat stress in a separate subset of adolescent mice (Group 5 in Table 1). This approach was taken because unlike most acute stressors, where maximal HPA activation occurs within the first 10 min post stress termination, there is a 20–40 min delay of maximal HPA activation after social conflicts (Heinrichs & Koob, 2006). Here, we found that adolescent mice exposed to social defeat stress displayed significantly higher levels of serum corticosterone when compared to non-defeated mice, reflecting the activation of the HPA axis as a result of social defeat exposure (Krishnan et al., 2007). This finding is likely mediating, at least in part, the depression- and anxiety-like phenotype observed across the different behavioral tests, since previous studies indicate that persistent activation of the HPA axis is associated with mood-related responses in rodents (Warren et al., 2013). While the neurobiological mechanism(s) underlying this defeat-induced depressive- and anxiogenic-output is not well understood, such behavioral responses in adult rodents have been correlated with alterations in post-receptor signaling molecules, namely brain derived neurotropic factor (BDNF)-related signaling (Eisch et al., 2003; Jiang & Salton, 2013), within the dopaminergic reward system (i.e. ventral tegmental area [VTA]–nucleus accumbens circuitry). In particular, stress-induced upregulation of VTA BDNF and several of its downstream effectors, including the extracellular signal-regulated kinase, have been found to mediate depression-like behaviors in both adult and adolescent rodents (Iñiguez et al., 2010b, 2014). Indeed, recent evidence links the stress-induced increases in glucocorticoids and depression-like behavior with dysregulation of dopaminergic neurons within the adolescent VTA (Niwa et al., 2013). Conversely, juvenile administration of the antidepressant fluoxetine (Prozac), the only Food and Drug Administration approved pharmaceutical for pediatric depression (Zito et al., 2006), regulates the activation of VTA BDNF-related molecules in an antagonistic manner (Warren et al., 2011; Iñiguez et al., 2014). However, we must emphasize that clinical diagnosis of depression- and anxiety-related syndromes are based on behavioral abnormalities only, with no known biologically based diagnostic distinctions (Krishnan & Nestler, 2008).
Murine models of depression have proven to be instrumental in the study of affective illnesses (Krishnan & Nestler, 2011). Here, we extend the social defeat paradigm as a valuable model to study adolescent depression- and anxiety-related syndromes. In adult rodents, social defeat stress induces, in most animals, the development of an array of behavioral and physiological changes that are reminiscent of depression- and anxiety-related symptoms. These animals are traditionally referred to as susceptible. However, social defeat also produces a small subgroup of animals that do not develop social avoidance, described as resilient mice (Krishnan et al., 2007). While the present results mirror those of adult rodents, future studies will be needed in order to examine if the resilient-like behavioral phenotype is evident in adolescent male c57BL/6 mice, as it is reported in early-(Huang et al., 2013) and mid-adulthood (Krishnan et al., 2007). Also, a limitation of the present study is that we did not assess the effects of social defeat stress in adolescent female subjects, thus hindering the interpretability of our results to the clinical setting, where twice as many girls (versus boys) are diagnosed with major depression (Hankin et al., 1998). Moreover, further characterization of social defeat stress on adolescent-specific behaviors, such as decision-making, play behavior, and sleep-wake patterns (Malkesman & Weller, 2009) will be required to further validate this paradigm as an adolescent model of affective distress. Meanwhile, it would be interesting to investigate the ability of potential antidepressant pharmaceuticals to acutely reverse the sequelae of juvenile social defeat stress, in a somewhat similar fashion as chronic administration of fluoxetine (Berton et al., 2006).
Conclusions
The use of animal models, though not without limitations (Krishnan & Nestler, 2011), is invaluable to our understanding of stress-induced mental illness (Krishnan & Nestler, 2008). However, until recently, most models of stress-induced depression have primarily implemented stressors in adulthood, rather than adolescence, the developmental stage when the first incidence of depression is most often reported (Kessler et al., 2001). Here, we demonstrate that exposure to 10 days of defeat is a potent social stressor in adolescent male c57BL/6 mice capable of inducing depression- and anxiety-like symptomatology (Krishnan & Nestler, 2011), making the social defeat paradigm a potential novel preclinical model to study adolescent stress-induced psychopathologies. Developing a suitable juvenile animal model of mood-related disorders is critical to understand and identify the basic neurobiological mechanisms underlying the etiology of anxious-depression (Das-Munshi et al., 2008), which in turn, will further assist in the development of more effective and safer pharmacological therapies for juvenile populations (Findling et al., 2006).
Acknowledgments
The authors are grateful to Tiffany Aiello, Raisa Ahmed, and Victor Cao for excellent technical assistance.
Footnotes
Declaration of interest
The National Institutes of Health (NIH) provided funding for this study. The NIH had no involvement in the design of the study, data collection process, or interpretation of the results. The funding sources did not play any role in writing the research manuscript or the decision to submit the paper for publication. This work was supported by a grant from the National Institute on Drug Abuse (R24DA033877 to SDI), a California State University Program for Education and Research in Biotechnology grant (to SDI), and the Associated Students Incorporated grants from CSUSB (to LMR, SJN, GD, NNZ, KLS, and BC).
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. DSM-IV Edition. [Google Scholar]
- Axelson DA, Birmaher B. Relation between anxiety and depressive disorders in childhood and adolescence. Depress Anxiety. 2001;14:67–78. doi: 10.1002/da.1048. [DOI] [PubMed] [Google Scholar]
- Backström T, Winberg S. Central corticotropin releasing factor and social stress. Front Neurosci. 2013;7:117. doi: 10.3389/fnins.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Beuke CJ, Fischer R, McDowall J. Anxiety and depression: why and how to measure their separate effects. Clin Psychol Rev. 2003;23:831–48. doi: 10.1016/s0272-7358(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Burke KC, Burke JD, Jr, Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Arch Gen Psychiatry. 1990;47:511–18. doi: 10.1001/archpsyc.1990.01810180011002. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience. 2013;249:258–70. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, et al. Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–20. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;225:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Das-Munshi J, Goldberg D, Bebbington PE, Bhugra DK, Brugha TS, Dewey ME, Jenkins R, et al. Public health significance of mixed anxiety and depression: beyond current classification. Br J Psychiatry. 2008;192:171–7. doi: 10.1192/bjp.bp.107.036707. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–71. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Findling RL, McNamara NK, Stansbrey RJ, Feeny NC, Young CM, Peric FV, Youngstrom EA. The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J Child Adolesc Psychopharmacol. 2006;16:131–45. doi: 10.1089/cap.2006.16.131. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Malhi GS. Do bullied children become anxious and depressed adults?: a cross-sectional investigation of the correlates of bullying and anxious depression. J Nerv Ment Dis. 2006;194:201–8. doi: 10.1097/01.nmd.0000202491.99719.c3. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–40. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Application of experimental stressors in laboratory rodents. Curr Protoc Neurosci. 2006;Chapter 8(Unit 8):4. doi: 10.1002/0471142301.ns0804s34. [DOI] [PubMed] [Google Scholar]
- Huang GB, Zhao T, Muna SS, Bagalkot TR, Jin HM, Chae HJ, Chung YC. Effects of chronic social defeat stress on behaviour, endoplasmic reticulum proteins and choline acetyl-transferase in adolescent mice. Int J Neuropsychopharmacol. 2013;16:1635–47. doi: 10.1017/S1461145713000060. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Bolaños-Guzmán CA. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry. 2010a;67:1057–66. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolaños-Guzmán CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–24. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010b;30:7652–63. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, Wright KN, et al. Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci. 2014;34:1007–21. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Salton SR. The role of neurotrophins in major depressive disorder. Transl Neurosci. 2013;4:46–58. doi: 10.2478/s13380-013-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49:1002–14. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–47. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, Berton O, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–20. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macri S, Adriani W, Morley Fletcher S. Psychobiological determinants of risk behavior in adolescence. Ann Ist Super Sanita. 2002;38:279–87. [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychophathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J Abnorm Psychol. 1993;102:133–44. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Weller A. Two different putative genetic animal models of childhood depression – a review. Prog Neurobiol. 2009;88:153–69. doi: 10.1016/j.pneurobio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–60. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–9. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–9. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P, Rogers J, Ferrari PF. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 1999;23:957–69. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. Neural systems underlying motivated behavior in adolescence: implications for preventative medicine. Prev Med. 2012;55:s7–16. doi: 10.1016/j.ypmed.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–5. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychos-timulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–78. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–56. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S. Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience. 2013;249:63–73. doi: 10.1016/j.neuroscience.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wals M, Verhulst F. Child and adolescent antecedents of adult mood disorders. Curr Opin Psychiatry. 2005;18:15–19. [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolaños-Guzmán CA. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–58. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Zito JM, Tobi H, de Jong-van den Berg LT, Fegert JM, Safer DJ, Janhsen K, Hansen DG, et al. Antidepressant prevalence for youths: a multi-national comparison. Pharmacoepidemiol Drug Saf. 2006;15:793–8. doi: 10.1002/pds.1254. [DOI] [PubMed] [Google Scholar]